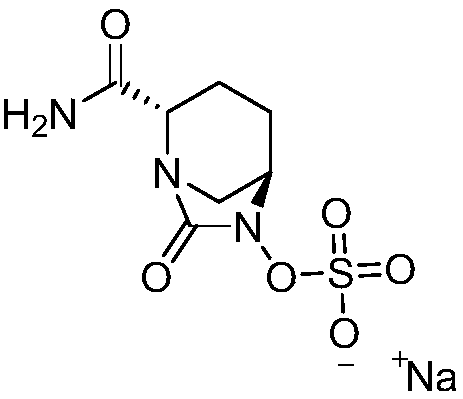
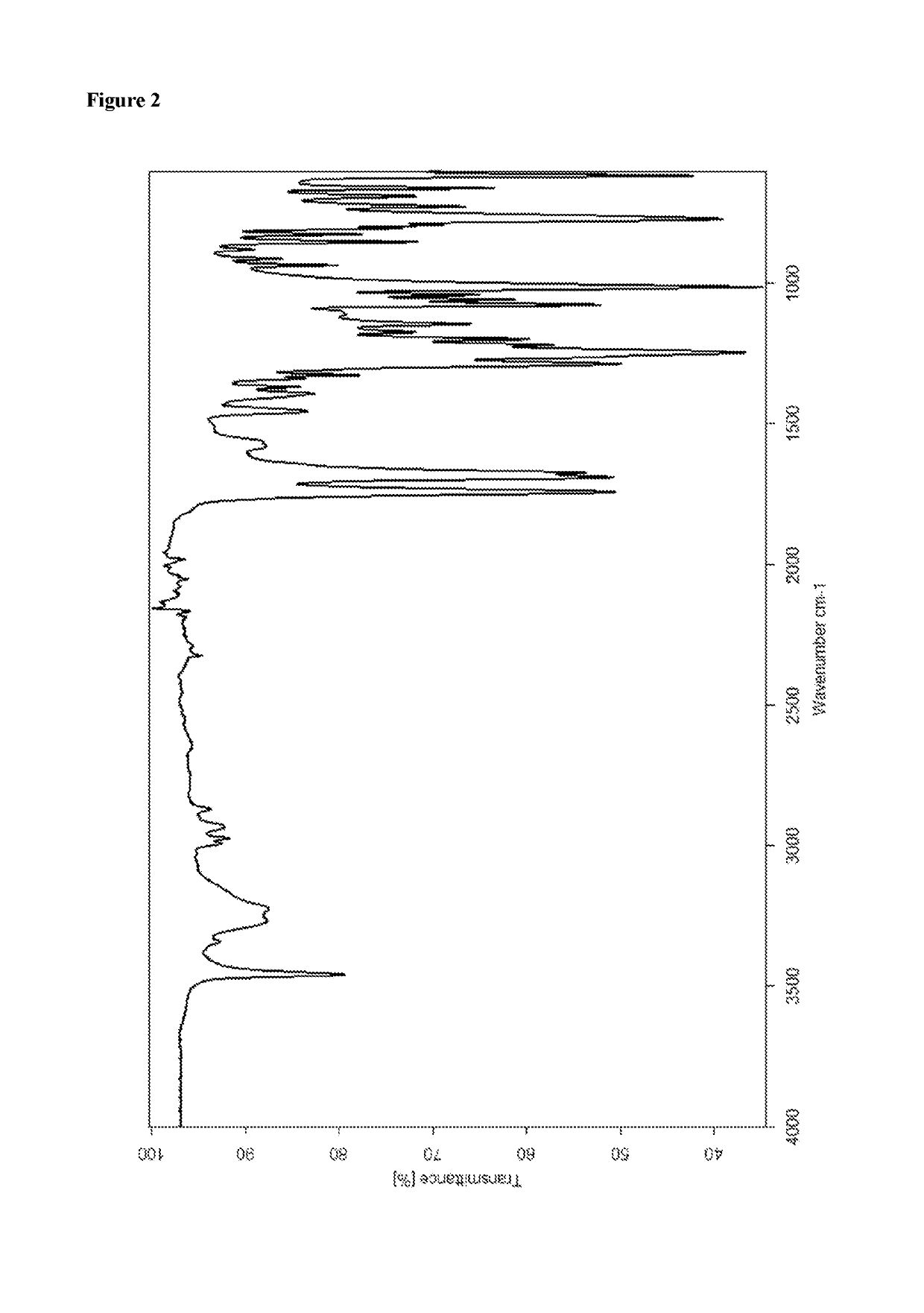
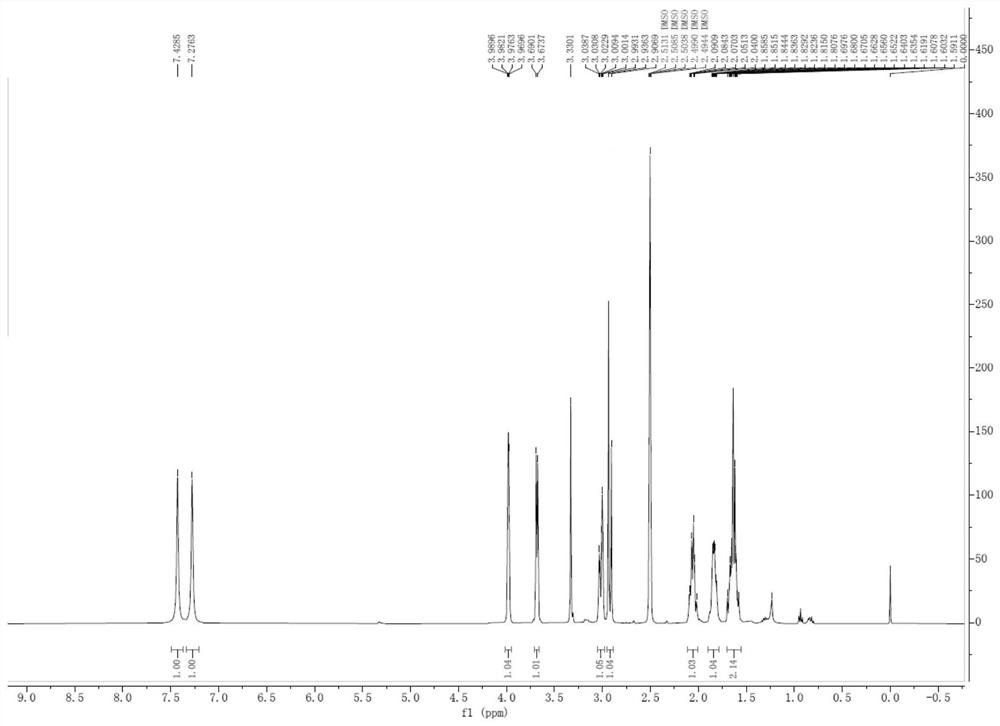
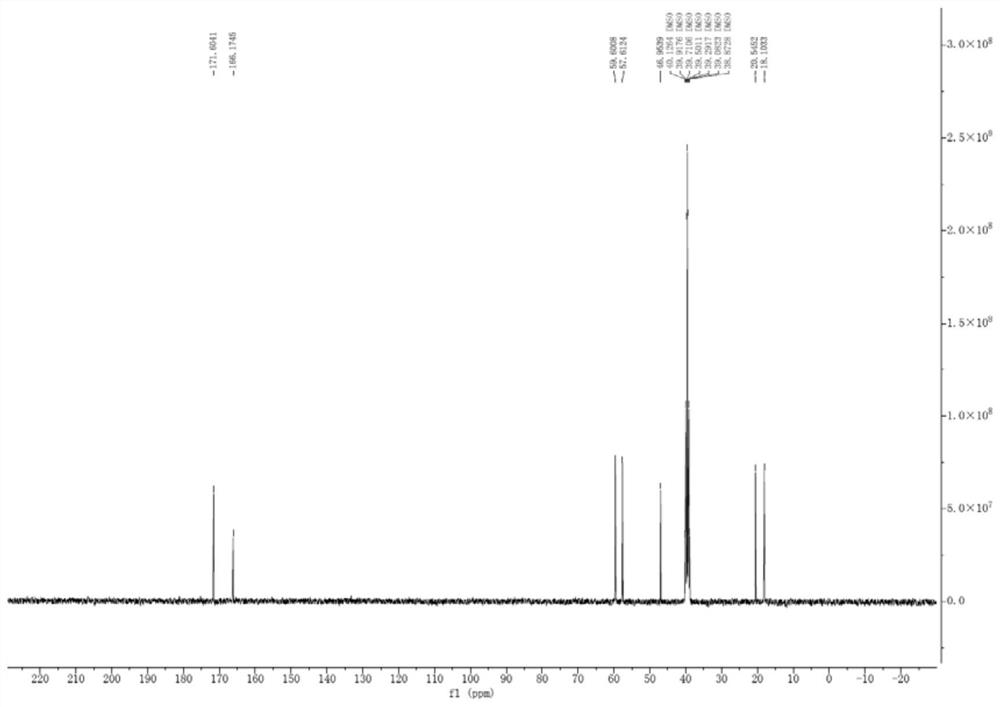
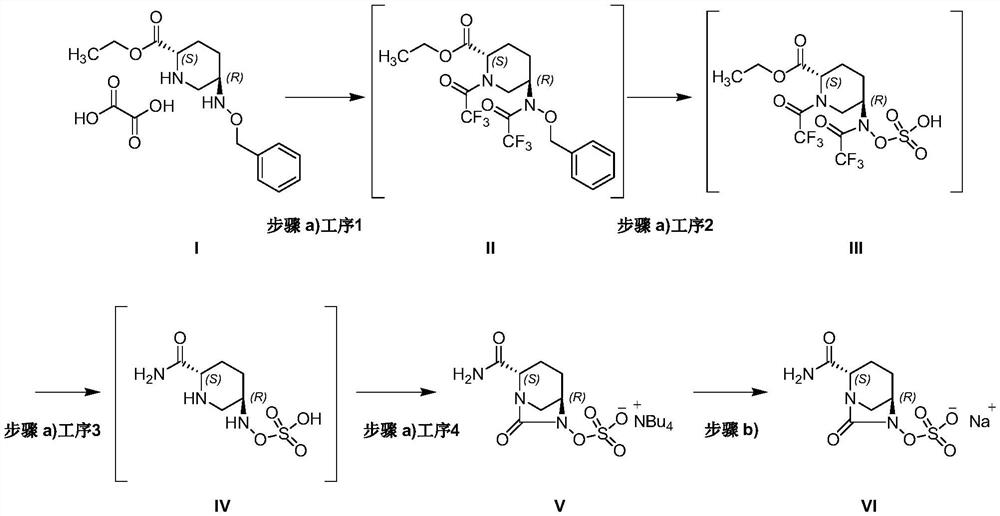
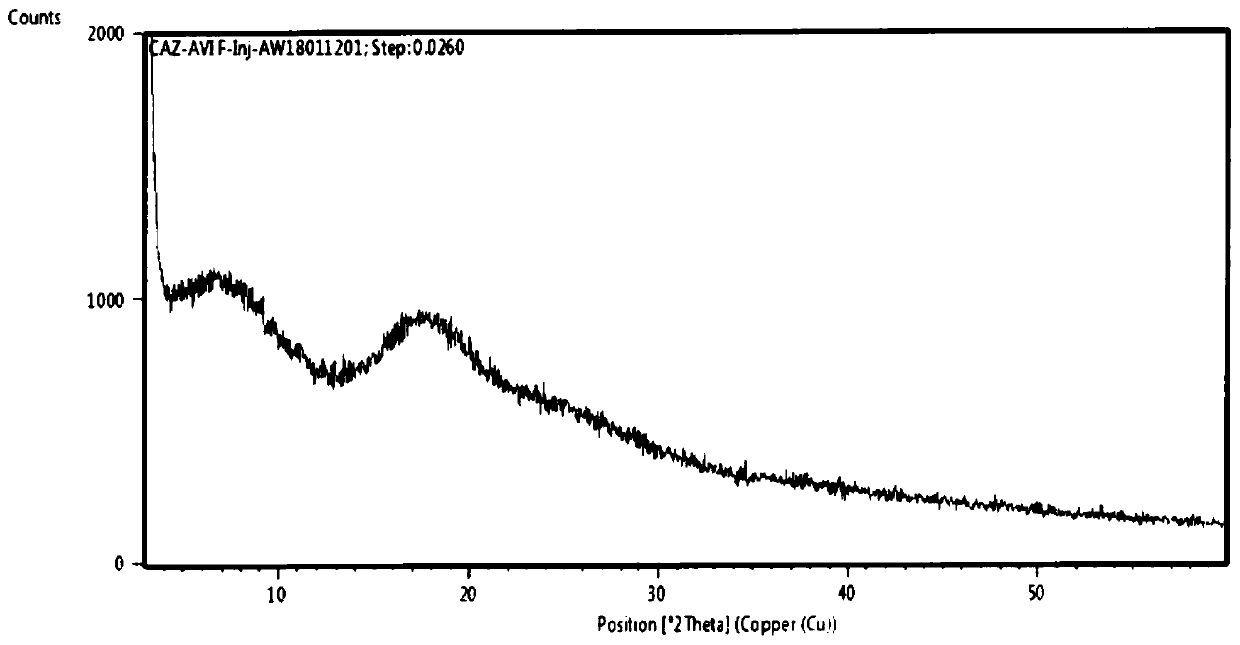
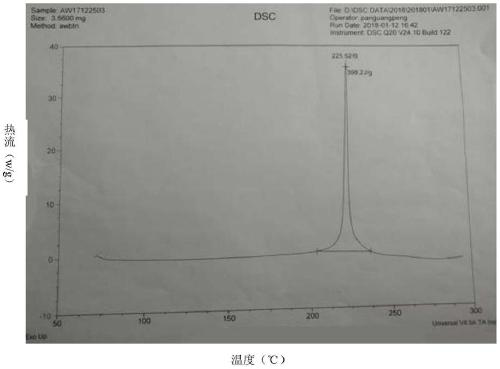
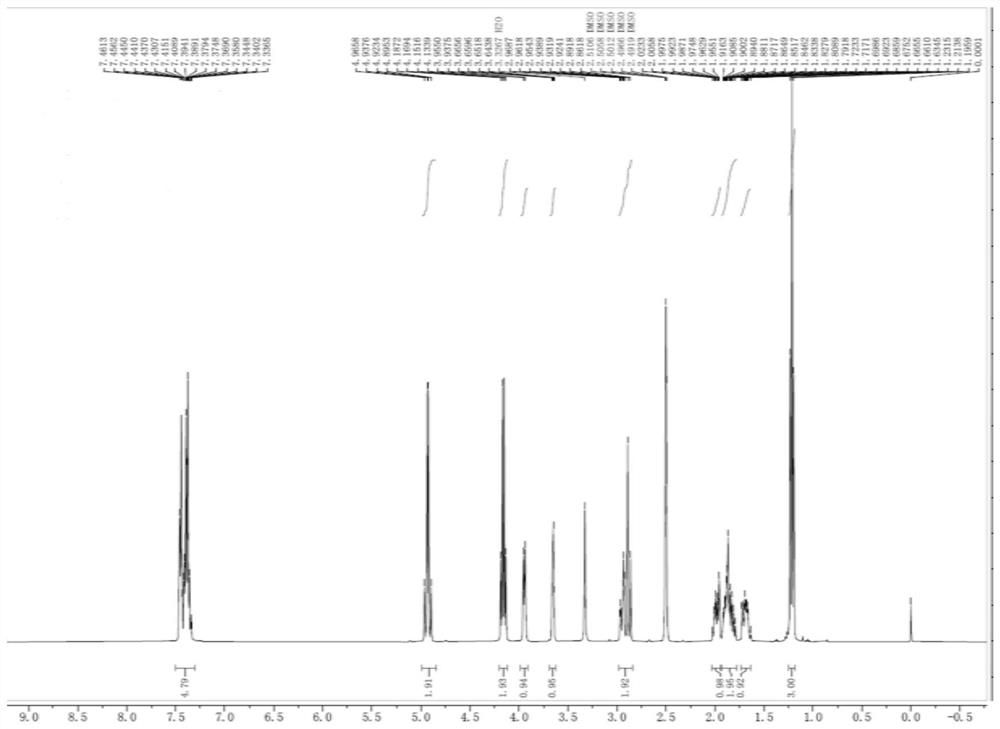
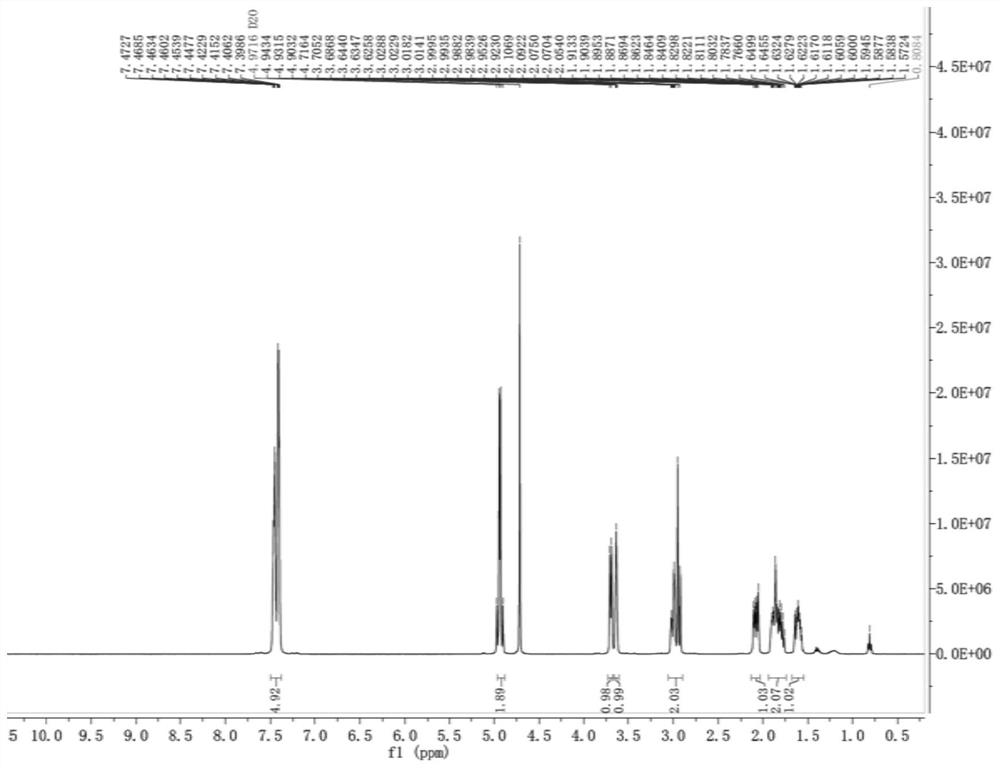
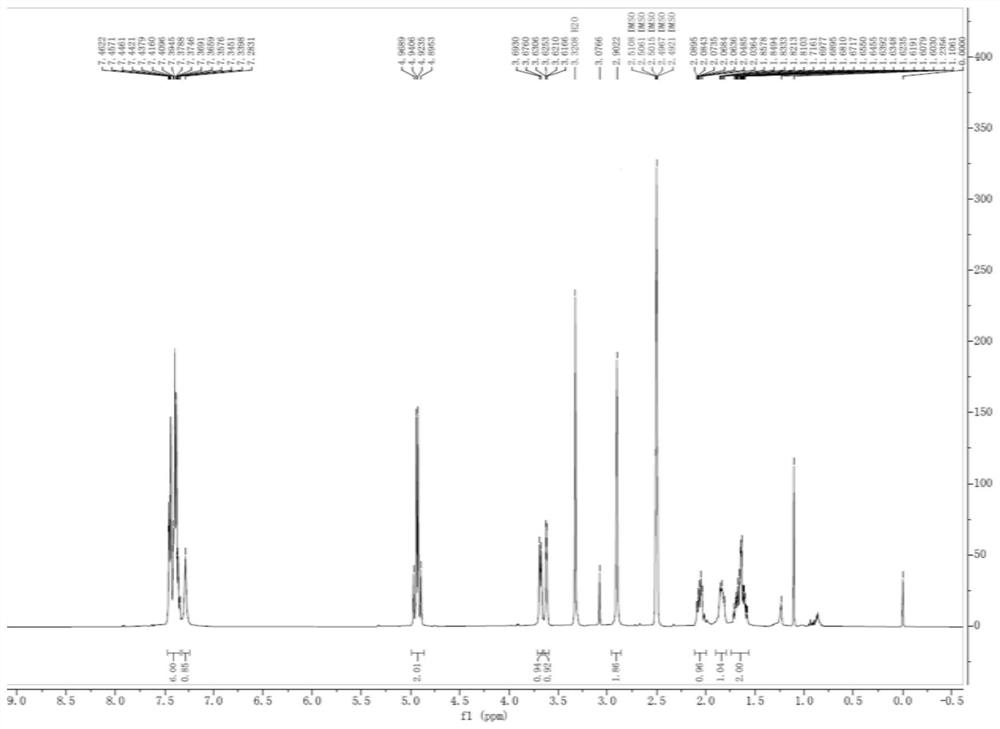
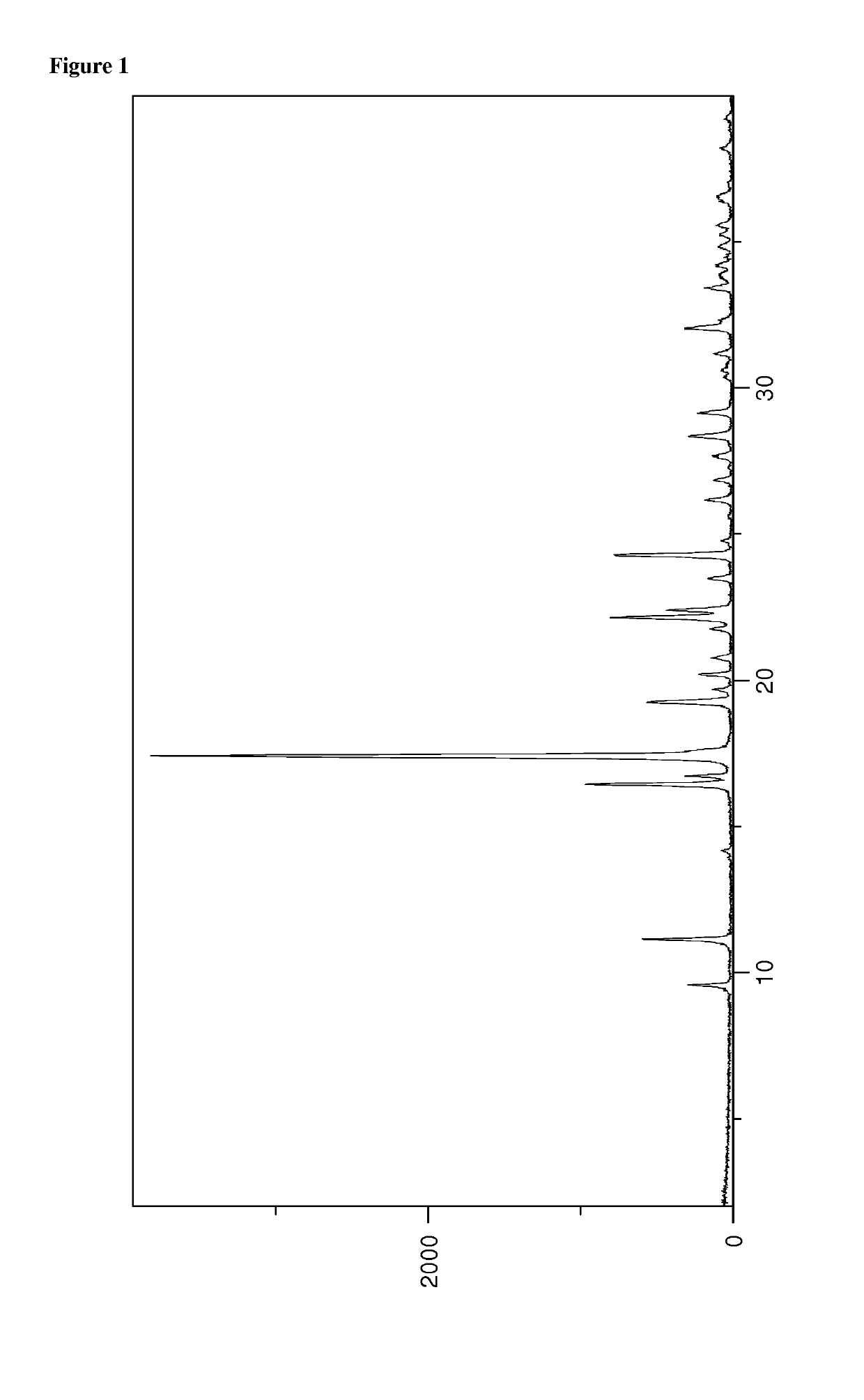
