Orm C of avibactam sodium
一种阿维巴坦钠、晶型的技术,应用在阿维巴坦钠的晶型C及其制备领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
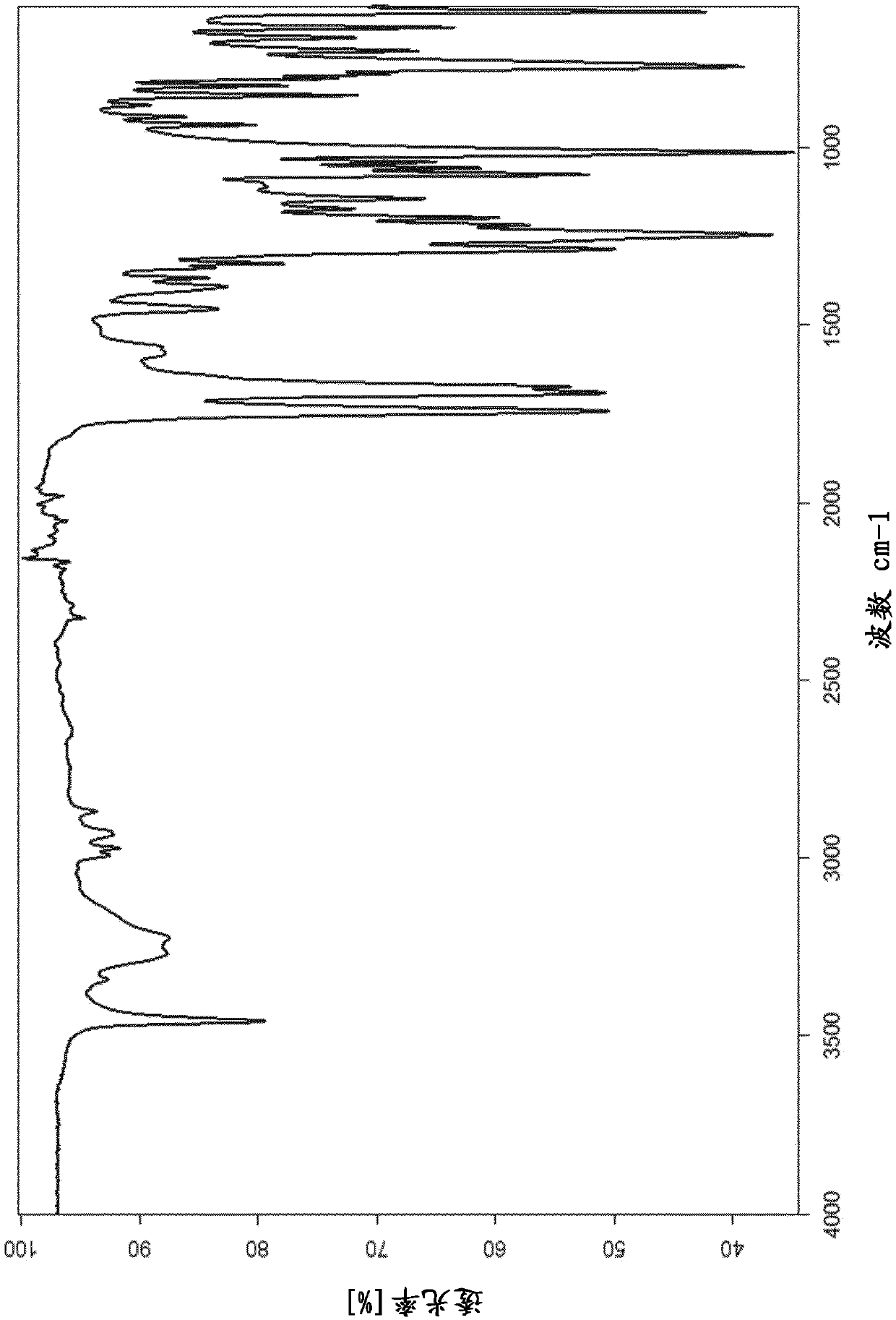
[0177] Example 1: Form C of Avibactam Sodium
[0178] Sodium avibactam (160 mg, for example the crystal form D prepared according to Example 5 of WO 2011 / 042560 A1) was dissolved in 2 mL of water. To this solution was added 3 mL of isobutanol, and the biphasic mixture was heated to a bath temperature of 119° C. to remove water azeotropically. After removal of water, precipitation occurred. The suspension was allowed to cool to room temperature and the solid was collected by filtration and blotted dry on the filter.
