Fluorocarbon chain-free hydrophobic fabric as well as preparation method and application thereof
A technology of fluorocarbon chains and fabrics, which is applied in plant fibers, textiles, papermaking, and liquid-repellent fibers, and can solve problems such as environmental pollution and low surface energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0025] The synthesis of 1,2-bis(p-toluenesulfonyl)hydrazine (TsNHNHTs) is shown in the following formula:
[0026]
[0027] Synthetic steps. Under nitrogen protection, 18.64 g (100.00 mmol) p-toluenesulfonyl hydrazide (p-Toluenesulfonyl hydrazide) and 28.60 g (150.00 mmol) p-toluenesulfonyl chloride (p-toluene sulfochloride) were added to a 1000 ml three-necked flask, and 120 mL Dichloromethane (except water) was used as a solvent, and 11.96 g (150.00 mmol) of pyridine was added dropwise in 10 minutes under nitrogen protection. After stirring at room temperature for 3 h, the solution became turbid after adding 300 mL of anhydrous diethyl ether. After cooling down to 0°C, 200 mL of deionized solution was added, and a light yellow floc was obtained by suction filtration, which was then continued to be filtered with 150 mL of anhydrous diethyl ether to obtain It is a white solid, which is dried in an oven at 30°C. Dissolve the dried white solid in 400 mL of methanol, heat to...
Embodiment 1
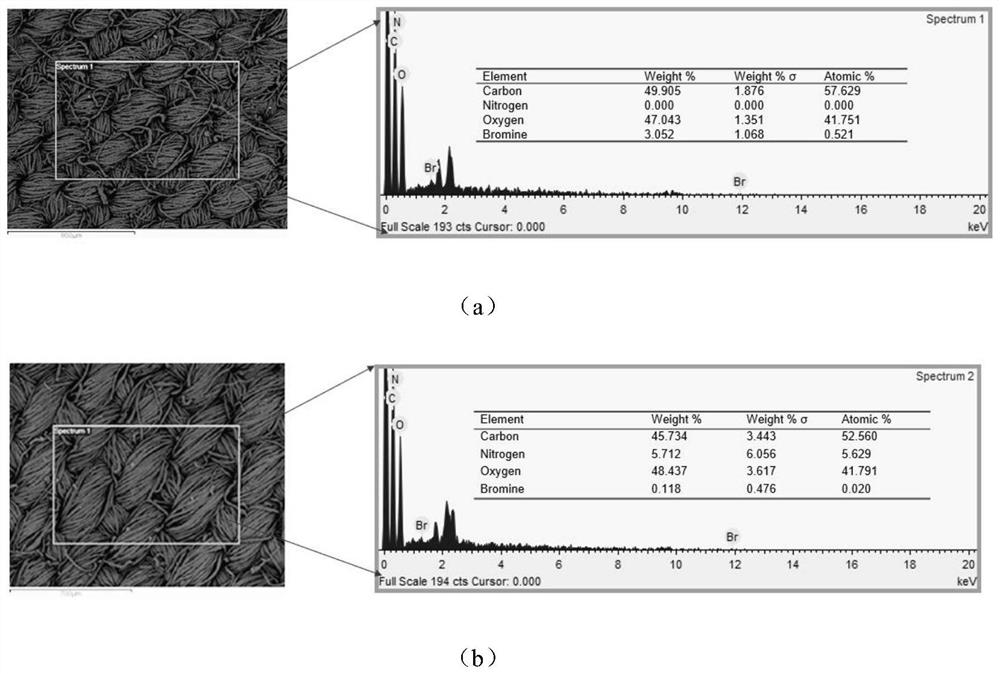
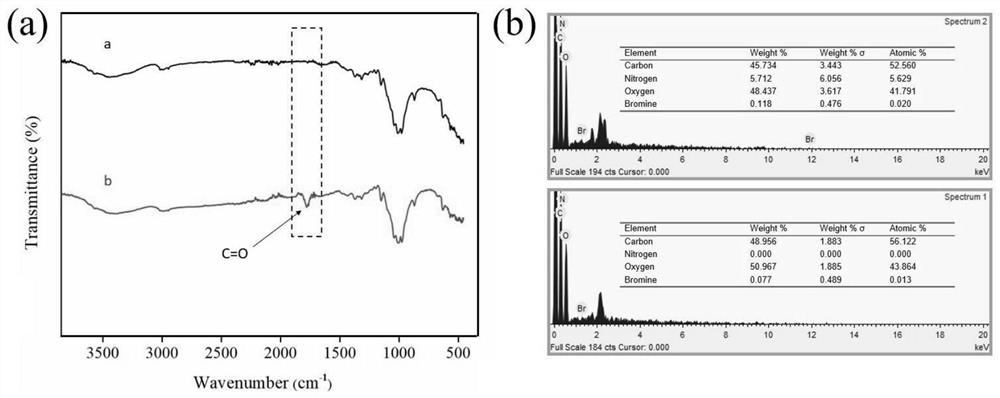
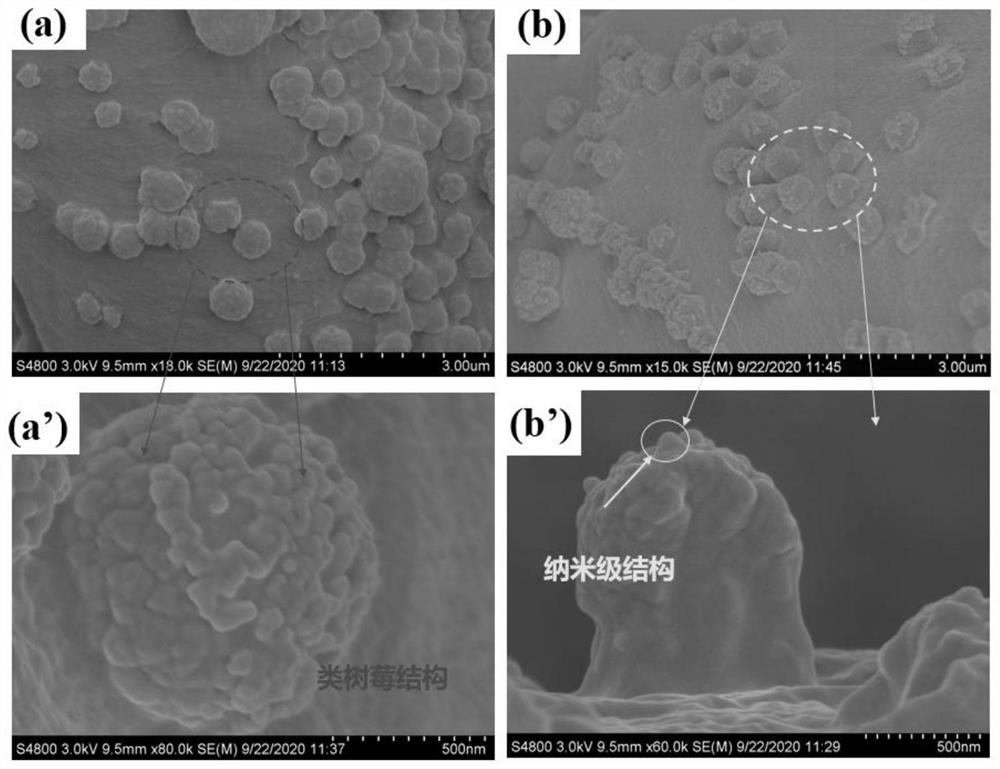
[0049] Add 100g sodium hydroxide and 500ml deionized water into a 1000ml beaker, stir until dissolved, soak the untreated raw cotton fabric in the solution for 1 hour, take it out and wash it with deionized water five times, then put the alkalized cotton fabric into the solution for 5 % glacial acetic acid for 30 min, and then washed five times with deionized water to obtain pretreated fabrics, which were dried at room temperature and then used.