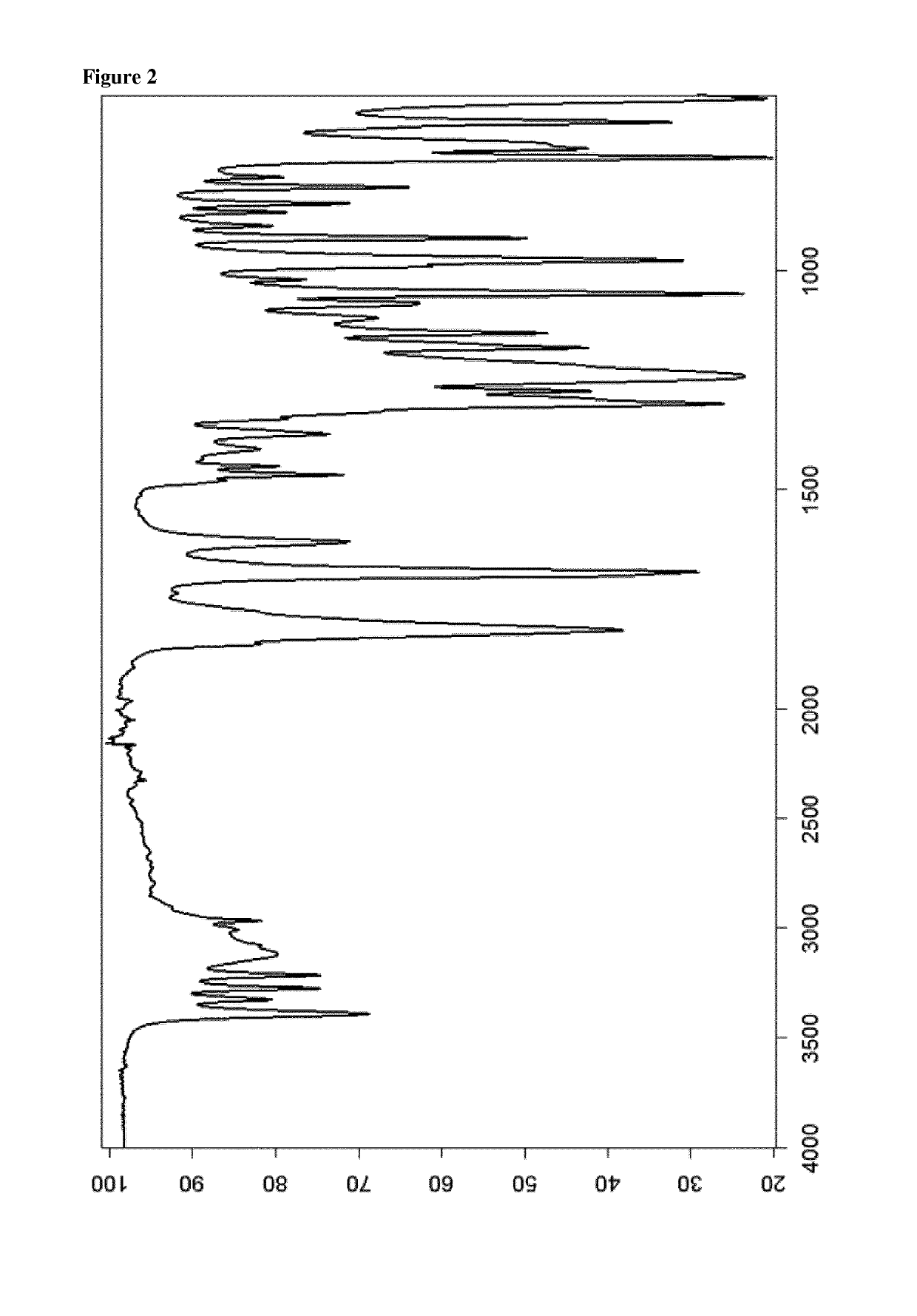
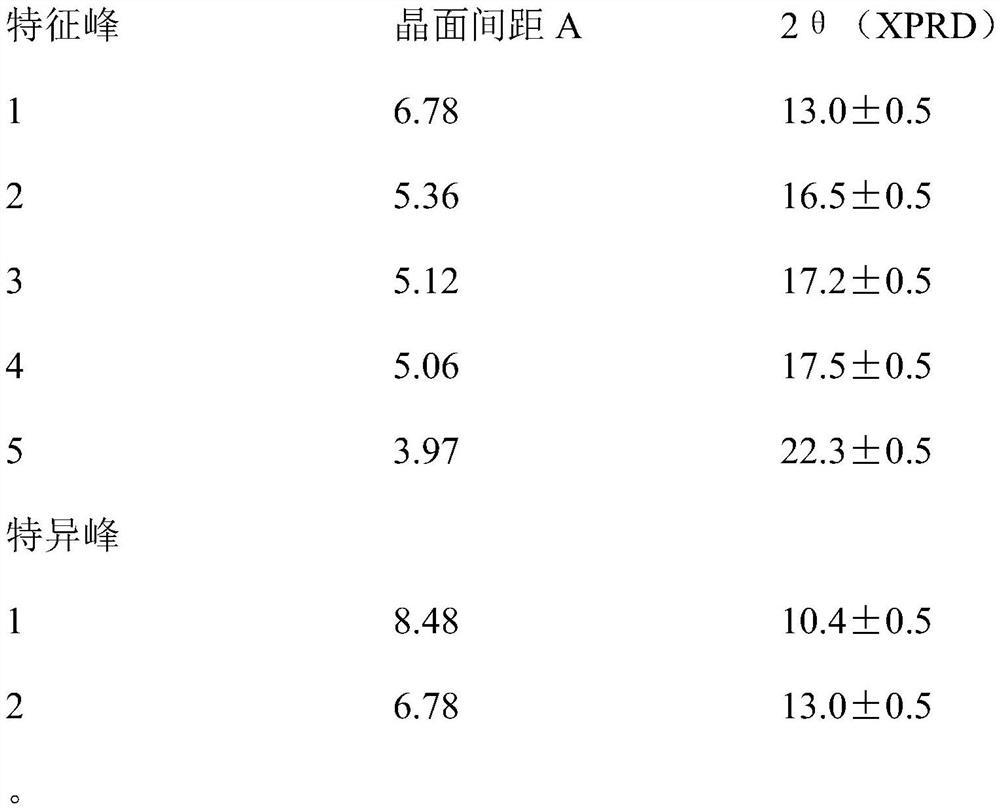
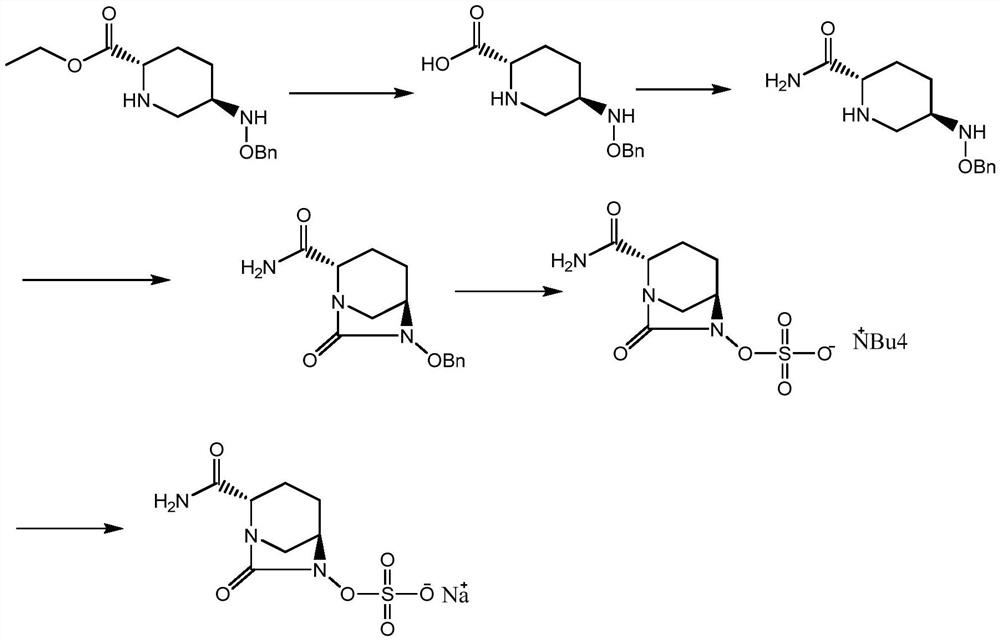
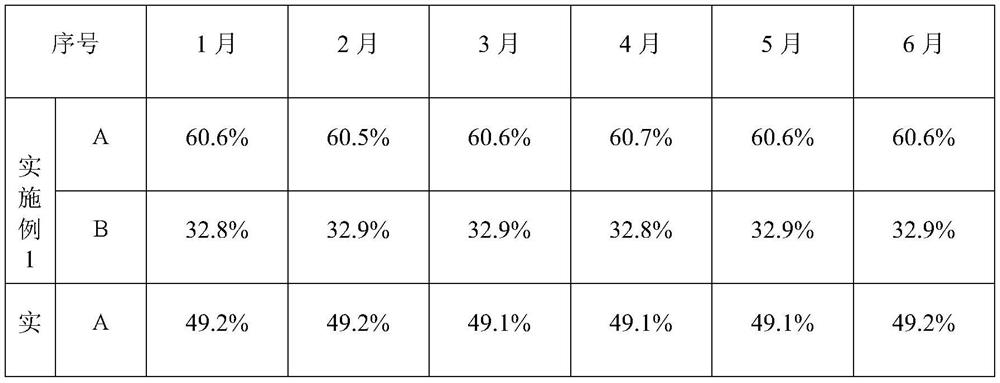
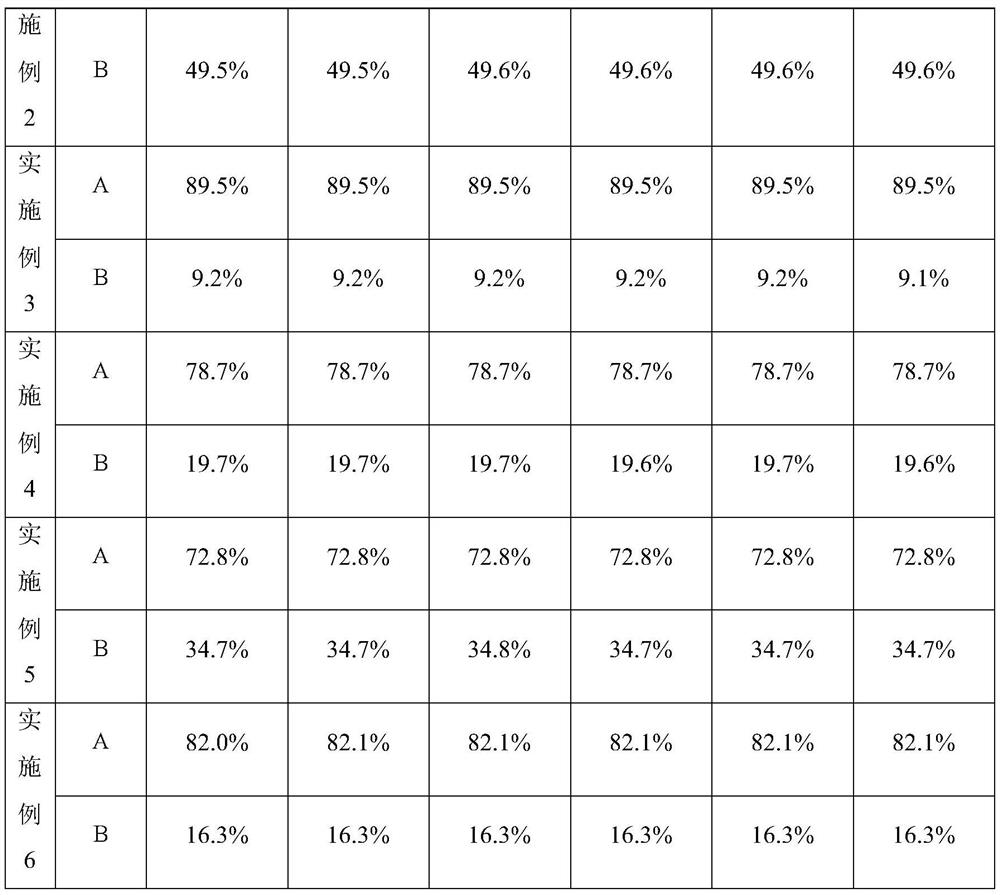
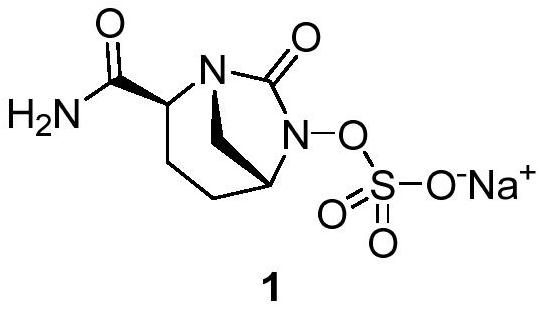
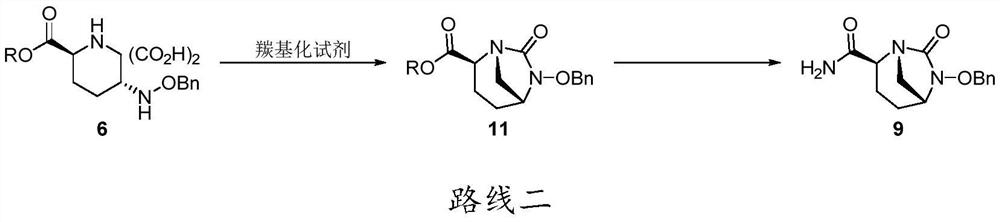
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
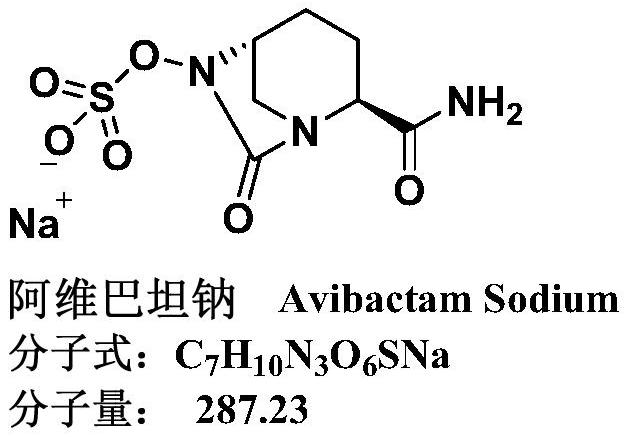
36 results about "Avibactam sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
