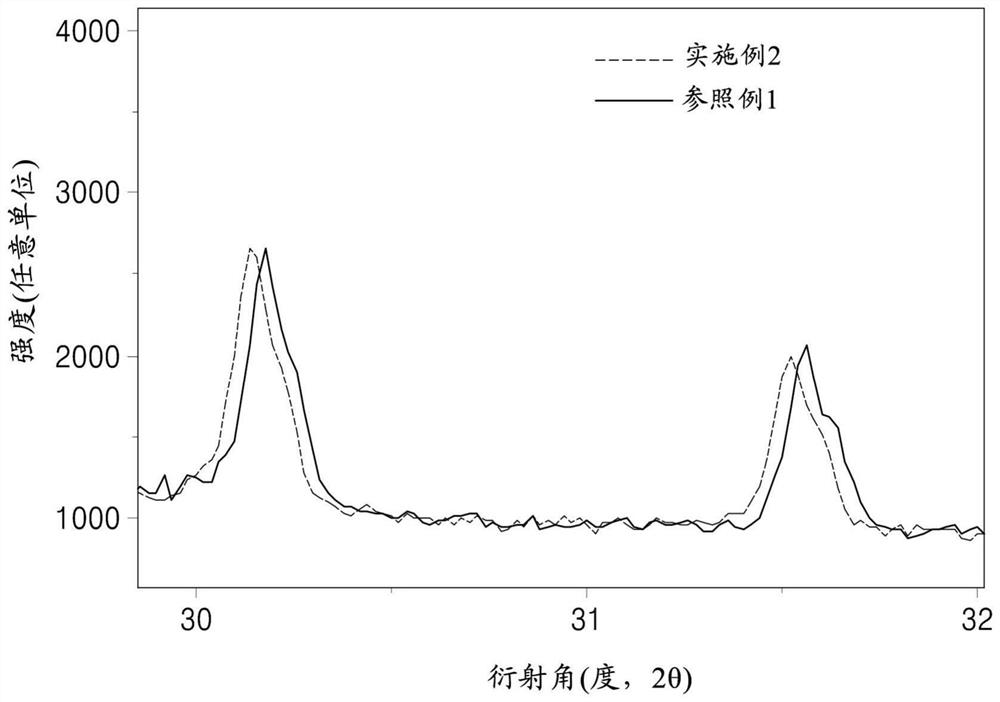
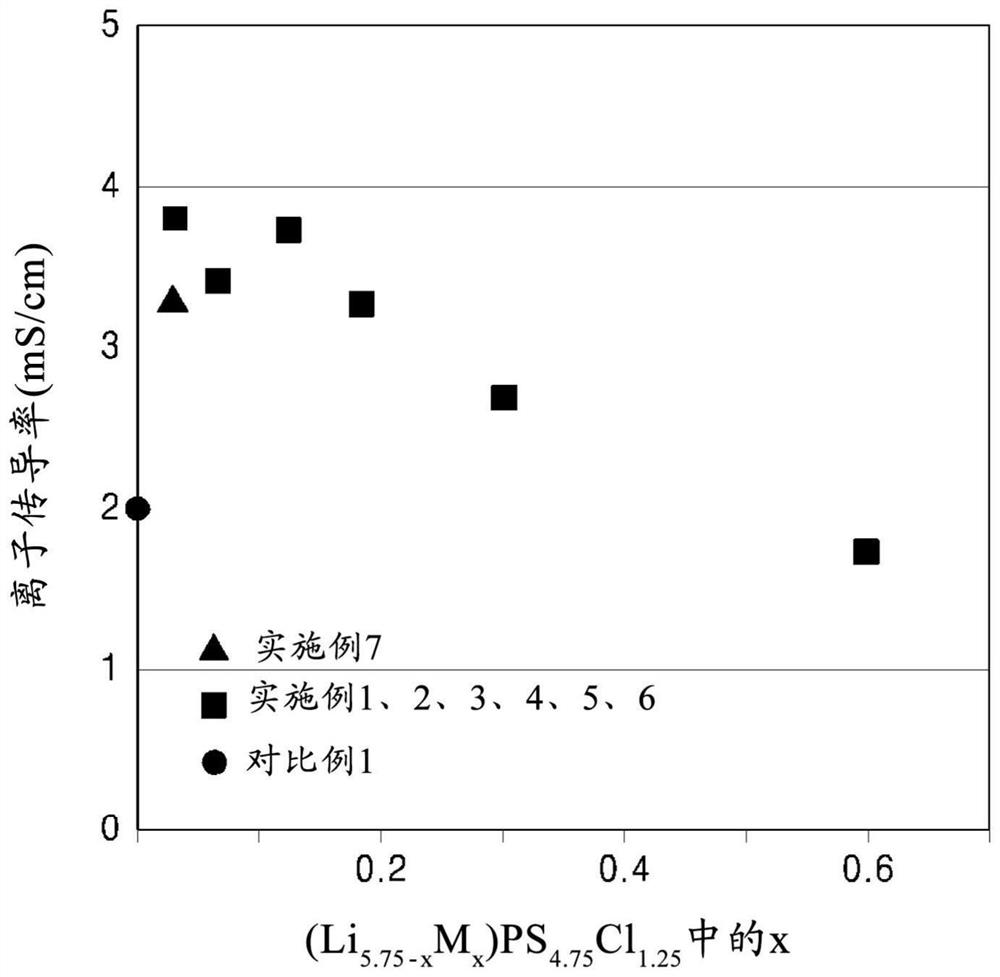
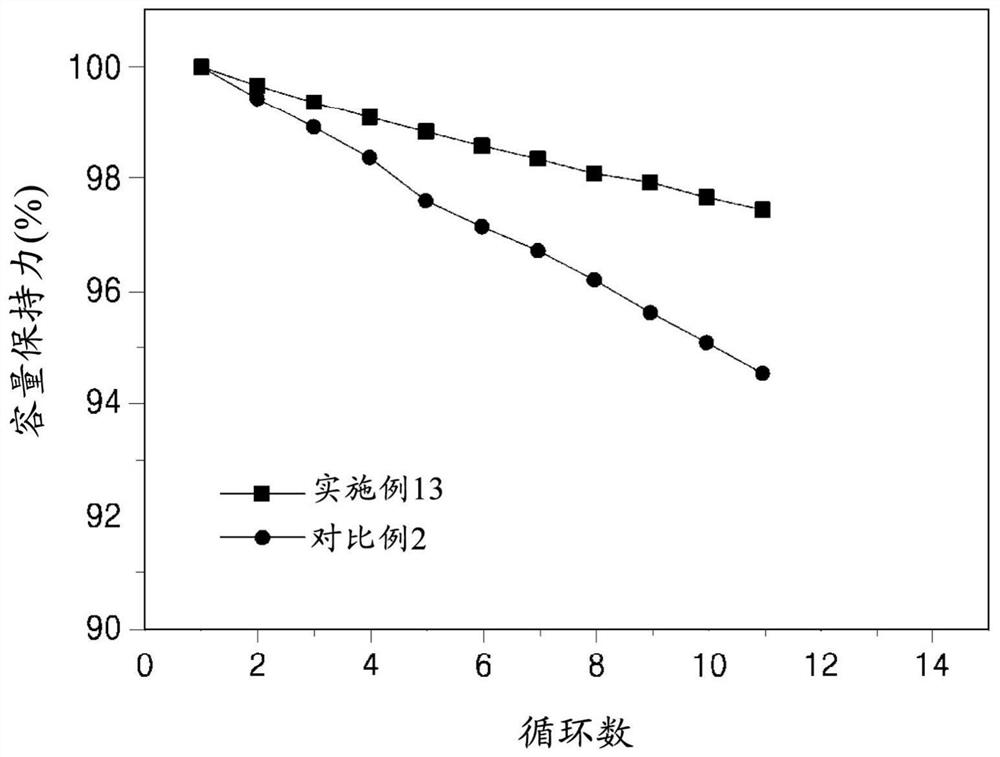
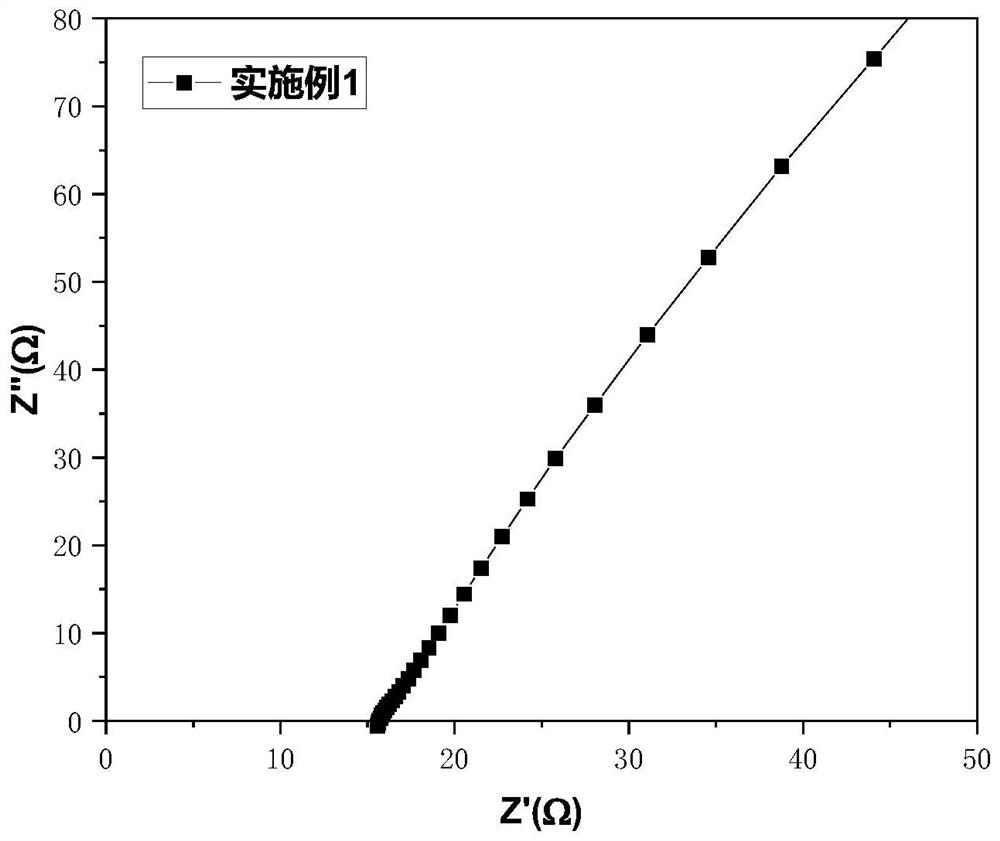
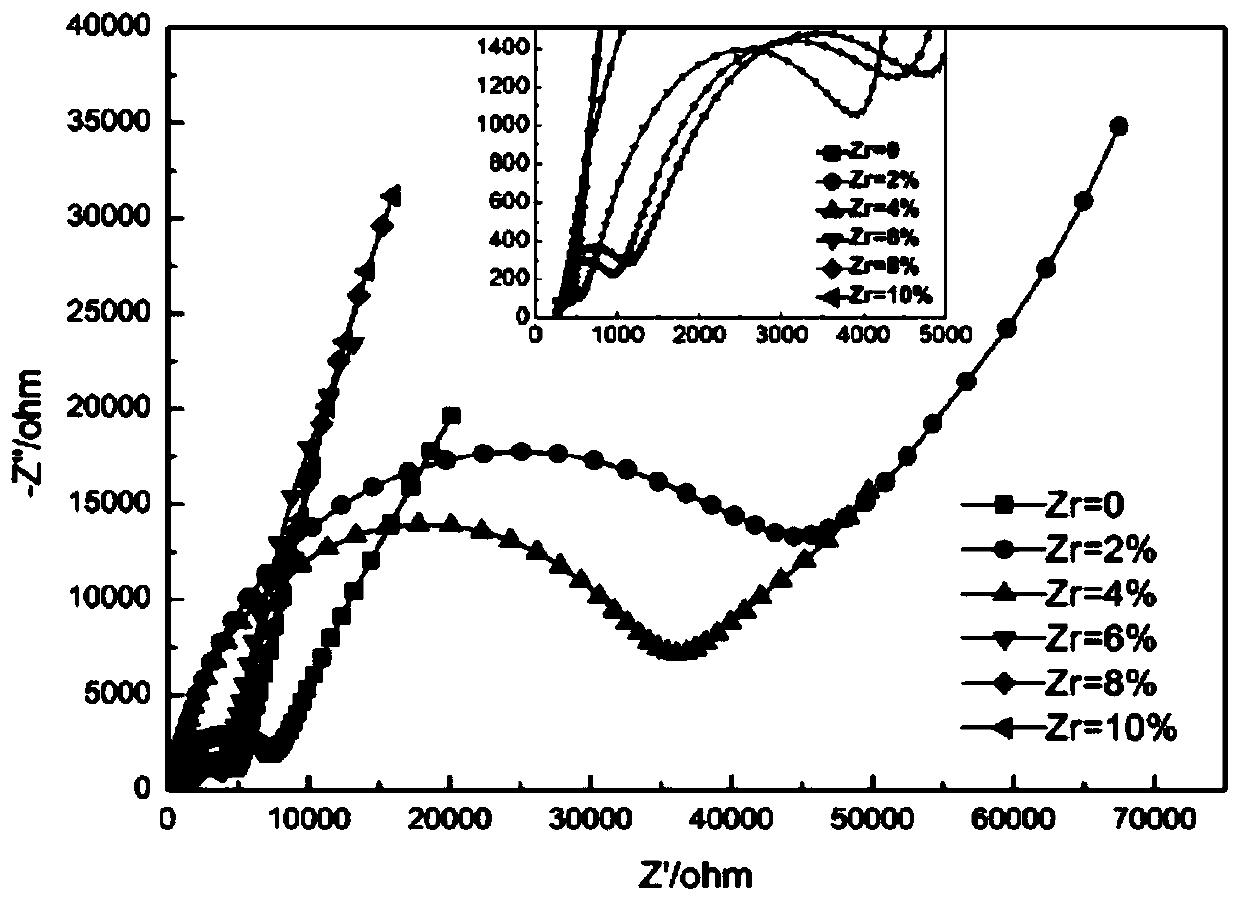
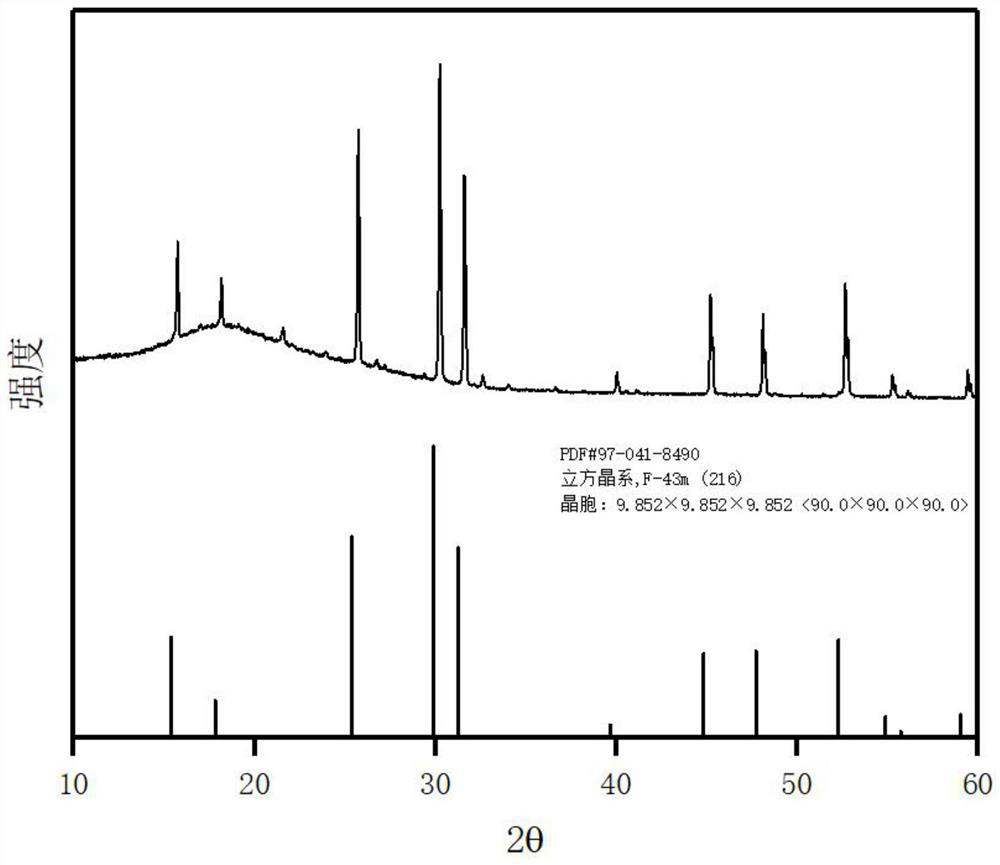
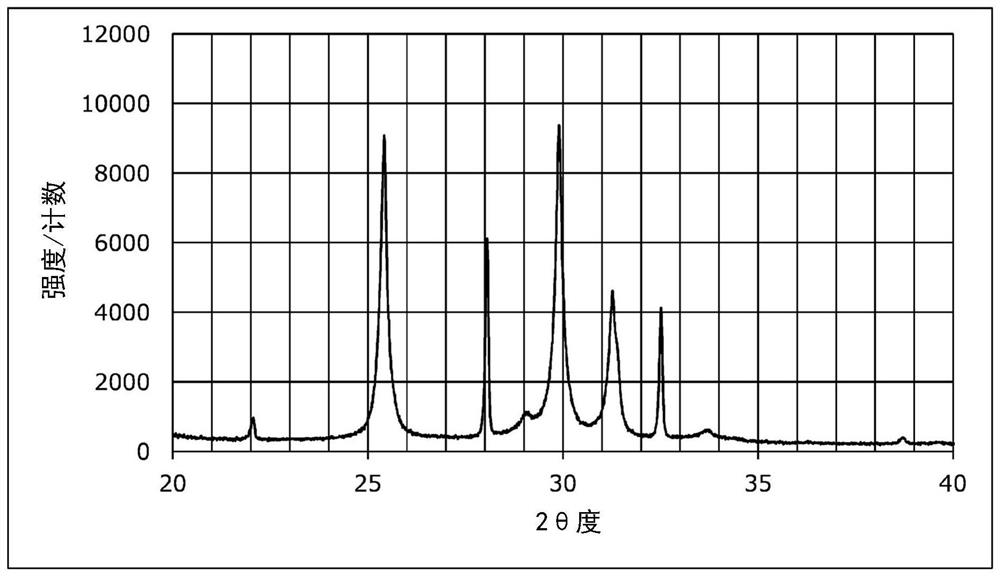
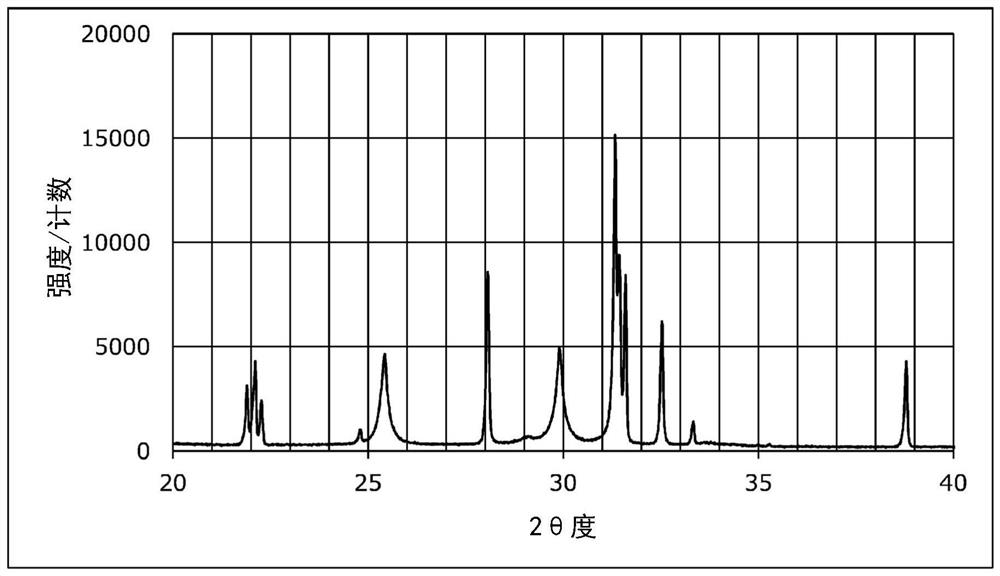
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Argyrodite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Argyrodite is an uncommon silver germanium sulfide mineral with formula Ag₈GeS₆. The color is iron-black with a purplish tinge, and the luster metallic. Discovered by Clemens Winkler in 