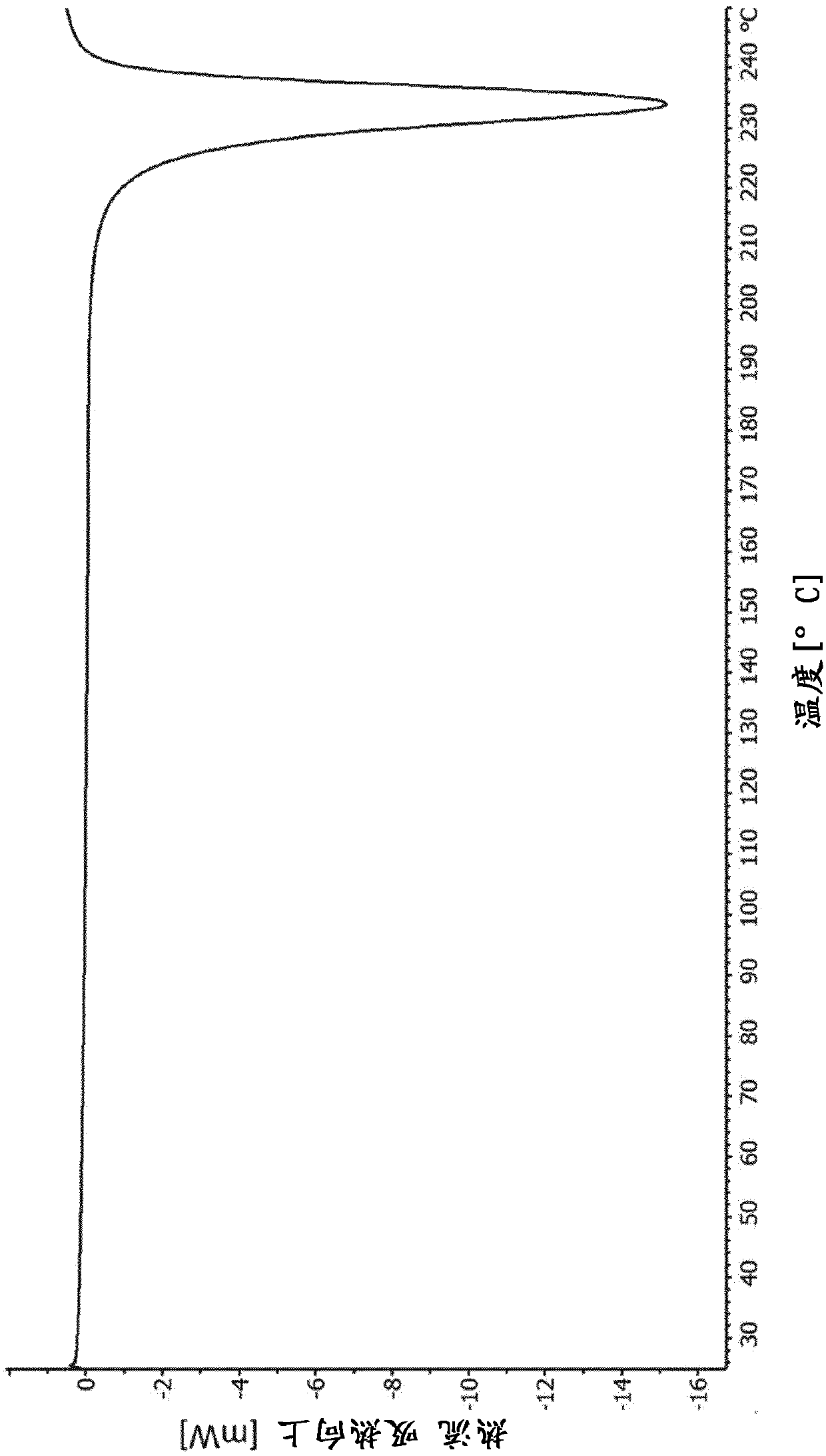
[0179] Yield: 98 mg (61% of theoretical value), DSC (10K / min): exothermic, initial temperature is 227 ° C,
[0180] TGA (10K / min): 0.5% mass change by weight from about 25 to 200°C
[0181] The powder X-ray diffraction pattern of the obtained material is as figure 1 As shown, the reflection list is shown in Table 1.
[0182]
[0183] Table 1: List of reflections and corresponding relative intensities for Form C at 2.0 to 30.0° 2-theta
[0184] The obtaine...
Embodiment 2
[0187] Example 2: Form C of Avibactam Sodium
[0188] Avibactam sodium (164 mg, crystal form D, prepared for example according to Example 5 of WO 2011 / 042560 A1) was dissolved in 2 mL of water. To this solution was added 3 mL of 2-butanol, and the biphasic mixture was heated to a bath temperature of 135° C. to remove water azeotropically. Precipitation occurs after removal of water. The suspension was allowed to cool to room temperature and the solid was collected by filtration and blotted dry on the filter. Powder X-ray diffraction confirmed Form C.
[0189] Yield: 102 mg (62% of theory),
Embodiment 3
[0190] Example 3: Stress Test
[0191] Form C (prepared according to Example 1 herein) was stored in a desiccator at room temperature with an open opening, and different relative humidity was generated in a saturated saline solution. The samples were analyzed by powder X-ray diffraction and the results are summarized in Table 2 below:
[0192]
[0193]
[0194] Table 3: Summary of stress tests performed with Form C as starting material
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com