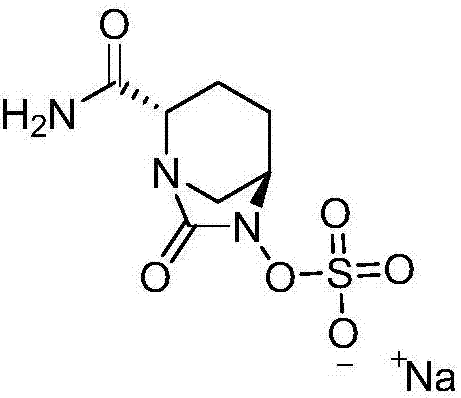
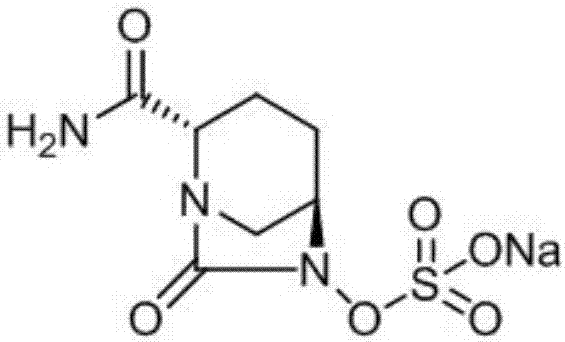
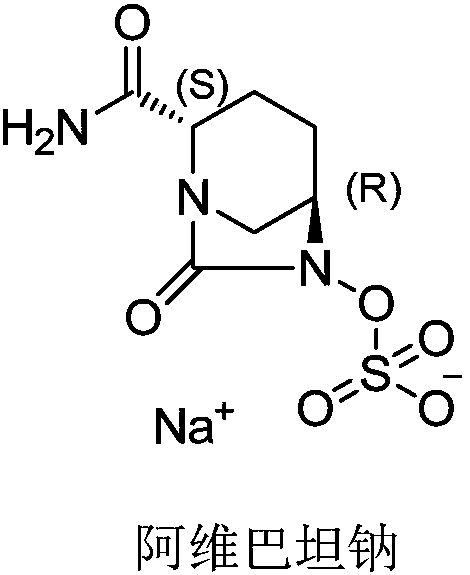
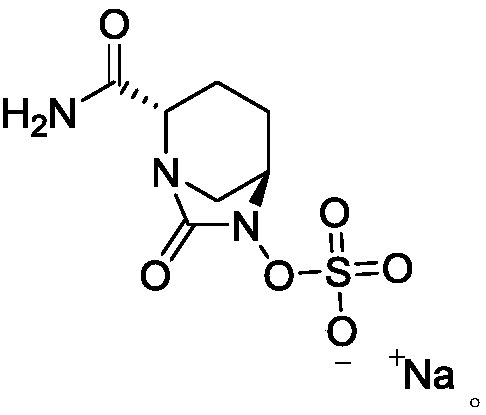
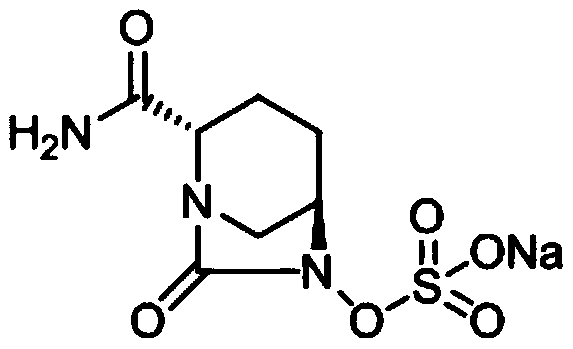
Avibactam sodium is an organic sodium salt that is the monosodium salt of avibactam. Used in combination with ceftazidime pentahydrate for the treatment of complicated urinary tract infections including pyelonephritis.