1886, it is of interest as it was described shortly after the element germanium was isolated, 15 years after it had been postulated by Mendeleev. It was first described for an occurrence in the Himmelsfürst Mine, Erzgebirge, Freiberg, Saxony, Germany.
Argyrodite thermoelectric material and preparation method thereof
InactiveCN106098923AImprove thermoelectric performanceLow thermoelectric performanceThermoelectric device manufacture/treatmentThermoelectric device junction materialsArgyroditeSlow cooling
The invention relates to an argyrodite thermoelectric material, the chemical formula of which is Ag8Sn(1-x)NbxSe6, x=0-0.05. The preparation method of the argyrodite thermoelectric material is characterized by, with simple substances being raw materials, carrying out material blending according to stoichiometric ratio of the chemical formula; after vacuum packaging, melting reaction quenching and thermal treatment quenching, grinding ingots into powders; and carrying out vacuum high-temperature hot-pressure sintering, and after slow cooling, obtaining a block material, which is the argyrodite thermoelectric material. Compared with the prior art, the high-performance thermoelectric material, which is low in heat conduction and high in thermoelectric performance, is prepared, and the method for preparing the thermoelectric material, which is high in density, high in mechanical strength and high in thermoelectric performance, is explored; the thermoelectric material has very low lattice thermal conductivity (0.2-0.4 W / m.K) in a whole-temperature range; when the temperature is 900 K, thermoelectric peak of the thermoelectric material reaches 1.2; when the temperature is 300-850 K, the average thermoelectric figure of merit zTave of the thermoelectric material is infinity-0.8; and the argyrodite thermoelectric material is a potential thermoelectric material.
