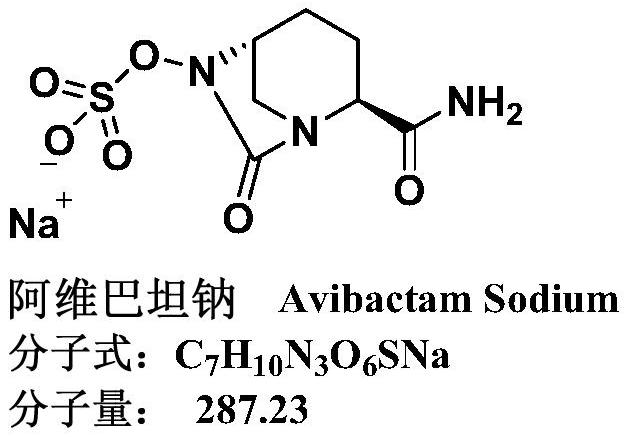
Refining method of avibactam sodium intermediate
A technology of avibactam sodium and a purification method, applied in the field of drug synthesis, can solve the problems of limited removal of impurities, large ring-opening impurities, inability to guarantee high purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
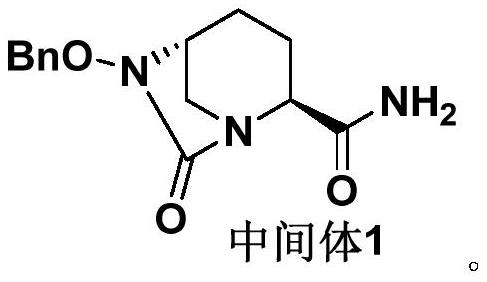
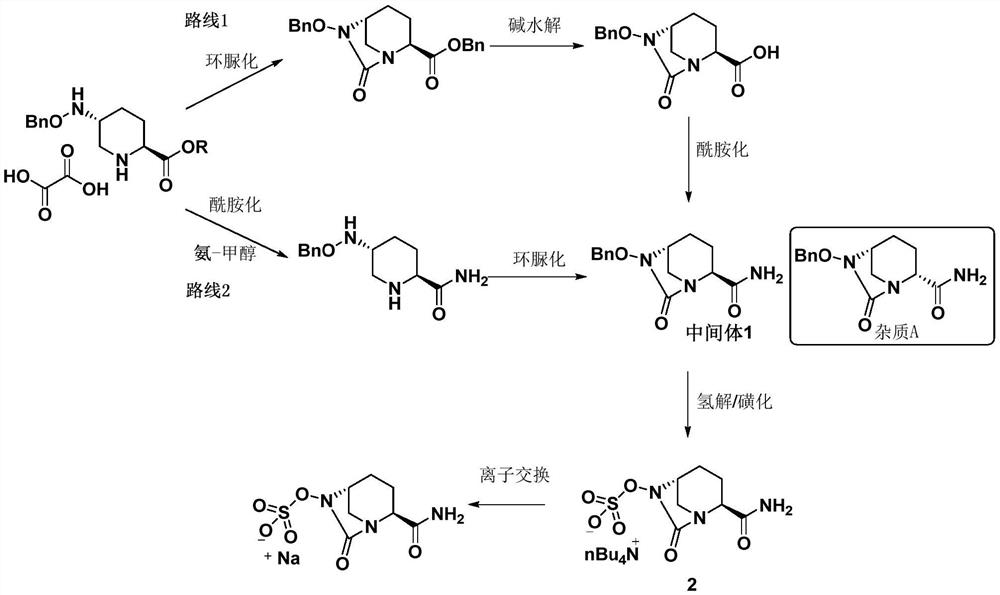
[0045] Preparation Example 1: Preparation of Avibactam Sodium Intermediate 1 Crude Product:
[0046] The crude product of avibactam sodium intermediate 1 was prepared with reference to the method disclosed in Example 3c of patent document CN201280029765.7 (page 30 of the description).
[0047] The obtained avibactam sodium intermediate 1 crude product is carried out HPLC purity detection, and its total purity is 88.37%, and impurity A content is 2.33%, and impurity B content is 0.66%, and total impurity content is 11.63%, as shown in table 1 Show. The obtained avibactam sodium intermediate 1 is used in the following examples or test examples as the avibactam sodium intermediate 1 crude product.
[0048] Table 1 Avibactam sodium intermediate 1 crude product HPLC detection result
[0049]
[0050]
Embodiment 1
[0051] Embodiment 1: the refining of avibactam sodium intermediate 1
[0052] Step (1): Take 20g of the crude product of avibactam sodium intermediate 1 (based on the pure product of avibactam sodium intermediate 1), add 60mL of dichloromethane, and heat up to 35-45°C under stirring;
[0053] Step (2): Add 180mL of methyl tert-butyl ether dropwise, dropwise for 0.5-1h, solids precipitate out, cool down to -10-0°C, stir for 1-2h, filter with suction, wash with 20mL of methyl-tert-butyl ether , and dried to obtain 19.0 g of avibactam sodium intermediate 1 (yield 95%).
[0054] The refined product of avibactam sodium obtained in this embodiment was detected by HPLC, and the experimental data are shown in Table 2. The total purity is 99.92%, impurity A is not detected, the content of impurity B is 0.011%, and the content of other single impurities is less than 0.03%, meeting the pharmaceutical requirement of less than 0.10%.
[0055] The avibactam sodium intermediate 1 refined p...
Embodiment 2
[0068] Embodiment 2: the refining of avibactam sodium intermediate 1
[0069] Step (1): Take 20g of the crude product of avibactam sodium intermediate 1 (based on the pure product of avibactam sodium intermediate 1), add 80mL of dichloromethane, and heat up to 35-45°C under stirring;
[0070] Step (2): Add 200mL of methyl tert-butyl ether dropwise, dropwise for 1-1.5h, solids precipitate out, cool down to -10-0°C, stir for 1-2h, suction filter, 40mL of methyl tert-butyl ether Washing, drying to obtain 18.6g avibactam sodium intermediate 1 (yield 93%).
[0071] The refined product of avibactam sodium obtained in this embodiment was detected by HPLC, and the experimental data are shown in Table 5. The total purity is 99.97%, the content of impurity A is 0.017%, the content of impurity B is 0.0095%, and other single impurities are not detected.
[0072] The avibactam sodium intermediate 1 refined product HPLC detection result that table 5 embodiment 2 makes
[0073] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com