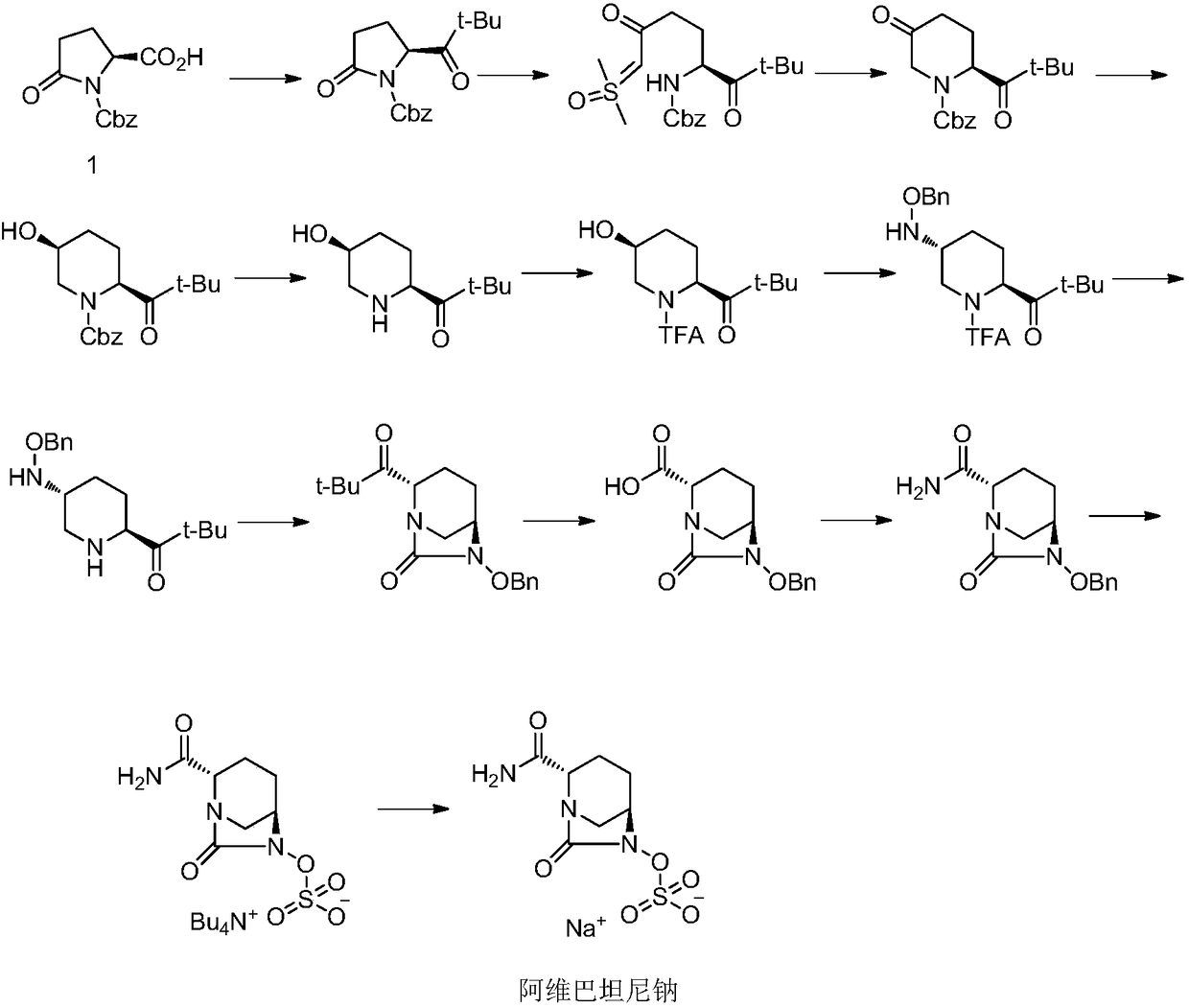
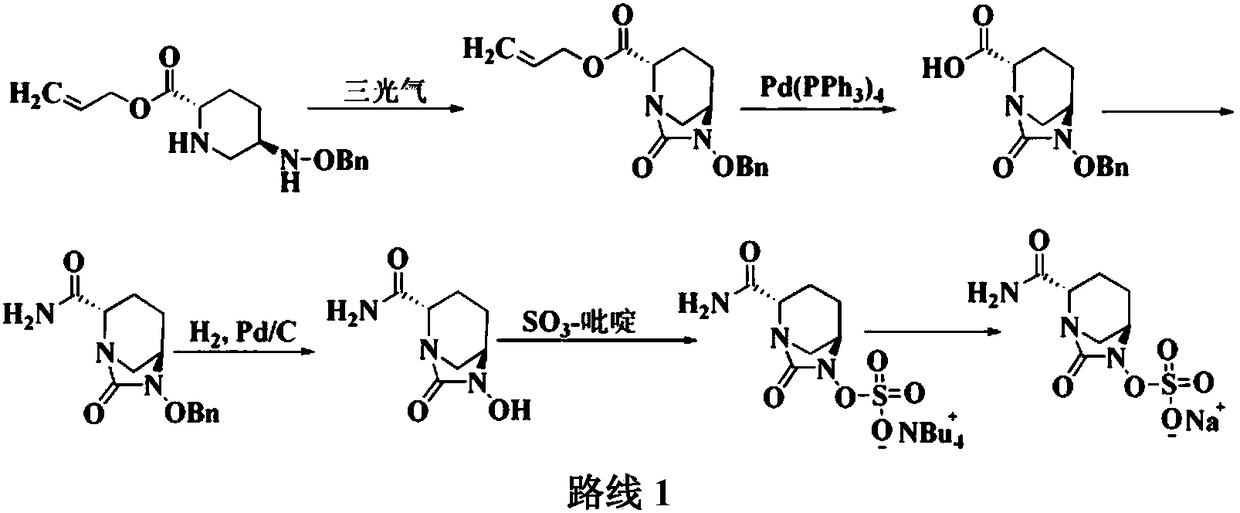
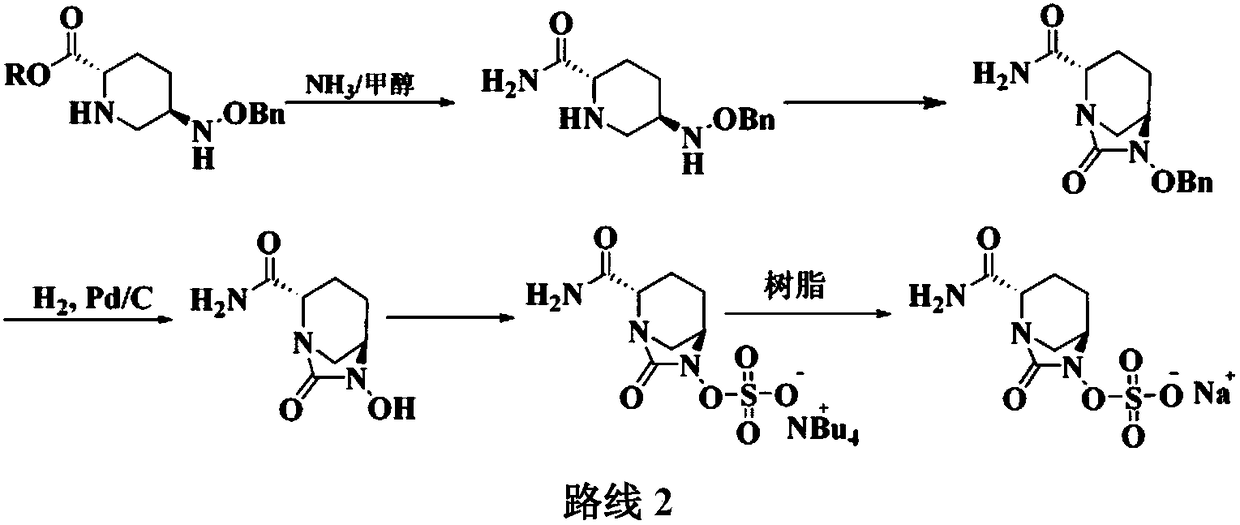
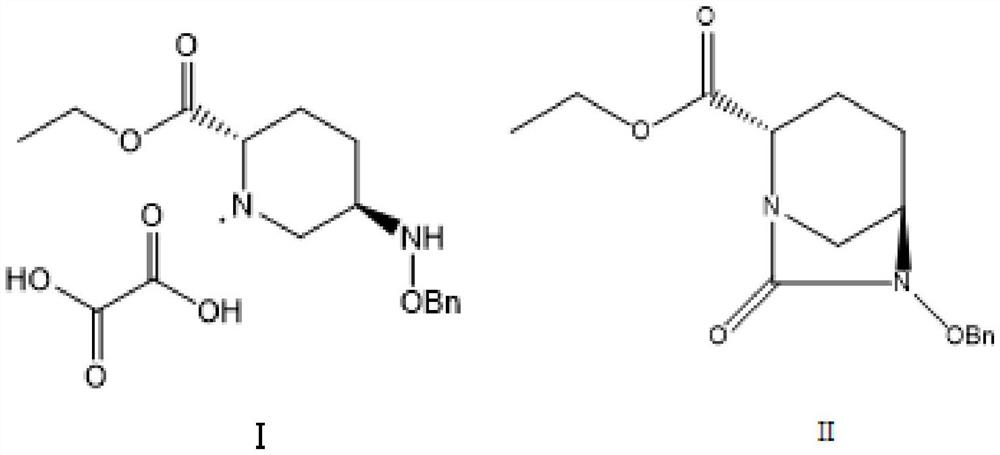
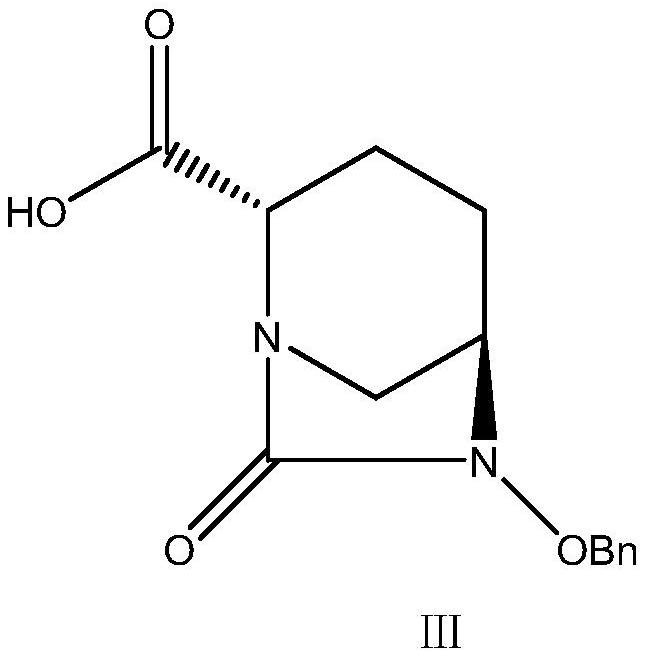
Method for preparing avibactam sodium through one-pot method
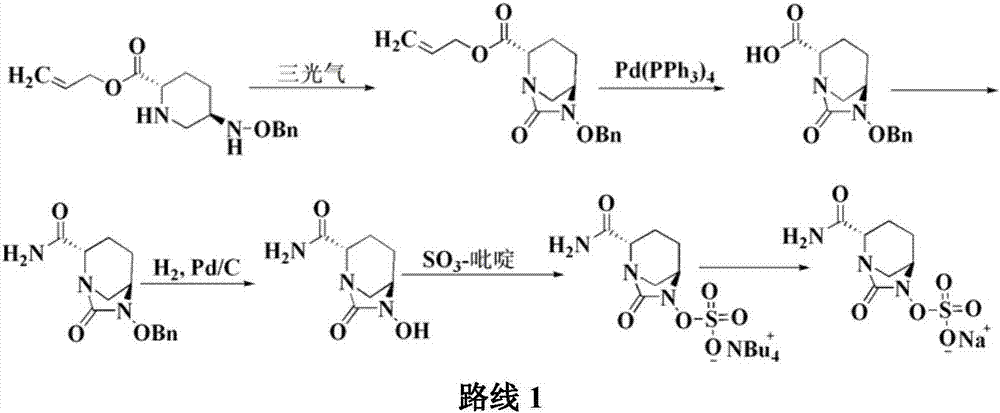
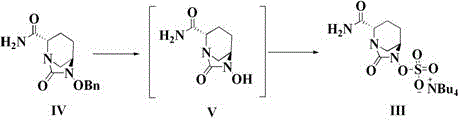
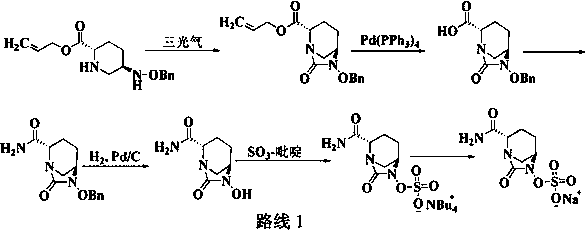
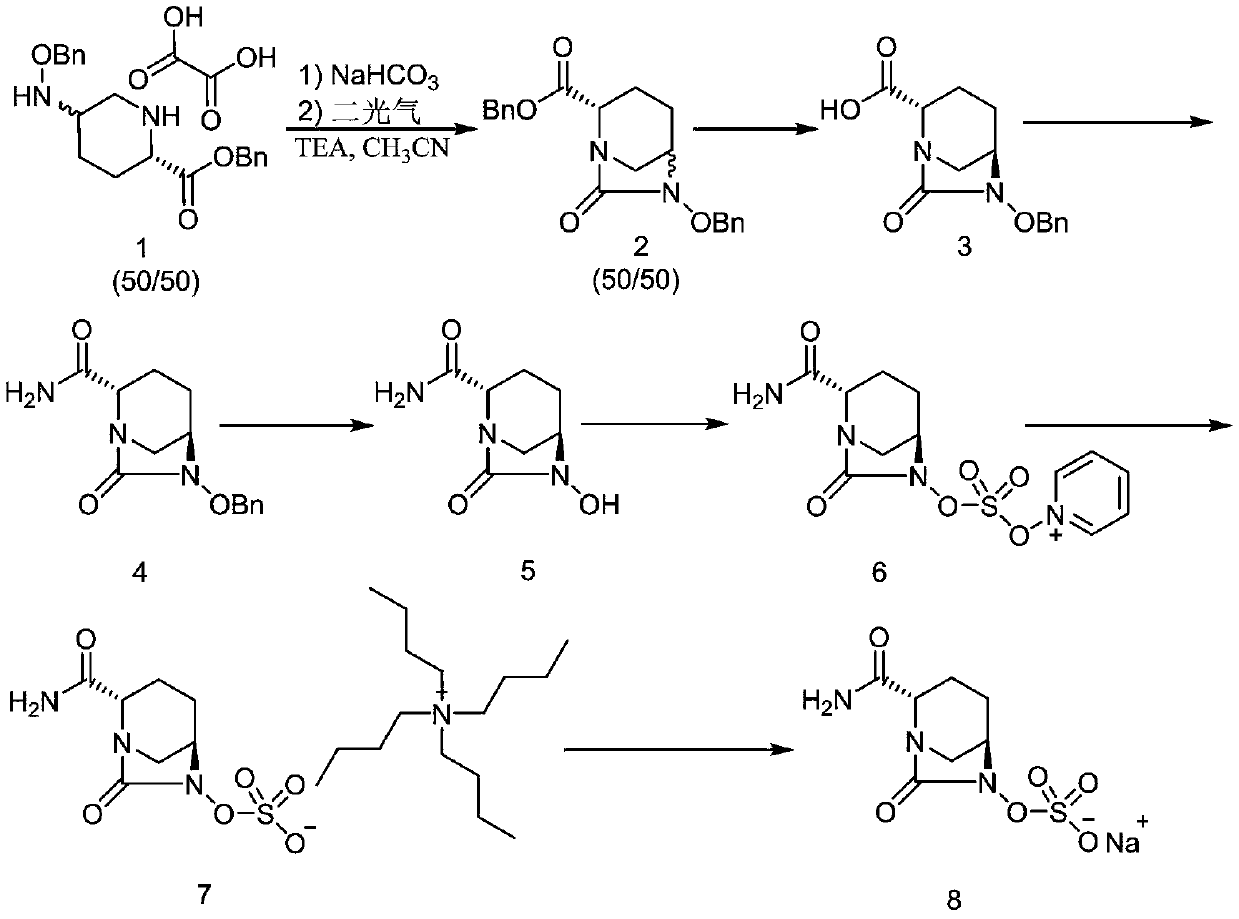
The invention discloses a method for preparing avibactam sodium through a one-pot method. The method comprises the following steps: by taking (2S,5R)-5-[(benzyloxy)amino]pyridine-2-ethyl carboxylate oxalate as a starting material, firstly generating a compound II by reacting with triphosgene, hydrolyzing, adding ammonia water to perform ammoniation, thereby obtaining a compound III, performing hydrogenation by taking formic acid, ammonium formate or hydrazine hydrate as a hydrogen donor in hydrogenation reaction, and then salifying to obtain the avibactam sodium. The avibactam sodium is prepared by use of the one-pot method, the raw material is cheap and easy to obtain, the reaction condition is mild, the operation is simple, the safety is higher, the yield is high, the purity is good, and the method is suitable for large scale industrial production. Formulae are shown in the description.
Owner:QILU TIANHE PHARMA
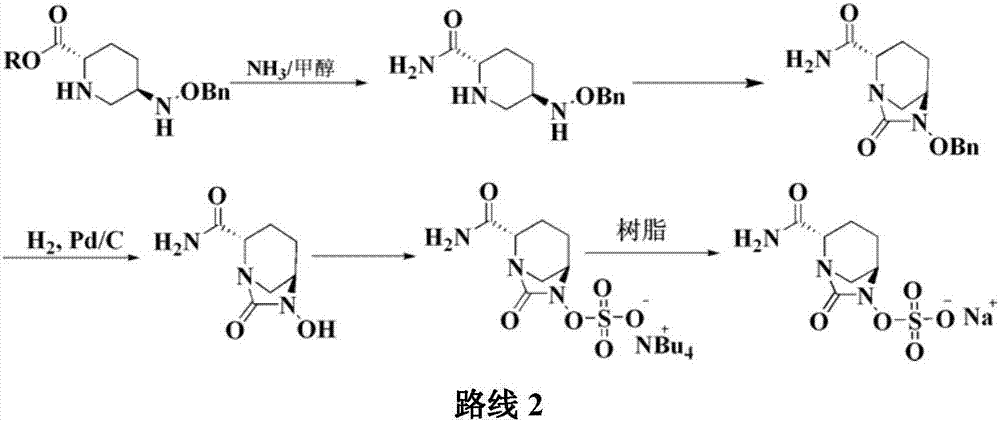
Preparation method of improved avibactam sodium intermediate compound
ActiveCN105753867AAvoid Hydrocatalytic OperationsMild reaction conditionsOrganic chemistryTriethylsilaneHydrogenation catalysis
The invention belongs to the technical field of medical chemistry and particularly relates to a preparation method of beta-lactamase inhibitor drug avibactam sodium and an intermediate of beta-lactamase inhibitor drug avibactam sodium. Triethyl-silane is adopted to realize benzyl removal, hydrogenation catalysis operation in the prior art is omitted, the reaction condition is milder, safety risk is reduced, the product yield and the purity are greatly improved, and the preparation method is more suitable for large-scale production.