Owner:TONGJI UNIV
Preparation method of sulfide solid electrolyte
PendingCN111908437AReduce cakingUniform sizeSolid electrolytesPhosphorus sulfur/selenium/tellurium compoundsSolid state electrolyteArgyrodite
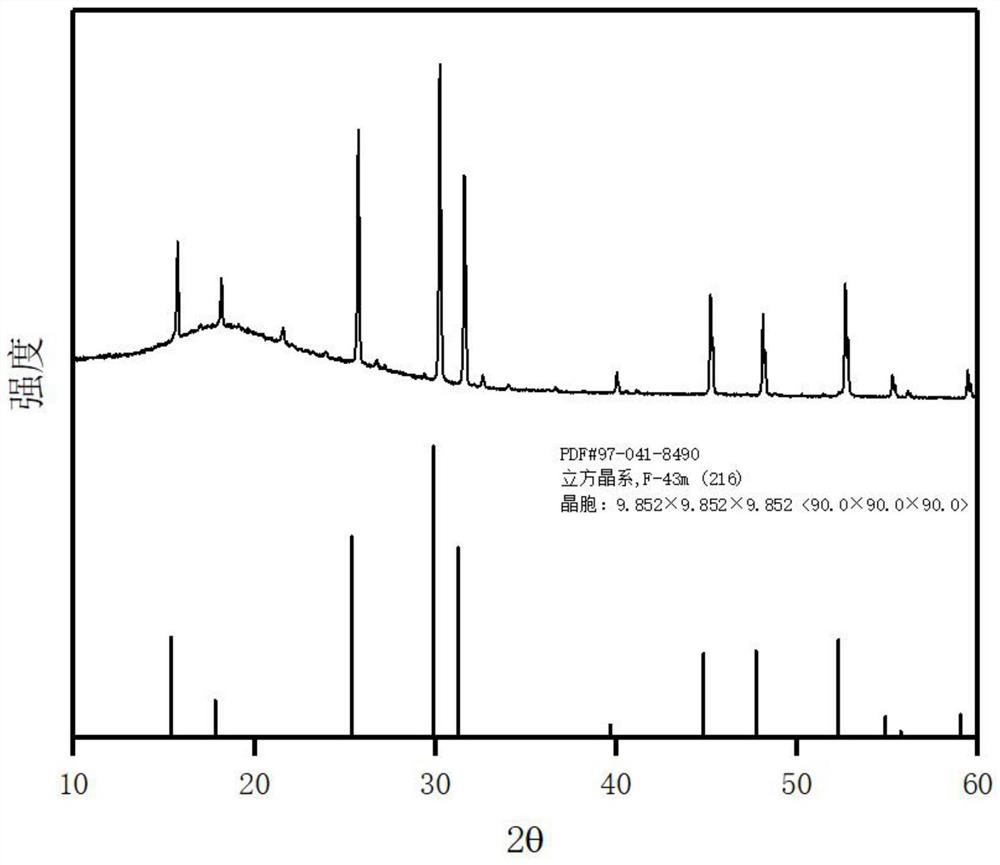
The invention discloses a preparation method of sulfide solid electrolyte, which comprises: (1) weighing Li2S, P2S5 and an X-containing lithium salt according to a required stoichiometric ratio, and uniformly mixing, with the X comprising one or a plurality of Cl, Br and I; (2) grinding and screening the uniformly mixed mixture to obtain a uniformly mixed precursor; and (3) putting the precursor into a ceramic vibration tank in microwave equipment, vibrating and overturning, carrying out microwave sintering at the temperature of 150 to 400 DEG C for 10 min to 1 hour, and cooling to obtain theargyrodite type solid electrolyte containing Li, P, S and X elements. The method is simple in process and low in sintering temperature, product components are easy to control, the argyrodite type cubic phase solid electrolyte stable at the room temperature can be obtained, and the method can be applied to industrial large-scale production.
Owner:湖南恩捷前沿新材料科技有限公司
Full solid-state lithium-sulfur battery and manufacturing method thereof
InactiveCN109638240AImprove cycle lifeImprove Coulombic efficiencySolid electrolytesFinal product manufactureArgyroditeLithium sulfur
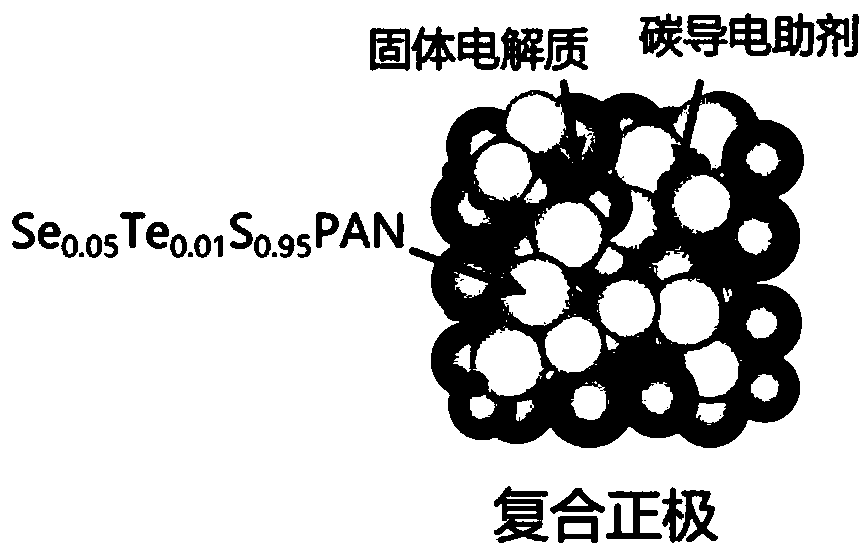
The invention belongs to the technical field related to the preparation of the lithium ion battery, and discloses a full solid-state lithium-sulfur battery. The full solid-state lithium-sulfur batterycomprises a metal negative electrode, a sulfide solid electrolyte and a composite positive electrode; the material of the metal negative electrode is selected from one of Li metal, Li-In alloy, Li-Alalloy, Li-Si alloy or Li-Sn alloy; the sulfide solid electrolyte is selected from one or combination of Li10GeP2S12 type solid electrolyte, Li2S-P2S5 glass-state electrolyte, and argyrodite type solid electrolyte; the composite positive electrode is commonly composed of selentellurium-doped sulfide polyacrylonitrile, a positive solid electrolyte with components same as that of the sulfide solid electrolyte, and a carbon-based conductive additive in a specific proportion. The invention further discloses a corresponding manufacturing method. Through the full solid-state lithium-sulfur battery disclosed by the invention, the defect that the conventional full solid-state lithium-sulfur battery is low in rate performance and low in active substance utilization efficiency can be well overcome,and a three-phase interface of the active substance, the solid electrolyte and the conductive auxiliary is optimized at the same time.
Owner:HUAZHONG UNIV OF SCI & TECH
Sulfide solid electrolyte of three-layer core-shell structure, preparation method thereof and all-solid-state battery
PendingCN110400967AGuaranteed stabilityReduce the likelihood of a reactionSecondary cellsAll solid stateWater vapor
The invention discloses a sulfide solid electrolyte of a three-layer core-shell structure, and relates to the field of all-solid-state batteries. From inside to outside, the Li-Argyrodite solid electrolyte or LGPS-type solid electrolyte is adopted as a nuclear body, the Li-P-S is adopted as an intermediate layer, and the Li-P-S-O is adopted as a shell layer. In this way, the stability of the sulfide solid electrolyte to lithium metal can be guaranteed. Meanwhile, the possibility of reaction between the sulfide solid electrolyte and water vapor in air can be reduced. The probability that the sulfide solid electrolyte is oxidized when making contact with the positive electrode material is reduced. Moreover, the preparation method is relatively simple and is suitable for large-scale production. In addition, the electrical performance of the all-solid-state battery can be effectively improved by combining the all-solid-state battery with a metal lithium negative electrode and a high-voltage positive electrode.