[0050] The pretreated fabric (0.415 g) was soaked in anhydrous tetrahydrofuran, ultrasonically cleaned for 30 min, and dried at room temperature for later use. Put the pretreated fabric into an Erlenmeyer flask containing 100 mL of anhydrous tetrahydrofuran. After the fabric is completely soaked, add 0.83 g of sodium bicarbonate as an acid binding agent. After stabilization, dissolve 3.25 g of bromoacetyl bromide in 5 ml of dehydrated tetrahydrofuran, add it into the Erlenmeyer flask through a syringe to react for 1 h, raise the temp...
Embodiment 2
[0054] Take Example 1 diazotized cotton fabric 0.26 g (containing 2 mmol of hydroxyl) and put it into a 150 ml Erlenmeyer flask, add 40 mmol of hexyl diazoacetate monomer, and use 100 mL of anhydrous tetrahydrofuran as a solvent, monomer in the bottle and The molar ratio of hydroxyl groups on the surface of diazotized cotton fabric is 20:1, under nitrogen condition, add 9.15 mg (0.025 mmol) (π-allylPdCl) 2 , cooled to -10°C, added NaBPh 4 32.5 mg (0.09 mmol), heated to 0°C, 5°C and 15°C and reacted for 1 h successively after dissolving, and finally transferred the Erlenmeyer flask to a shaking water bath, raised the temperature to 30°C and reacted for 12 h, and the treated fabric was dissolved in tetrahydrofuran Ultrasonic cleaning in medium for 2 min, and drying in an oven at low temperature to obtain a hydrophobic fabric with a water contact angle of 110.4°.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com