Owner:QILU PHARMA HAINAN +1
Synthetic method for avibactam sodium salt
ActiveCN107417686ALow priceReduce manufacturing costOrganic chemistryIon exchangeCarbonyldiimidazole
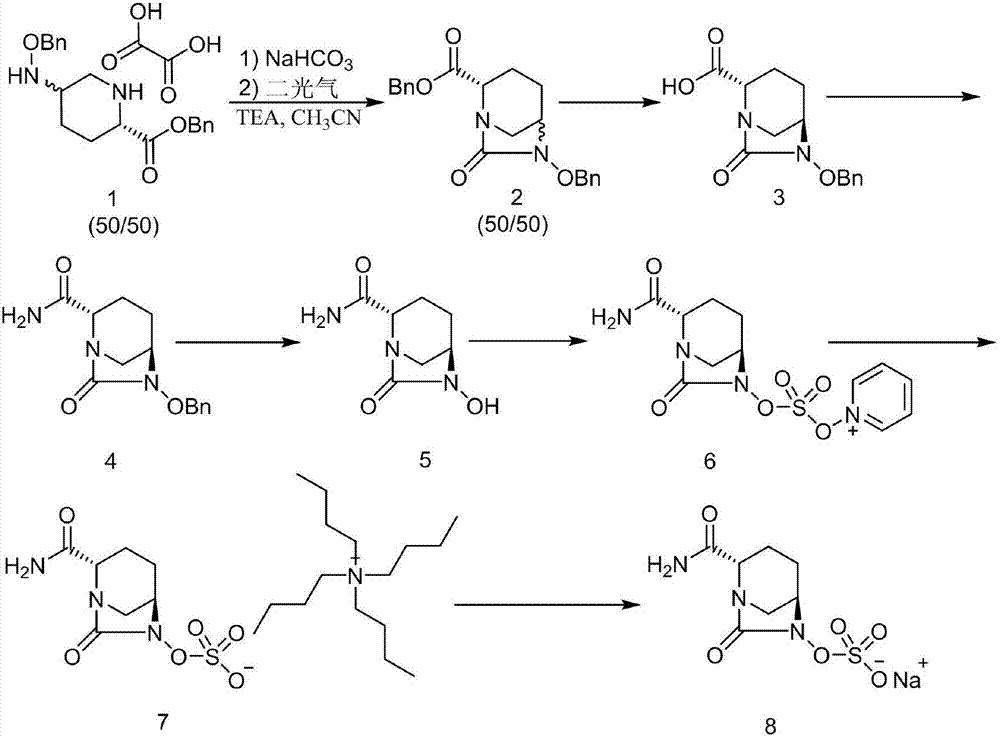
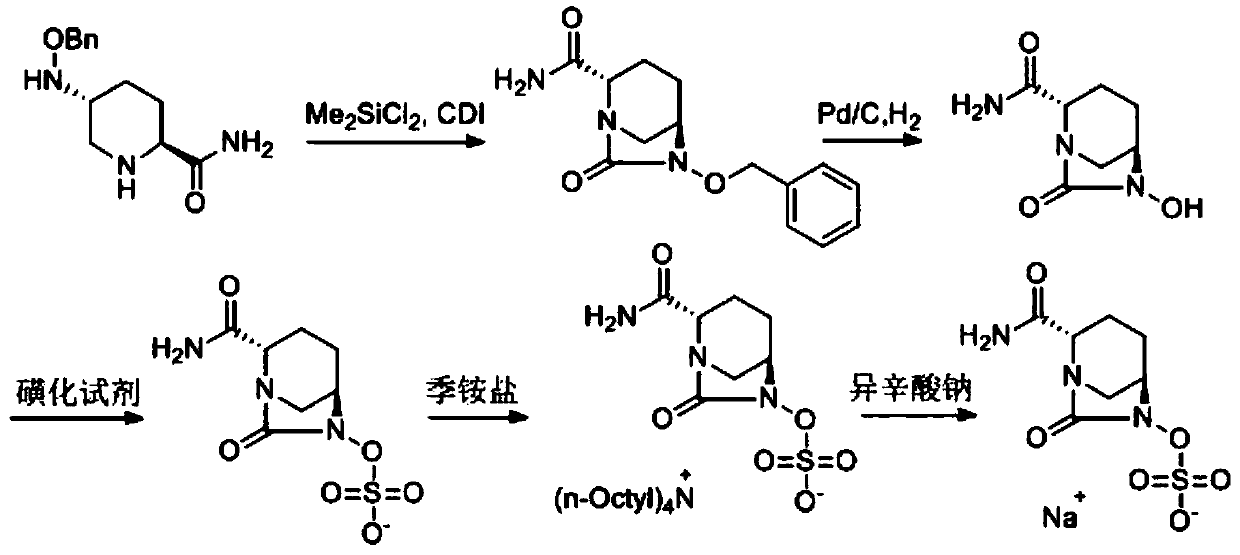
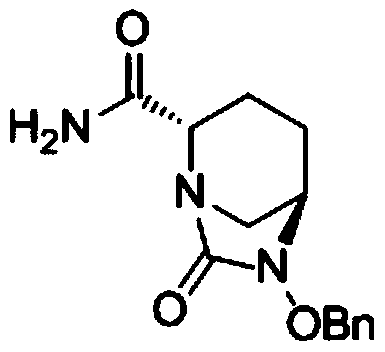
The invention discloses a synthetic method for avibactam sodium salt. The method comprises the following steps: taking (2S, 5R)-5-[(benzyl oxyl) amino] piperidine-2-formamide as a starting material; constructing a urea ring by carbonyl diimidazole under the effect of dimethyldichlorosilance to obtain (2S, 5R)-6-(benzyl oxyl)-7-oxo-1,6-diazabicyclo [3.2.1] octane-2-formamide; then carrying out hydrogenation to remove benzyl; carrying out sulfonation reaction on the compound and a sulfonated reagent; synthesizing into a quaternary ammonium salt intermediate by using quaternary ammonium salt; and finally carrying out ion exchange to obtain the avibactam sodium salt. The improved process is low in cost, simple and convenient to operate, good in product quality and suitable for industrial production. In a process of synthesizing the intermediate (2S, 5R)-6-(benzyl oxyl)-7-oxo-1,6-diazabicyclo [3.2.1] octane-2-formamide, dimethyldichlorosilance which is low in price is used, and therefore, the production cost is greatly reduced.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Beta-lactamase inhibitor
InactiveCN105801579AExtended half-lifeLower minimum inhibitory concentrationAntibacterial agentsOrganic active ingredientsHalf-lifeAvibactam sodium
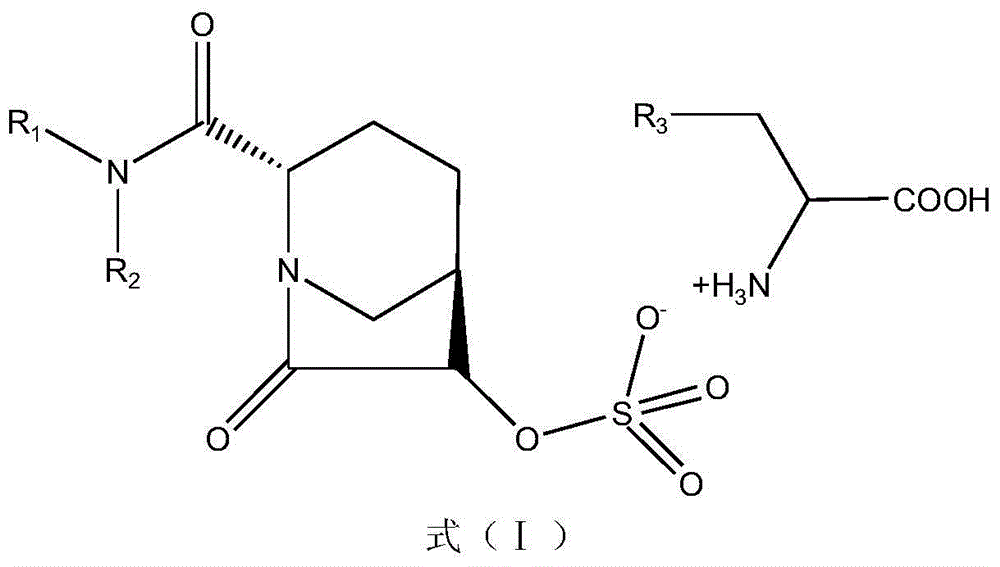
The invention discloses a beta-lactamase inhibitor. The beta-lactamase inhibitor can inhibit A-type beta-lactamases (such as SHV, TEM and CTX), C-type beta-lactamase (mainly comprising an AmpC enzyme) and D-type beta-lactamase (such as OXA), has good inhibition effects and can reduce the lowest ceftazidime bacteriostasis concentration through combination with ceftazidime. Compared with tazobactam, the beta-lactamase inhibitor has a longer half life. A part of the beta-lactamase inhibitor has a half life longer than that of avibactam sodium. The beta-lactamase inhibitor provides more possibility for antibiotic development.
Owner:卢来春
Avibactam sodium synthesis method
ActiveCN108239089AThe synthesis steps are simpleReduce the difficulty of synthesisOrganic chemistryElectrophilic additionSynthesis methods
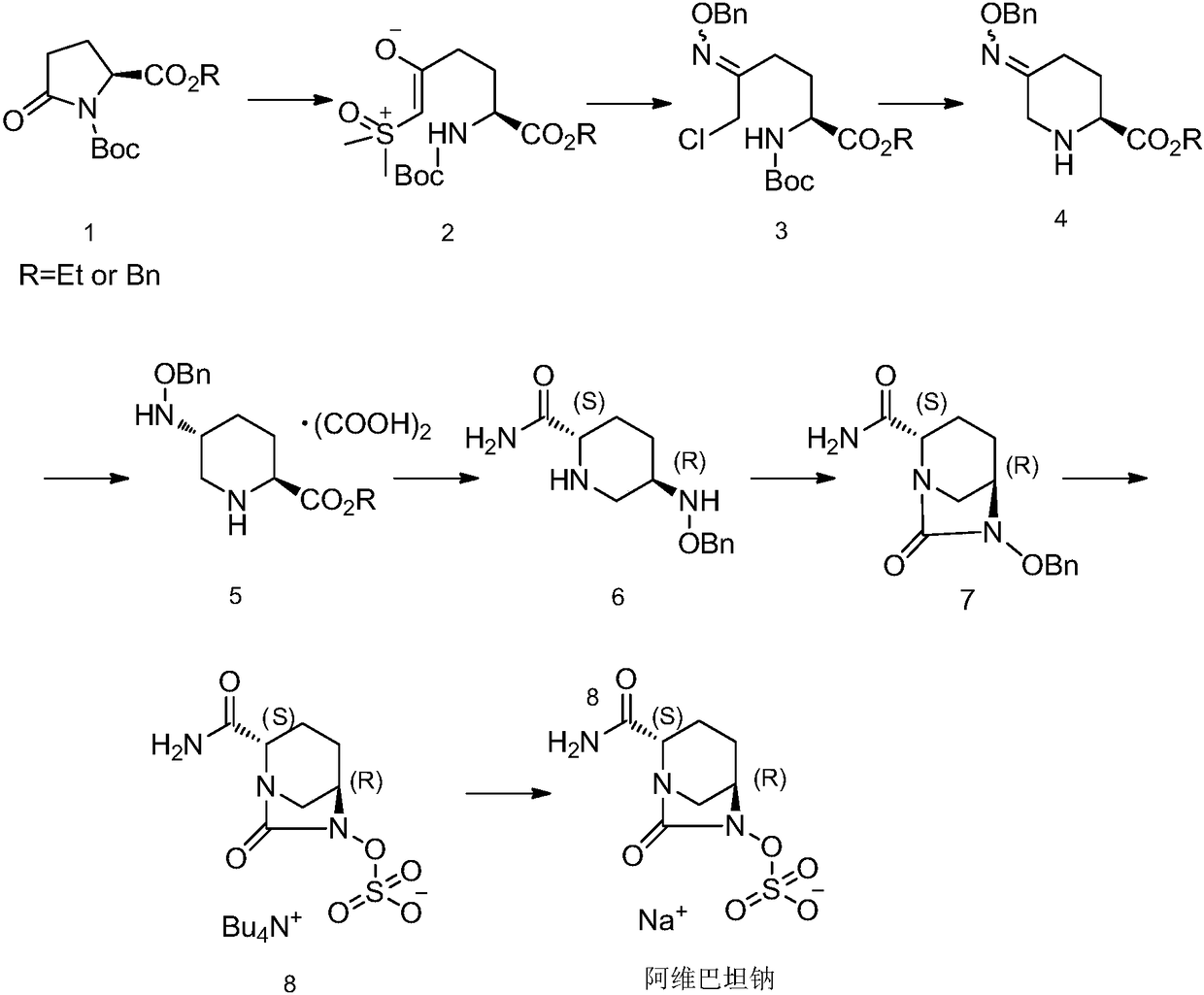
The invention discloses an avibactam sodium synthesis method. In the existing synthesis method, the production cost is high, the selectivity of the intermediate is low during the reducing so as to cause the reduced yield, and the large-scale production is limited by the high toxicity of the side-product generated in the reaction, no environment protection and other factors. According to the present invention, 5-hydroxy-2-pyridine ethyl formate is used as a starting raw material, and 11 steps such as reduction hydrogenation, biological lipase resolution, electrophilic addition, nucleophilic substitution, Boc removing, aminolysis, intramolecular urealysis, debenzylation, sulfonic acid esterification, salt formation and cation exchange are performed to obtain avibactam sodium; and the synthesis method has advantages of high yield, short route, mild reaction conditions, low environmental pollution, easy large-scale preparation and the like.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Method for preparing amorphous avibactam sodium by spray-drying
ActiveCN108409736AReduce generationThe method steps are simpleOrganic chemistryAvibactam sodiumPurified water
The invention discloses a method for preparing amorphous avibactam sodium by spray-drying. According to the invention, solution of sodium iso-octoate is dropwise added into solution of avibactam tetrabutylammonium salt, after dropwise adding is completed, the reaction is performed for 3 to 4h, and purified water is added for extraction; after a water phase is subjected to spray-drying, the amorphous avibactam sodium is obtained. The method disclosed by the invention is simple in step and easy for industrial production, the purity of a product is greater than or equal to 98.0%, and yield is greater than or equal to 90.0%.
Owner:山东安信制药有限公司
Preparation method of crystal form B avibactam sodium
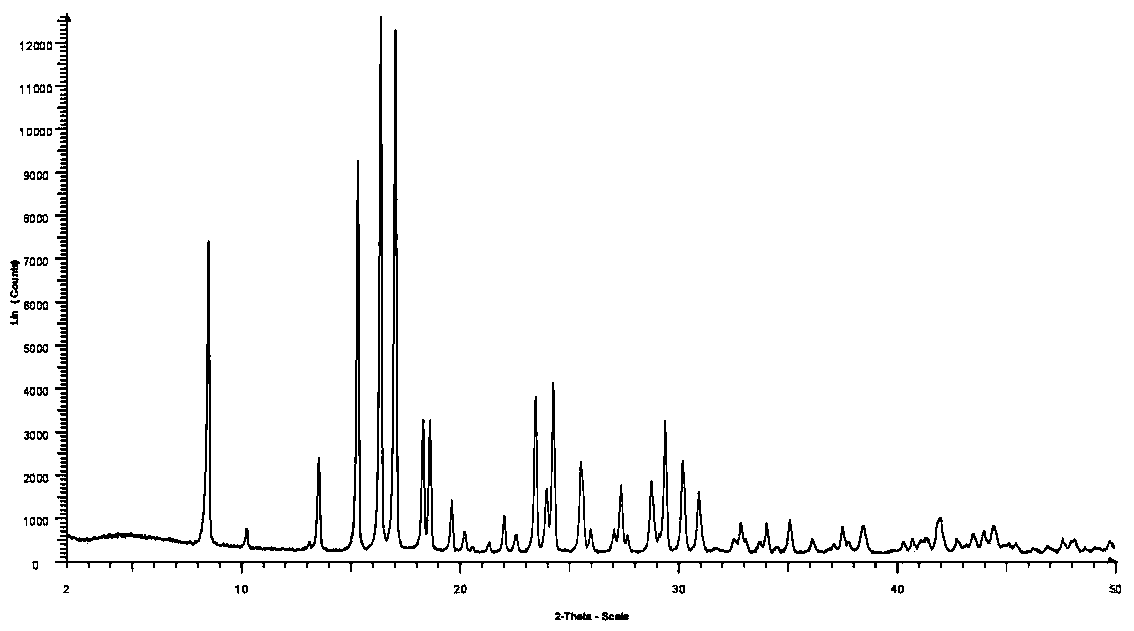
The invention discloses a preparation method of crystal form B avibactam sodium, which comprises the following steps: S100, adding methanol and water into avibactam sodium, stirring, heating and dissolving to obtain an avibactam sodium solution; and S200, adding an alcohol poor solvent into the avibactam sodium solution, cooling to a first temperature, stirring for growing crystals, and then continuously cooling to a second temperature for devitrification.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD +1
Orm C of avibactam sodium
ActiveCN107922411AStable temperature stressIncrease humidityAntibacterial agentsOrganic active ingredientsPharmaceutical drugCombinatorial chemistry
The present invention relates to crystalline form C of avibactam sodium and to a process for its preparation. The invention also concerns a pharmaceutical composition comprising form C and one or moreantibacterial agents, wherein at least one antibacterial agent is a beta-lactam antibiotic. The pharmaceutical composition of the present invention can be used as medicament, in particular for treatment and / or prevention of bacterial infections.