Owner:ZHEJIANG FUNLITHIUM NEW ENERGY TECH CO LTD
Sulfide-Based Solid Electrolyte Particles
ActiveUS20210013542A1Improve rate characteristicExcellent cycle characteristicsSolid electrolytesPhosphorus sulfur/selenium/tellurium compoundsHalogenArgyrodite
A sulfide-based solid electrolyte particle having a crystal phase of a cubic argyrodite-type crystal structure composed of Li, P, S and a halogen (Ha. The proposed sulfide-based solid electrolyte particle has a feature such that the ratio (ZHa2 / ZHa1) of an element ratio ZHa2 of the halogen (Ha) at the position of 5 nm in depth from the particle surface to an element ratio ZHa1 of the halogen (Ha) at the position of 100 nm in depth from the particle surface is 0.5 or lower, as measured by XPS; and the ratio (ZO2 / ZA2) of an element ratio ZO2 of oxygen to the total ZA2 of element ratios of phosphorus (P), sulfur (S), oxygen (O) and the halogen (Ha) at the position of 5 nm in depth from the particle surface is 0.5 or higher, as measured by XPS.
Owner:MITSUI MINING & SMELTING CO LTD
Preparation method of argyrodite type sulfide solid electrolyte
ActiveCN113321485ALow costImprove solubilitySolid electrolytesLithium compoundsSolid state electrolyteArgyrodite
Owner:湖南恩捷前沿新材料科技有限公司
Novel low-thermal-conductivity argyrodite thermoelectric material and preparation method thereof
InactiveCN107359231ALower lattice thermal conductivityHigh purityThermoelectric device manufacture/treatmentThermoelectric device junction materialsAdditive ingredientArgyrodite
The invention relates to a novel low-thermal-conductivity argyrodite thermoelectric material and a preparation method thereof. A chemical formula of the thermoelectric material is Ag9Ga1-XMx(Se1-YSy)6, wherein, M is one selected from Cr, Cd, Zn or Ge, 0< / =x< / =0.06, and 0< / =y< / =0.10; during preparation, a simple substance with the purity of more than 99.99% is used as a raw material, and ingredients are weighed according to the stoichiometric ratio and placed in a sealed quartz tube for vacuum packaging; a muffle furnace is used for heating, so that the high-purity raw material is subjected to a melt reaction at a high temperature and then to rapid quench cooling, to obtain a first ingot; the first ingot is vacuum packaged in the quartz tube, and is subjected to high temperature annealing treatment and then to rapid quench cooling, to obtain a second ingot; the second ingot is grinded into powder which is placed in a graphite mold, hot pressed sintering is carried out throguh induction heating and temperature rise in a vacuum atmosphere, then slow cooling is performed, and completing the preparation. Compared with the prior art, the thermoelectric material of the invention has the advantages of stable mechanical property, very low lattice thermal conductivity (0.2W / m K), and good application prospect.
Owner:TONGJI UNIV
Compound, solid electrolyte, electrochemical cell, method for preparing compound, and protected positive electrode active material
PendingCN112777625ASolid electrolytesPhosphorus sulfur/selenium/tellurium compoundsArgyroditeCrystal structure
The invention relates to a compound, a solid electrolyte, an electrochemical cell, a method for preparing the compound, and a protected positive electrode active material. The compound is represented by Formula 1 and has an argyrodite-type crystal structure. In Formula 1, M1 is at least one metal element of Group 1 to Group 15 of the periodic table except Li, M2 is SOn, and M3 is at least one element of Group 17 of the periodic table; and 4 < = x < = 8, 0 < = v< 1, 3 < = y < = 7, 0<w<2; 0 < = z < = 2, and 1.5 < = n < = 5. Formula 1: Li<x> M1<v> PS<y> M2<w> M3 .
Owner:SAMSUNG SDI CO LTD
Compound, solid electrolyte including the same, electrochemical cell including the same, and method for preparing solid ion conductor
The present invention relates to a compound, a solid electrolyte comprising the same, an electrochemical cell comprising the same, and a method for preparing a solid ion conductor. The compound is represented by Formula 1 and has a argyrodite-type crystal structure, wherein M1 is at least one element of Group 2 or Group 11 of the periodic table, M2 is at least one metal element of Group 1 of the periodic table other than Li, M3 is at least one element of Group 17 of the periodic table, and 4<=a<=8, 0<x <0.5, 0 <=w<0.5, 3<=y<=7, and 0<=7<=2, Formula 1 LiaM1xM2wPSyM3z.
Owner:SAMSUNG SDI CO LTD
Argyrodite type sulfide electrolyte and preparation method thereof
PendingCN114725494AAccelerate real-world application developmentImprove ionic conductivityFinal product manufactureSecondary cellsArgyroditePhysical chemistry
The invention discloses argyrodite type sulfide solid electrolyte and a preparation method thereof, and belongs to the technical field of battery materials. The sulfide electrolyte in the product developed by the invention is Li < 6 + x > Zr < x > P < 1-x > S5PF < 6 >. The preparation method comprises the following steps: manually grinding and mixing the raw materials in a glove box filled with inert gas, and then filling the mixture into a ball milling tank for ball milling; then tabletting the sample subjected to ball milling; and finally, sintering to obtain the target sulfide solid electrolyte. The obtained product has higher ionic conductivity, so that the practical application and development of the high-performance solid-state battery are accelerated.
Owner:SHANGHAI FIRM-LITHIUM NEW ENERGY TECH CO LTD
Argyrodite-doped perovskite solid electrolyte and preparation method thereof
ActiveCN111129580AImprove compactnessAccelerated degradationFinal product manufactureSecondary cells servicing/maintenanceArgyroditeChemical engineering
The invention discloses argyrodite-doped perovskite solid electrolyte and a preparation method thereof. The chemical formula of the electrolyte material is Li<0.33>La<0.57>(Ti(1-x), Ag9GeSe<6-x-y>Te<y>)O<3>, wherein x is greater than or equal to 0 and less than or equal to 0.1, y is greater than or equal to 0 and less than or equal to 0.75, and x and y are different and are 0. The preparation method comprises the following steps: proportionally weighing ingredients according to the chemical formula; performing mixing and ball-milling of the ingredients; presintering the powder obtained by ballmilling to obtain a precursor with a crystal structure; carrying out secondary ball milling on the precursor obtained by presintering to obtain fine particles, and pressing the particles into sheets;carrying out rubber discharging treatment on the pressing sheet; and sintering the pressed sheet after rubber discharging to obtain the argyrodite doped perovskite type solid electrolyte material. The conductivity of the solid electrolyte can be improved, and the performance of a power battery and an electric energy storage battery using the all-solid-state electrolyte is improved.