Owner:SANDOZ LTD
Preparation method of avibactam sodium key intermediate
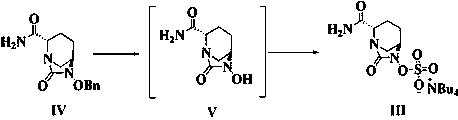
ActiveCN111646991AOrganic chemistryBulk chemical productionChiral selectivityCombinatorial chemistry
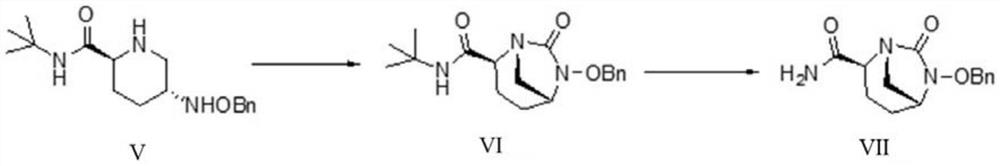
The invention relates to a preparation method of an avibactam sodium key intermediate compound (VII). The compound (VII) is obtained by cyclization and deprotection reactions of a compound (V). The preparation method provided by the invention has the advantages of good chiral selectivity, simple and feasible operation, high yield, good purity, cheap and easily available raw materials, and industrial value.
Owner:ZHEJIANG HISOAR PHARMA +1
The method for preparing avibactam sodium by one-pot method
The invention discloses a method for preparing avibactam sodium through a one-pot method. The method comprises the following steps: by taking (2S,5R)-5-[(benzyloxy)amino]pyridine-2-ethyl carboxylate oxalate as a starting material, firstly generating a compound II by reacting with triphosgene, hydrolyzing, adding ammonia water to perform ammoniation, thereby obtaining a compound III, performing hydrogenation by taking formic acid, ammonium formate or hydrazine hydrate as a hydrogen donor in hydrogenation reaction, and then salifying to obtain the avibactam sodium. The avibactam sodium is prepared by use of the one-pot method, the raw material is cheap and easy to obtain, the reaction condition is mild, the operation is simple, the safety is higher, the yield is high, the purity is good, and the method is suitable for large scale industrial production. Formulae are shown in the description.
Owner:QILU TIANHE PHARMA
Form C of avibactam sodium
ActiveUS10265326B2Stable against moistureHighly stable against temperature stressAntibacterial agentsOrganic active ingredientsMedicineAvibactam sodium
The present invention relates to crystalline form C of avibactam sodium and to a process for its preparation. The invention also concerns a pharmaceutical composition comprising form C and one or more antibacterial agents, wherein at least one antibacterial agent is a beta-lactam antibiotic. The pharmaceutical composition of the present invention can be used as medicament, in particular for treatment and / or prevention of bacterial infections.
Owner:SANDOZ AG
A kind of preparation method of improved avibactam sodium intermediate compound
ActiveCN105753867BAvoid Hydrocatalytic OperationsMild reaction conditionsOrganic chemistrySilanesTriethylsilane
The invention belongs to the technical field of medical chemistry and particularly relates to a preparation method of beta-lactamase inhibitor drug avibactam sodium and an intermediate of beta-lactamase inhibitor drug avibactam sodium. Triethyl-silane is adopted to realize benzyl removal, hydrogenation catalysis operation in the prior art is omitted, the reaction condition is milder, safety risk is reduced, the product yield and the purity are greatly improved, and the preparation method is more suitable for large-scale production.
Owner:QILU PHARMA HAINAN +1
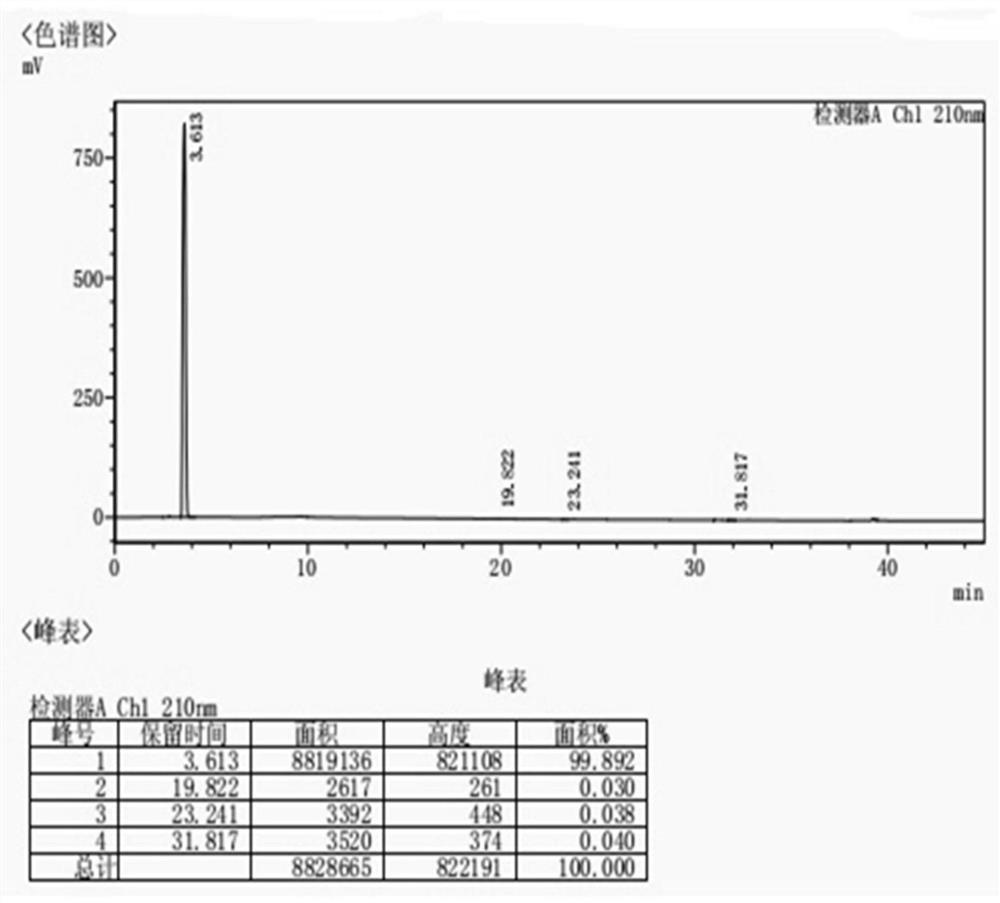
Avibactam sodium substance analysis method
PendingCN113984929AHigh detection sensitivityImprove stabilityComponent separationMonopotassium phosphateGradient elution
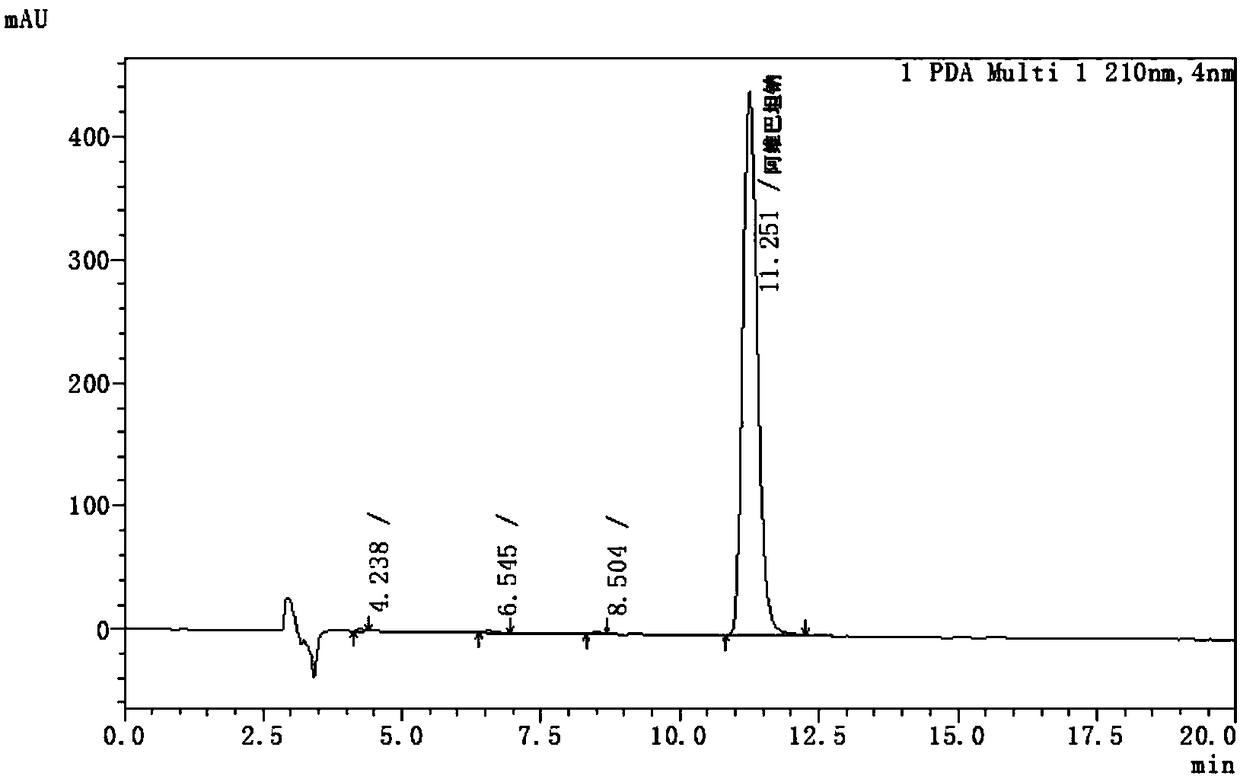
The invention discloses an avibactam sodium analysis method, relates to the field of medicine detection, and aims to provide an avibactam sodium related substance analysis method with high sensitivity and good stability. The chromatographic conditions are that a hydrophilic C18 column is adopted as a chromatographic column; the mobile phase A is a 10 mmol / L-50 mmol / L monopotassium phosphate solution, the mobile phase B is 10 mmol / L-50 mmol / L monopotassium phosphate-acetonitrile (80: 20-30: 70), and gradient elution is carried out; the column temperature is 25-40 DEG C; the flow velocity is 0.5 mL / min to 1.2 mL / min; the sample size is 20 mu L; and the sample concentration is 1 mg / mL to 10 mg / mL. The method has the advantages of high detection sensitivity, good reproducibility, high stability and the like, and is suitable for detecting the avibactam sodium related substances.
Owner:HARBIN PHARMA GROUP TECH CENT
Method for preparing crystal form A or crystal form D type avibactam product through crystallization
The invention discloses a method for preparing a crystal form A or crystal form D avibactam product through crystallization. The method comprises the following steps: preparing an avibactam sodium salt solution by adopting a first solvent; weighing a second solvent; mixing the avibactam sodium salt solution and the second solvent by adopting a dropwise adding manner; after dropwise adding, stirring at room temperature; then cooling to 0 to 5 DEG C and continually stirring; filtering to obtain a filter cake; washing the filter cake by adopting the second solvent; after washing, drying in vacuumto obtain a white solid, namely the product. According to the method disclosed by the invention, the avibactam product is prepared through a crystallization manner; the yield is high, the operation method is simple and large-scale industrial production is easy to realize; the solvents used in a preparation process are adjusted, so that the crystal form A and crystal form D avibactam products canbe obtained respectively; when the large-scale industrial production is carried out, the avibactam products with different crystal forms can be obtained respectively only if simple solvent adjustmentis carried out; the method has a wide application range and a wide market prospect.
Owner:上海龙翔生物医药开发有限公司
Preparation method of avibactam sodium
PendingCN111777607AHigh yieldSuitable for industrial productionOrganic chemistryBulk chemical productionSodium saltEster hydrolysis
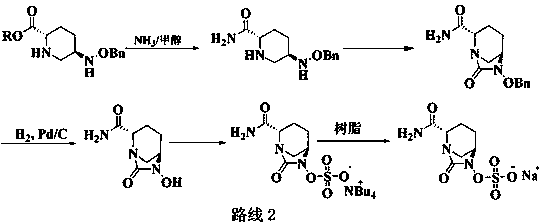
The invention discloses a synthesis method of avibactam sodium. (2S,5R)-benzyloxyamino piperidine-2-ethyl formate oxalate (I) is used as an initial raw material; and the method comprises the followingsteps: reacting the raw material with a protecting group, carrying out carbonylation cyclization, carrying out hydrolysis of ester, ammoniating, sulfonating with a sulfur trioxide complex, salifyingwith an ammonium ion source, and salifying with a sodium salt to obtain avibactam sodium, and has the advantages of simple operation, easily controlled conditions, easy industrial production and wideapplication prospect.
Owner:HAINAN HAILING CHEMIPHARMA CORP
Preparation method of avibactam sodium
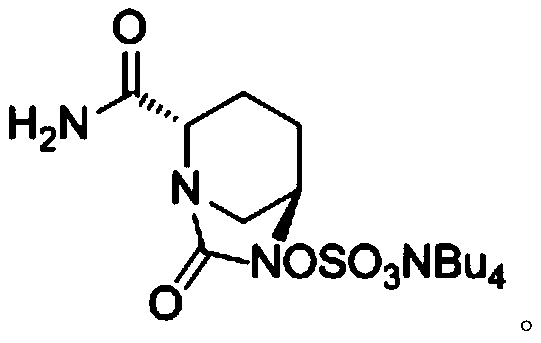
The invention provides a novel preparation method of (2S, 5R)-2-carbamoyl-7-oxo-1, 6-diazabicyclo [3.2. 1]-octane-6-yl] sodium sulfate, and belongs to the technical field of chemical drug synthesis. In the construction process of avibactam sodium molecules, the mode of firstly introducing sulfonic groups, then carrying out aminolysis and then constructing diazo heterocyclic ring is adopted for the first time, and the construction thought is essentially different from original research and other preparation method patents. The initial raw material used in the method is a chemical product easily obtained in the market, and guarantees are provided for process production conversion. The novel preparation process is mild in condition, simple to operate, high in total yield, short in construction period and suitable for commercial production of the avibactam sodium bulk drug. An original preparation route is successfully avoided, so that the limitation of the protection period of an original preparation method patent is overcome, and the problem of medication accessibility of the medicine in China can be solved quickly.
Owner:BEIJING JIMEITANG MEDICINE RES CO LTD
A method for preparing amorphous avibactam sodium by spray drying
ActiveCN108409736BReduce generationThe method steps are simpleOrganic chemistryAvibactam sodiumPurified water
The invention discloses a method for preparing amorphous avibactam sodium by spray-drying. According to the invention, solution of sodium iso-octoate is dropwise added into solution of avibactam tetrabutylammonium salt, after dropwise adding is completed, the reaction is performed for 3 to 4h, and purified water is added for extraction; after a water phase is subjected to spray-drying, the amorphous avibactam sodium is obtained. The method disclosed by the invention is simple in step and easy for industrial production, the purity of a product is greater than or equal to 98.0%, and yield is greater than or equal to 90.0%.
Owner:山东安信制药有限公司
Preparation method of avibactam sodium
PendingCN114685499AHigh yieldSimple post-processingOrganic chemistryPalladium on carbonPtru catalyst
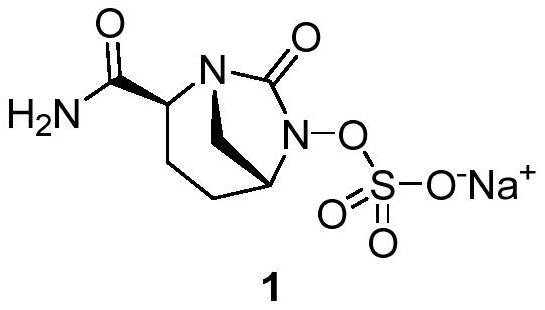
The invention discloses a preparation method of avibactam sodium, which comprises the following steps: carrying out salt decomposition on a compound 1, carrying out ring-closure reaction under the action of triphosgene to generate a compound 2, hydrolyzing the compound 2 to prepare a brand new intermediate alkali metal salt compound 3, and further condensing with ammonia water to prepare a compound 4. The compound 4 uses palladium on carbon as a catalyst, cyclohexene or 1, 4-cyclohexadiene is used for replacing hydrogen with high production safety risk as a reducing reagent, after reduction, the compound 4 reacts with sulfur trioxide trimethylamine and tetrabutylammonium acetate to generate a compound 5, and then the finished product avibactam sodium is obtained through salifying and crystal transformation.