Owner:GUANG DONG DONGBOND TECH CO LTD
A kind of preparation method of sulfur silver germanium ore type sulfide solid state electrolyte
ActiveCN113321485BLow costImprove solubilitySolid electrolytesLithium compoundsSolid state electrolyteArgyrodite
The invention discloses a CS 2 The invention discloses a preparation method of a sulfide solid state electrolyte which combines wet ball milling and solid phase ball milling as raw materials and solvents. The present invention uses the cheap Li 2 o 2 or Li 2 O, P or P 2 o 5 、CS 2 And LiX (X is Cl, Br or I) as raw materials, avoiding the expensive Li 2 The use of S, the preparation of solid electrolytes by the combination of vacuum wet ball milling and solid phase ball milling, CS 2 Even the raw material is also a solvent, the raw material has strong reactivity and high utilization rate, and the preparation method is simple without high-temperature sintering. The prepared sulfur-argentite-type solid electrolyte has high ion conductivity and a wide electrochemical window. It is applied to the preparation of all-solid-state batteries, which has high safety, high energy density, and excellent cycle stability.
Owner:湖南恩捷前沿新材料科技有限公司
Lithium-argyrodite-based super-ionic conductors containing fully filled halogens and method for preparing the same
Provided are a lithium-argyrodite ionic superconductor containing a halogen element and a method for preparing the same, wherein an argyrodite-type crystal structure can be maintained and lithium ion conductivity can be greatly improved by combining specific elements at a specific molar ratio.
Owner:KOREA INST OF SCI & TECH
Sulfide solid electrolyte
Provided is a sulfide solid electrolyte that has a diffraction peak A in a 2Theta range of 20.0-24.0 degrees inclusive and a diffraction peak B in a 2Theta range of 24.4-26.4 degrees inclusive in X-ray diffractometry using a CuKalfa1 ray, wherein, when the intensity of peak A is represented by IA and the intensity of peak B is represented by IB, then the ratio between IB and IA, i.e., IA / IB is not greater than 2.0. Preferably, the sulfide solid electrolyte contains lithium element, phosphorus element, sulfur element and a halogen element. Preferably, the sulfide solid electrolyte has an argyrodite crystal structure. Preferably, the sulfide solid electrolyte contains a lithium halide hydrate.
Owner:MITSUI MINING & SMELTING CO LTD
Preparation method of sulfide solid electrolyte
The invention relates to a preparation method of an inorganic sulfide solid electrolyte, and belongs to the field of lithium ion batteries. Aiming at the defects that a primary solid-phase sintering method of argyrodite solid electrolyte Li6PS5Cl is easy to deform, generate pores, curl and the like in the preparation process, improvement is carried out on the basis of a primary sintering preparation method, and a novel secondary sintering preparation method is obtained. The method is characterized in that a solid electrolyte sheet obtained by primary sintering is crushed and then tabletted again, and is sintered again at a slightly high temperature, and the novel process is beneficial to homogenization of the grain size, enlargement of the overall size of the grains and improvement of the crystallinity and density. Meanwhile, residual gas which is not volatilized in one-time sintering can be eliminated, deformation and defects of an electrolyte sheet are avoided, the obtained solid electrolyte is more uniform in surface and more compact in structure, subsequent characterization and electrochemical testing are facilitated, and the ionic conductivity of the sulfide solid electrolyte can also be improved.
Owner:UNIV OF SCI & TECH BEIJING
High-stability underground pipe culvert and preparing method thereof
The invention discloses a high-stability underground pipe culvert and a preparing method thereof. The preparing method comprises the steps of 1, calcinating plant straw, kieselguhr, argyrodite and potassium fluoxyniobate in the presence of shielding gas to obtain a calcination product; 2, heating and mixing pitch, polypropylene fiber, rubber particles, sodium gluconate and the calcination product in the presence of ultraviolet rays to obtain an activation product; 3, mixing, pouring and curing sand, cement, coal ash, water and the activation product to obtain the high-stability underground pipe culvert. The underground pipe culvert prepared with the method has excellent stability.
Owner:ANHUI YUTE CONCRETE STRUCTURE TECH
Sulfide electrolyte material and preparation method and application thereof
InactiveCN113363565AImprove structural stabilityImprove humidity stabilitySecondary cellsElectrolyte immobilisation/gelificationSulfate radicalsArgyrodite
The invention relates to a sulfide electrolyte material. The general formula of the sulfide electrolyte material is Li6PS5X1.xAgX2.yB2O3, wherein X1 is selected from one of chloride ions, bromide ions and iodide ions, and X2 is selected from one of chloride ions, bromide ions, fluoride ions, iodide ions and sulfate ions; 0 < x < = 0.5, and 0 < = y < = 0.5. By doping AgX2 in the argyrodite type sulfide electrolyte, the structural stability of the sulfide electrolyte material is improved; and boron oxide with a specific proportion is doped, so that the comprehensive performance of humidity stability and conductivity of the electrolyte material is improved.
Owner:KUNSHAN BAOTRON NEW ENERGY TECH CO LTD
Solid electrolyte, and electrode mixture, solid electrolyte layer, and solid-state battery using same
PendingCN114556492ASolid electrolytesPhosphorus sulfur/selenium/tellurium compoundsHalogenArgyrodite
The solid electrolyte according to the present invention contains a compound A that contains a crystal phase having an argyrodite-type crystal structure and that is represented by LiaPSbXc (X is at least one halogen element), and a compound B that contains a crystal phase having an argyrodite-type crystal structure. And a represents a number of 3.0 or more and 6.0 or less. And b represents a number of 3.5 or more and 4.8 or less. And c represents a number of 0.1 or more and 3.0 or less). The compound B is represented by LiX (in the formula, X is defined as above). The crystallite size of the compound B is 25 nm or more. The solid electrolyte is suitable when the BET specific surface area is 14.0 m2 / g or less.