Owner:BEIJING JIMEITANG MEDICINE RES CO LTD
Avibactam Free Acid
The present invention relates to avibactam free acid, a method for preparing avibactam free acid and a method for preparing avibactam sodium by further reacting avibactam free acid. The invention further refers to a pharmaceutical composition comprising avibactam free acid, one or more alkaline sodium salt(s) and one or more beta-lactam antibiotic(s). The pharmaceutical composition of the present invention can be used as medicament, in particular for treatment and / or prevention of bacterial infections.
Owner:SANDOZ AG
Preparation method of avibactam sodium
PendingCN114656465AReduce usageAvoid the disadvantages of long reaction time and volatile reagentsOrganic chemistry methodsPalladium on carbonPtru catalyst
The invention discloses a preparation method of avibactam sodium, which comprises the following steps: carrying out ammonolysis on (2S, 5R)-5-[(benzyloxy) amino] piperidine-2-carboxylic acid ethyl ester oxalate II serving as a starting raw material to generate a compound III, then carrying out cyclization to obtain a compound IV, reacting the compound IV with a hydrogen donor under the action of a catalyst to obtain a compound V, and carrying out post-treatment on the compound V to obtain the avibactam sodium. And sulfonating and forming sodium salt to obtain avibactam sodium. The ammonolysis reagent is selected from ammonium chloride, ammonium carbonate, ammonium sulfate, ammonium bicarbonate and ammonium formate; the catalyst is selected from palladium on carbon, platinum dioxide or Raney nickel, and the hydrogen donor is selected from cyclohexene, cyclohexadiene or tetrahydronaphthalene. The method is high in yield, good in safety and mild in reaction condition.
Owner:南京佰麦生物技术有限公司
Stable crystal form of avibactam sodium and preparation method thereof
PendingCN111689964ASimple methodHigh yieldOrganic chemistry methodsAvibactam sodiumPharmaceutical Substances
Owner:HAINAN HAILING CHEMIPHARMA CORP
Avibactam and cefmenoxime compound powder injection for injection and preparation method thereof
PendingCN113413367AGood dispersionImprove solubilityAntibacterial agentsOrganic active ingredientsBiochemistryAvibactam sodium
The invention relates to an avibactam and cefmenoxime compound powder injection for injection and a preparation method thereof. The compound powder injection is prepared by well mixing cefmenoxime hydrochloride and avibactam sodium, tabletting and crushing. The powder injection disclosed by the invention is easy to disperse and dissolve when being prepared into an injection, is convenient to use, cannot easily generate beta-lactamase bacterial drug resistance while ensuring the content of cefmenoxime, and has a good market application prospect.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
A kind of preparation method of Avibactam sodium intermediate
ActiveCN111646991BOrganic chemistryBulk chemical productionChiral selectivityCombinatorial chemistry
The invention relates to a preparation method of a key intermediate compound (VII) of avibactam sodium, wherein the compound (VII) is obtained from the compound (V) through cyclization and deprotection reactions. The preparation method of the present invention has good chiral selectivity, simple and easy operation, high yield, good purity, cheap and easy-to-obtain raw materials, and has industrial value
Owner:ZHEJIANG HISOAR PHARMA +1
Form C of Avibactam Sodium
ActiveUS20180193351A1Maintain antimicrobial activitySufficient protectionAntibacterial agentsOrganic active ingredientsCombinatorial chemistryAvibactam sodium
The present invention relates to crystalline form C of avibactam sodium and to a process for its preparation. The invention also concerns a pharmaceutical composition comprising form C and one or more antibacterial agents, wherein at least one antibacterial agent is a beta-lactam antibiotic. The pharmaceutical composition of the present invention can be used as medicament, in particular for treatment and / or prevention of bacterial infections.
Owner:SANDOZ AG
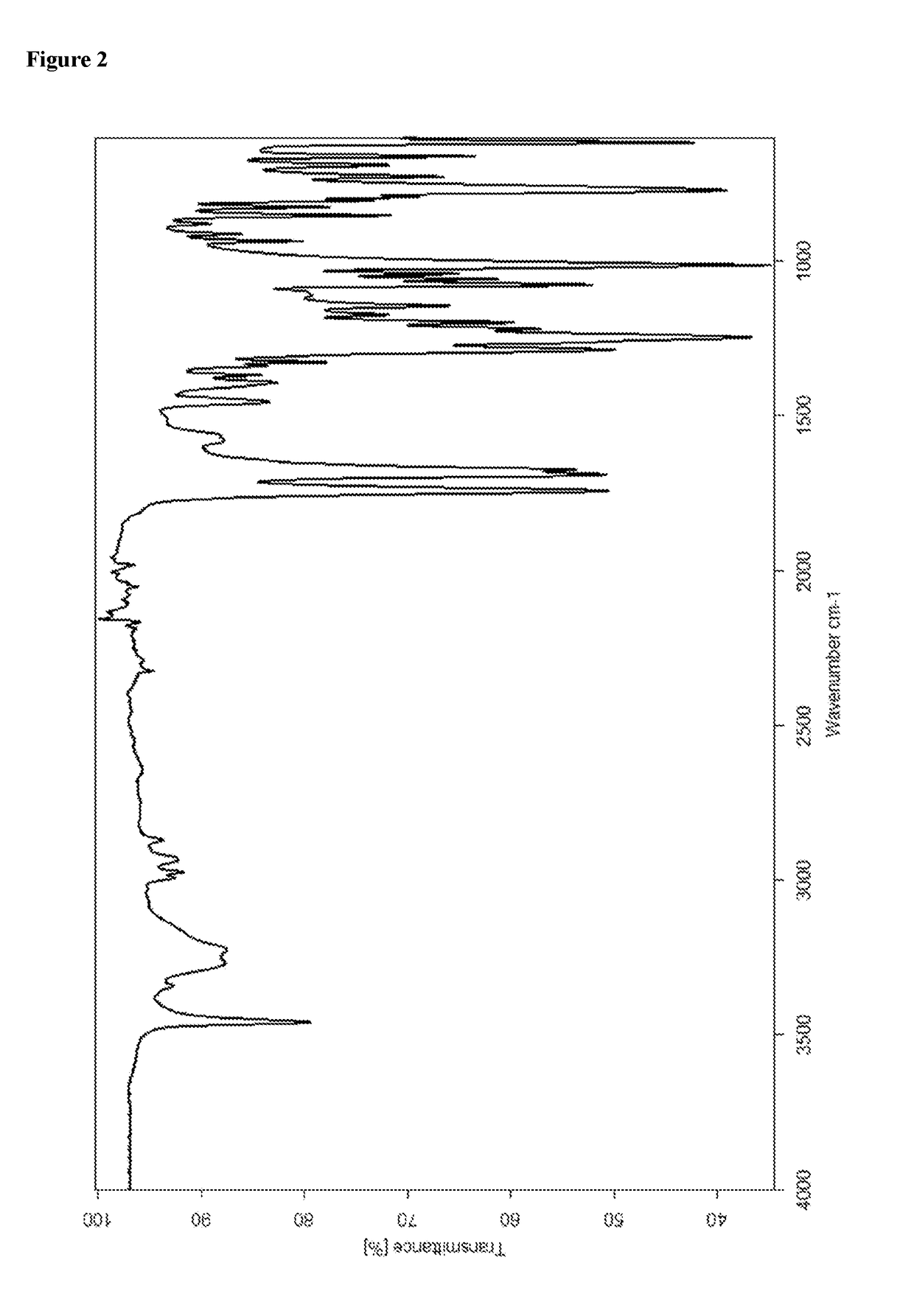
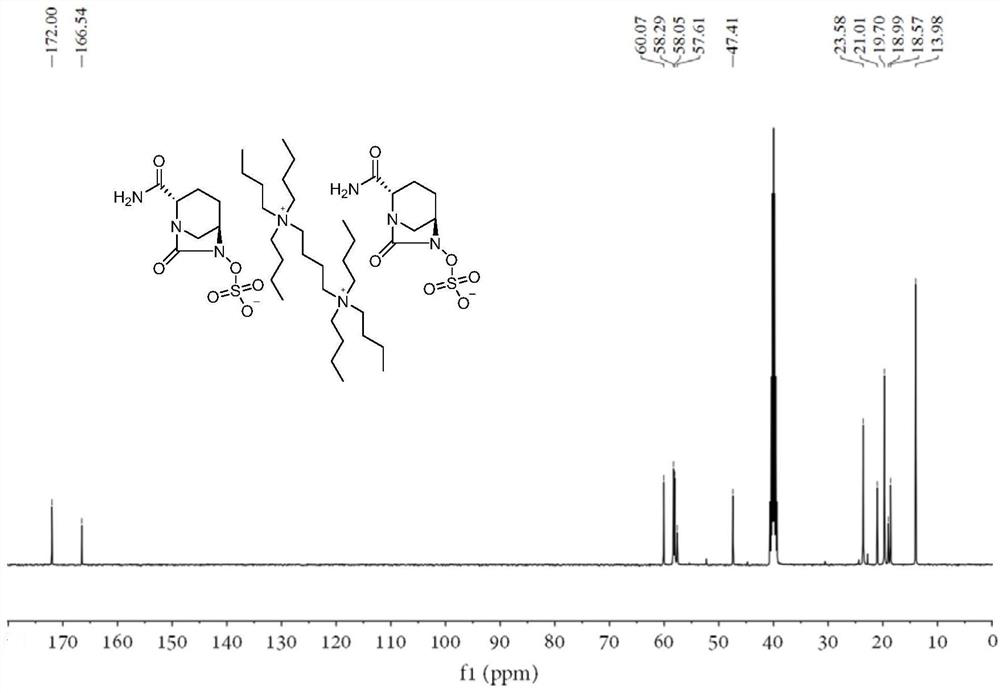
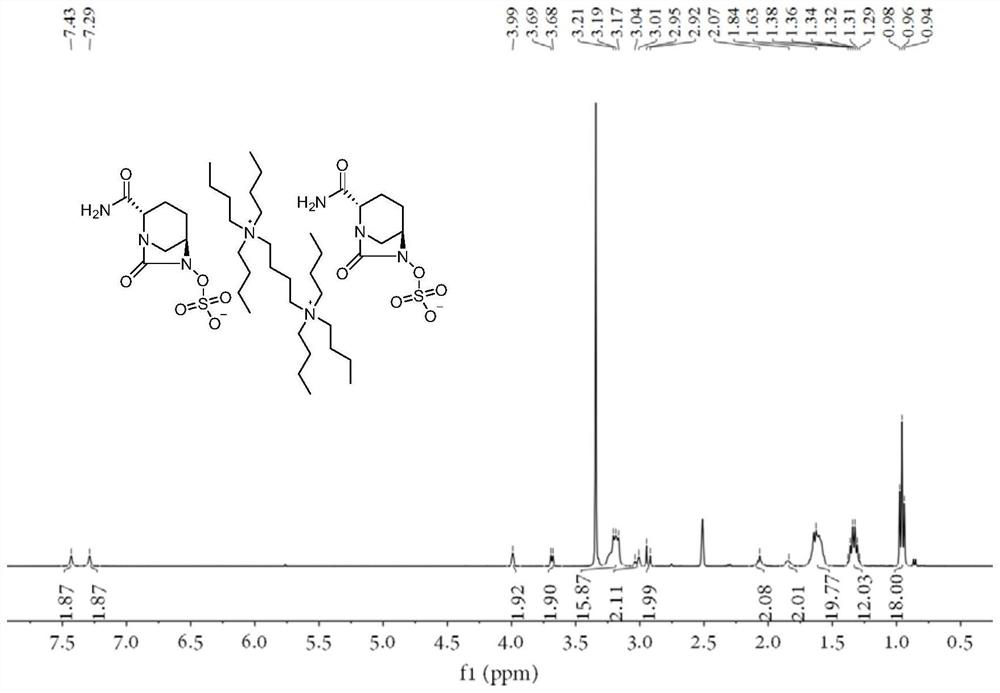
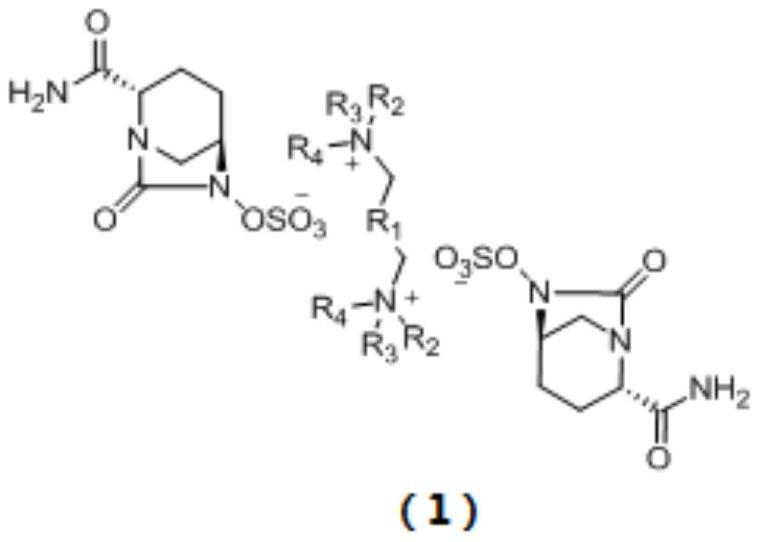
Avibactam intermediate compound disulfonic acid gemini quaternary ammonium salt and preparation method thereof
ActiveCN111732587AEasy to purifyEasy to operateOrganic compound preparationAmino compound preparationChemical synthesisSodium salt
The invention discloses an avibactam intermediate compound, disulfonic acid gemini quaternary ammonium salt, and a preparation method thereof, and relates to medical compounds and organic chemical synthesis, and the preparation method of the avibactam intermediate compound disulfonic acid gemini quaternary ammonium salt comprises the following steps: (1) carrying out hydrogenolysis sulfonation reaction on (2S,5R)-6-hydroxy-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-formamide; (2) after the reaction in the previous step is completed, performing suction filtration, washing filtrate once, and adding gemini quaternary ammonium salt for reaction; and (3) after the reaction in the previous step is completed, carrying out extraction, rotary evaporation and crystallization. Compared with the prior art,the method has the advantages that the operation is simple, the raw materials are easy to obtain, the cost is lower, the dosage of the gemini quaternary ammonium salt is lower, the purity of the obtained disulfonic acid gemini quaternary ammonium salt is higher, and the HPLC relative purity of avibactam sodium generated by sodium salt exchange of the disulfonic acid gemini quaternary ammonium salt is greater than 99.5%, so that the process is suitable for large-scale production.
Owner:山东大医精诚药业有限公司
A kind of synthetic method of avibactam sodium
ActiveCN107417686BLow priceReduce manufacturing costOrganic chemistryIon exchangeCarbonyldiimidazole
The invention discloses a synthetic method for avibactam sodium salt. The method comprises the following steps: taking (2S, 5R)-5-[(benzyl oxyl) amino] piperidine-2-formamide as a starting material; constructing a urea ring by carbonyl diimidazole under the effect of dimethyldichlorosilance to obtain (2S, 5R)-6-(benzyl oxyl)-7-oxo-1,6-diazabicyclo [3.2.1] octane-2-formamide; then carrying out hydrogenation to remove benzyl; carrying out sulfonation reaction on the compound and a sulfonated reagent; synthesizing into a quaternary ammonium salt intermediate by using quaternary ammonium salt; and finally carrying out ion exchange to obtain the avibactam sodium salt. The improved process is low in cost, simple and convenient to operate, good in product quality and suitable for industrial production. In a process of synthesizing the intermediate (2S, 5R)-6-(benzyl oxyl)-7-oxo-1,6-diazabicyclo [3.2.1] octane-2-formamide, dimethyldichlorosilance which is low in price is used, and therefore, the production cost is greatly reduced.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Preparation method of beta-lactamase inhibitor drug avibactam sodium intermediate
InactiveCN110590775AReduce security risksImprove reaction efficiencyOrganic chemistryDecompositionReaction intermediate
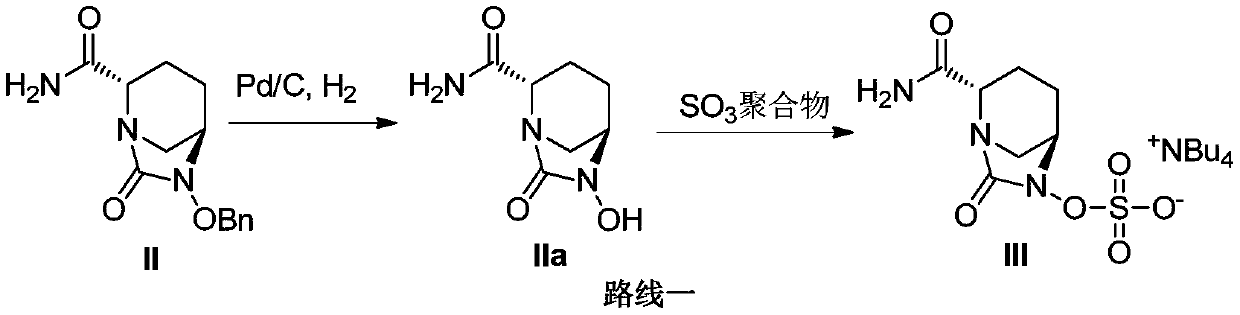
Belonging to the technical field of medicinal chemistry, the invention particularly relates to a preparation method of a beta-lactamase inhibitor drug avibactam sodium intermediate. The method includes: adding a reaction solvent into a reaction container, then sequentially adding a compound II, a sulfur trioxide polymer, alkali and a catalyst, and stirring the substances uniformly; setting the reaction conditions of a micro-channel reactor, pumping the reaction materials into the micro-channel reactor for reaction, and collecting the reaction liquid; performing filtering and collecting the filtrate, adding an ammonium salt aqueous solution into the filtrate, and stirring the substances uniformly, finally adding an extraction agent for extraction, performing liquid separation, then collecting an organic phase and performing concentration to obtain a compound III crude product, and carrying out aftertreatment on the crude product to obtain a compound III. The method provided by the invention utilizes the micro-channel reactor to combine hydrogenation and sulfonation reactions into one, avoids decomposition of the reaction intermediate, and increases the yield; and the method also realizes safe, rapid and continuous reaction at the same time, greatly improves the productivity, and is more suitable for large-scale production.
Owner:REYOUNG PHARMA
Avibactam sodium optical isomer high performance liquid chromatography detection method
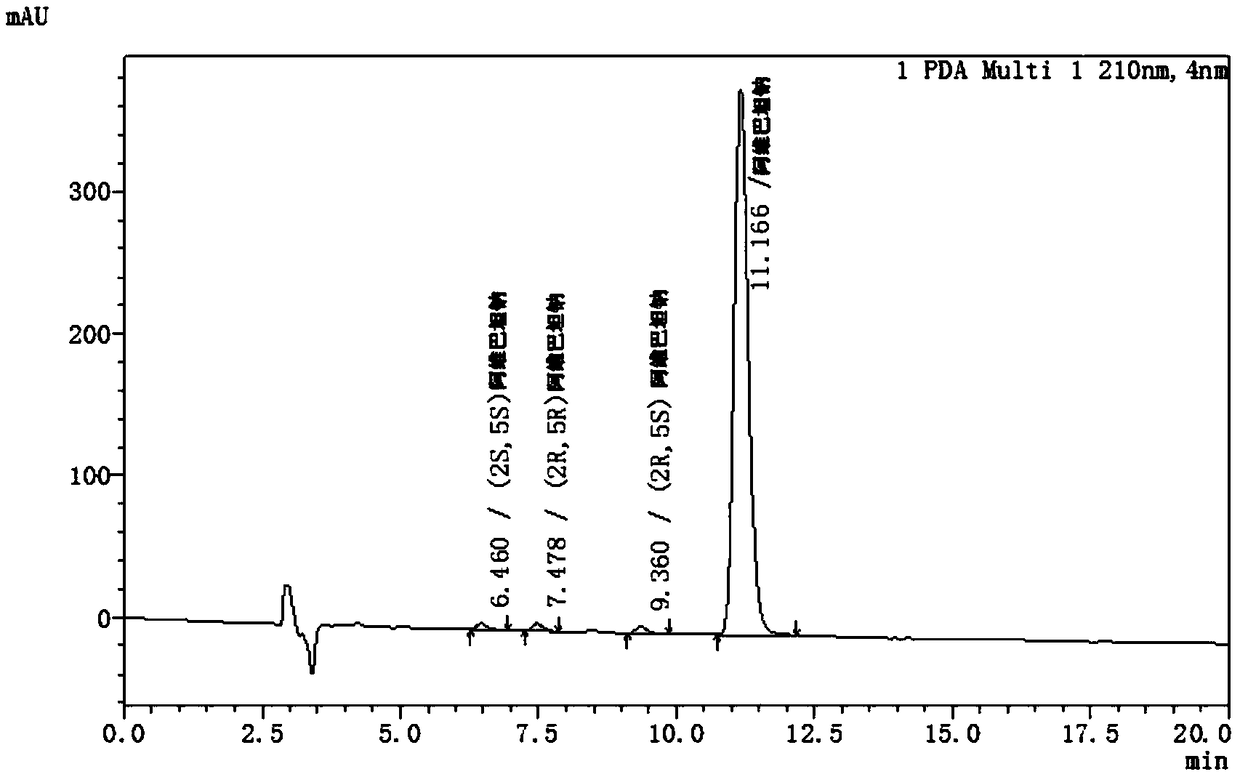
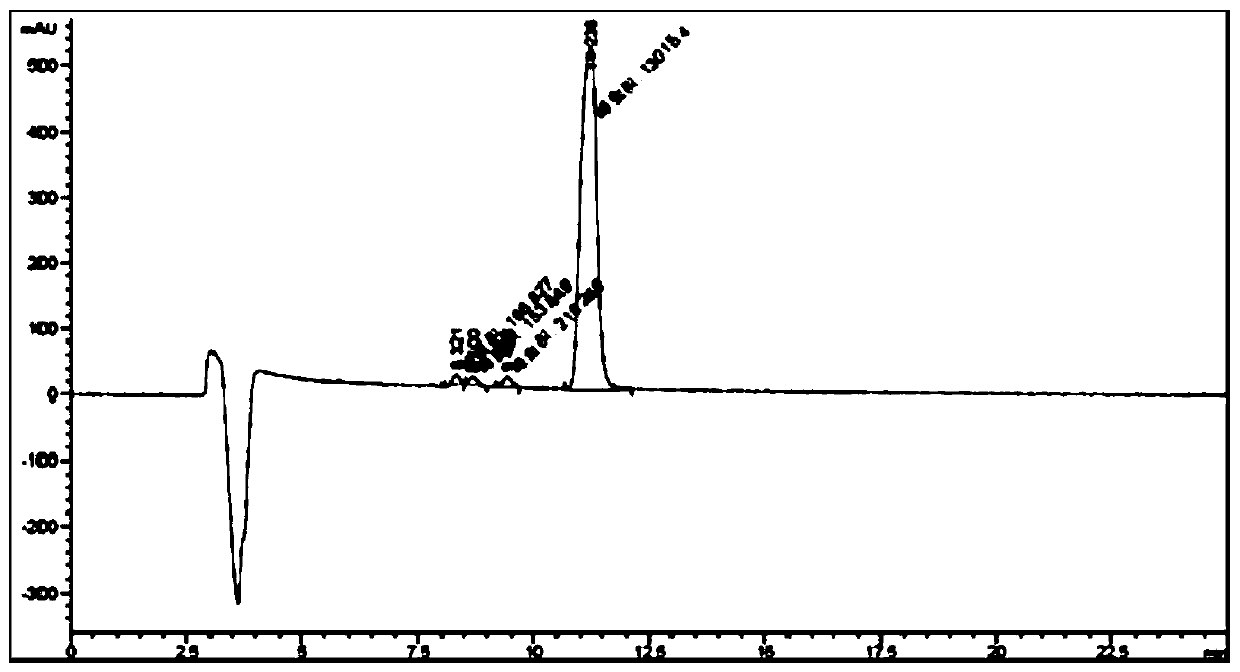
ActiveCN108362789ARealize separation detectionStrong specificityComponent separationTrifluoroacetic acidColumn temperature
The invention belongs to the technical field of pharmaceutical analysis detection, and more specifically relates to an avibactam sodium optical isomer high performance liquid chromatography detectionmethod. According to the invention, a celluloid IC column is taken as a chromatographic column, a mobile phase is a mixture of ethanol, diethylamine and trifluoroacetic acid with the volume ratio being 100: 0.15: 0.1, the column temperature is 25-40 DEG C, the flow velocity is 1ml / min, and the detection wavelength is 210 nm. The avibactam sodium optical isomer high performance liquid chromatography detection method has the advantages of good specialization, sensitivity and reappearance, can accurately detect the content of an optical isomer in avibactam sodium, and can guarantee good clinicaleffect.
Owner:珠海优润医药科技有限公司
Refining method of avibactam sodium intermediate
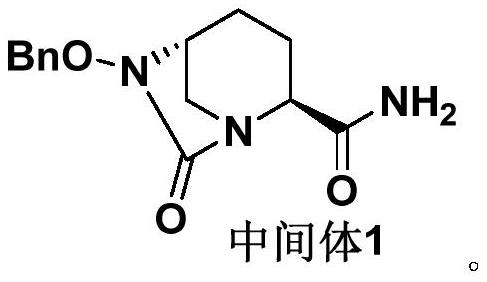
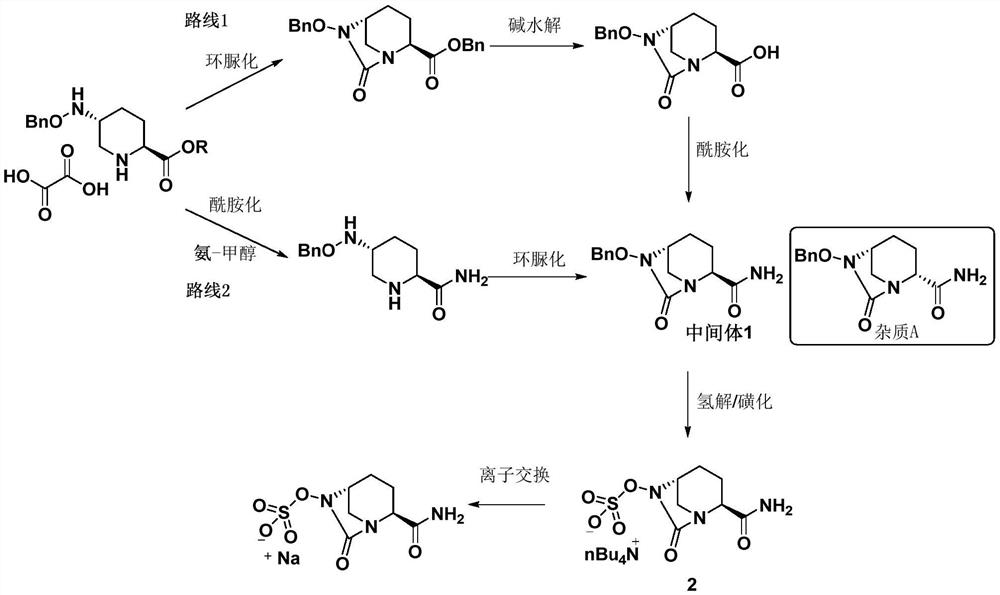
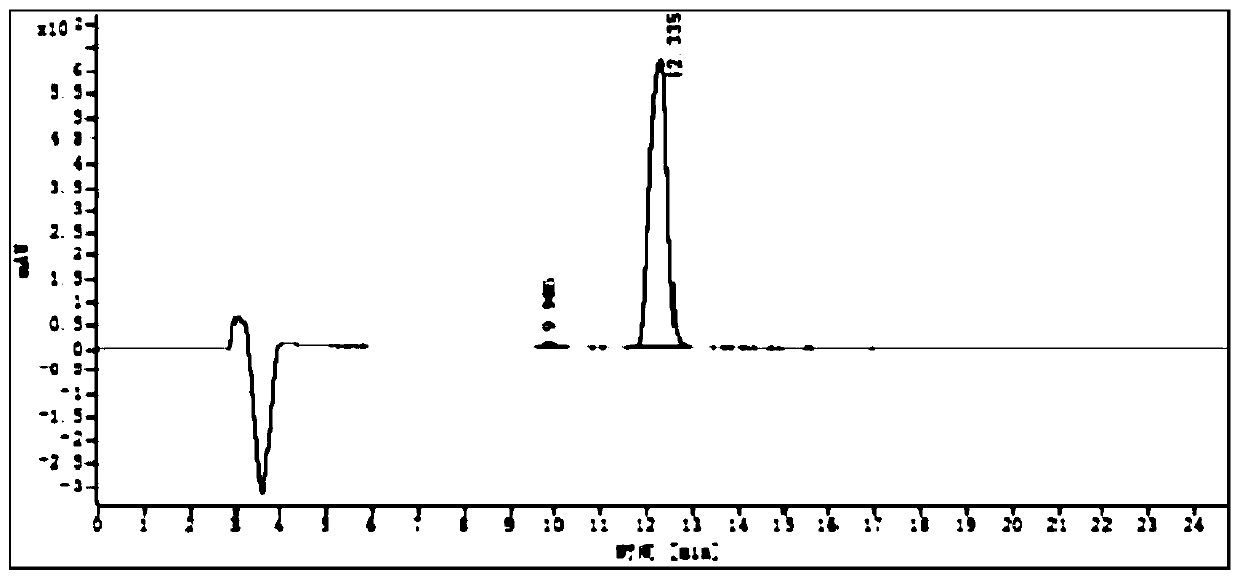
The invention discloses a refining method of an avibactam sodium intermediate 1. The method comprises the step of refining a crude product of the avibactam sodium intermediate 1 in a good solvent dichloromethane and poor solvent methyl tert-butyl ether system to obtain the high-purity avibactam sodium intermediate 1. According to the refining method provided by the invention, the content of an isomer impurity A and a ring-opening impurity B can be effectively reduced, the purity is improved, the total purity of the refined avibactam sodium intermediate 1 is greater than 99.9%, the isomer impurity A and the ring-opening impurity B are both less than 0.05%, the other single impurities are all less than 0.10%, the reagents are conventional and easy to obtain, the operation is simple and safe and the method is very suitable for industrial amplification under conventional production conditions.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD +1
A method for detecting chiral isomers of key intermediates of avibactam sodium
The invention provides a method for detecting chiral isomers of a key intermediate of avibactam sodium. The method specifically comprises the following steps: detection of content of the chiral isomers in benzyl(2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethanedioate with high performance liquid chromatography; effective separation of main peak. Adopted chromatographic conditions are as follows: a CHIRALPAK AD-H chromatographic column is adopted, the column temperature is 25-40 DEG C, detection wavelength is 280 nm, a mobile phase is a mixture of n-hexane, isoamyl alcohol, ethyl alcohol and diethylamine, and the flow rate is 0.8-1.2 mL / min. In an actual detection process, the chiral isomers higher than 0.066% can be detected from the key intermediate when the limit of detection inthe method reaches 0.33 mu g / mL, the practicability is high, and the detection process is simple and fast.
Owner:海南欣莱医药科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com