Owner:MITSUI MINING & SMELTING CO LTD
Sulfide-Based Solid Electrolyte Particles
PendingUS20220059870A1Improving the rate characteristic andReduce concentrationSolid electrolytesPhosphorus sulfur/selenium/tellurium compoundsHalogenArgyrodite
A sulfide-based solid electrolyte particle having a crystal phase of a cubic argyrodite-type crystal structure composed of Li, P, S and a halogen (Ha), wherein good contact between the sulfide-based solid electrolyte particles and positive or negative electrode active material particles is secured and improvements in the rate characteristic and the cycle characteristic are attained. The ratio (ZHa2 / ZHa1) of an element ratio ZHa2 of the halogen (Ha) at the position of 5 nm in depth from the particle surface to an element ratio ZHa1 of the halogen (Ha) at the position of 100 nm in depth from the particle surface is 0.5 or lower and the ratio (ZO2 / ZA2) of an element ratio ZO2 of oxygen to the total ZA2 of element ratios of P, S, O, and the halogen (Ha) at the position of 5 nm in depth from the particle surface is 0.5 or higher, as measured by XPS.
Owner:MITSUI MINING & SMELTING CO LTD
Solid electrolyte and method for producing same
PendingCN114207898AInhibitionImprove ionic conductivitySolid electrolytesPhosphorus sulfur/selenium/tellurium compoundsLithiumHalogen
The present invention relates to a method for producing a solid electrolyte having an argyrodite crystal structure, the method comprising: a mixing step for mixing raw materials such that lithium (Li), phosphorus (P), sulfur (S), oxygen (O), and halogen (X) satisfy formulae (11)-(14); and a heating step for heating the mixture obtained in the mixing step. 4.8 < = Li / P < = 5.3 (11) 3.8 < = S / P < = 4.4 (12) 0 < O / P < = 0.8 (13) 1.0 < X / P < = 2.0 (14) (formula (11) is the molar ratio of Li to P, formula (12) is the molar ratio of S to P, formula (13) is the molar ratio of O to P, and formula (14) is the molar ratio of halogen (X) to P).
Owner:IDEMITSU KOSAN CO LTD
Preparation method of sulfide solid electrolyte
PendingCN114709471AContraction reaction timeFacilitated contact responseSecondary cellsSolid state electrolyteArgyrodite
The invention relates to a preparation method of sulfide solid electrolyte. The preparation method comprises the following steps: firstly, dissolving argyrodite type sulfide solid electrolyte raw materials in an ultra-dry solvent according to a certain proportion, and violently stirring so that the raw materials are fully and uniformly mixed and react; after the reaction is finished, unreacted residual solids are removed through centrifugation, a solution is collected, and a solvent is removed to obtain powder; and finally, sintering the dried powder in a vacuum sealed quartz tube to obtain the argyrodite type sulfide solid electrolyte. The preparation method disclosed by the invention solves the problems of high energy consumption caused by a traditional solid-state method, non-uniform mixing of raw materials for preparing the sulfide electrolyte and the like, and can improve the conductivity of the prepared sulfide electrolyte.
Owner:SHANGHAI FIRM-LITHIUM NEW ENERGY TECH CO LTD
Method for liquid-phase preparation of doped argyrodite type sulfide solid electrolyte with high ionic conductivity
PendingCN114725511ASimple liquid phase reactionSmall particlesSecondary cellsSolid state electrolytePhosphorus pentasulfide
The invention belongs to the technical field of solid electrolyte, and discloses a method for preparing doped argyrodite type sulfide solid electrolyte with high ionic conductivity in a liquid phase. The method comprises the following steps: 1) dispersing lithium sulfide and phosphorus pentasulfide in an organic solvent, heating, stirring and reacting to obtain a precursor solution containing Li3PS4; 2) mixing lithium iodide, powdered sulfur and an additive with the precursor solution, heating and stirring for reaction, and removing the organic solvent to obtain powder; and 3) sintering the powder in a protective atmosphere to obtain the argyrodite type sulfide solid electrolyte, and the additive is one or more of SiS2, GeS2, SnS2, As2S3 and Sb2S3. The method is simple, the prepared argyrodite type sulfide electrolyte is subjected to element doping in the thermal crystallization process, and the ionic conductivity and air stability of the electrolyte are improved. The method is suitable for industrial production.
Owner:SOUTH CHINA UNIV OF TECH
Solid electrolyte, electrochemical cell including solid electrolyte, and method of preparing solid electrolyte
PendingCN112786952ASolid electrolytesPhosphorus sulfur/selenium/tellurium compoundsPseudohalogenArgyrodite
The present invention relates to a solid electrolyte, an electrochemical cell including solid electrolyte, and a method of preparing the solid electrolyte. The solid electrolyte includes a compound represented by Formula 1: Formula 1 (Li1-aMa)7-d+xPS6-d-x+kNxXdwherein, in Formula 1, M is Na, K, Ca, Fe, Mg, Ag, Cu, Zr, Zn, or a combination thereof; X is Cl, Br, F, I, a pseudohalogen, or a combination thereof; furthermore 0<x<1, 0<=a<1, 0<d<=1.8 and 0<=k<1, and wherein the compound has an argyrodite crystal structure. Formula1 (Li1-aMa)7-d+xPS6-d-x+kNxXd.
Owner:SAMSUNG SDI CO LTD
Quantum dot light emitting diode and preparation method thereof
PendingCN114695705ALower injection barrierSmall recessionSolid-state devicesSemiconductor/solid-state device manufacturingElectron holeArgyrodite
Owner:TCL CORPORATION
Method of manufacturing argyrodite-type solid electrolyte, argyrodite-type solid electrolyte, and all-solid-state battery comprising the solid electrolyte
PendingUS20210242493A1Solid electrolytesPhosphorus sulfur/selenium/tellurium compoundsAll solid stateHalogen
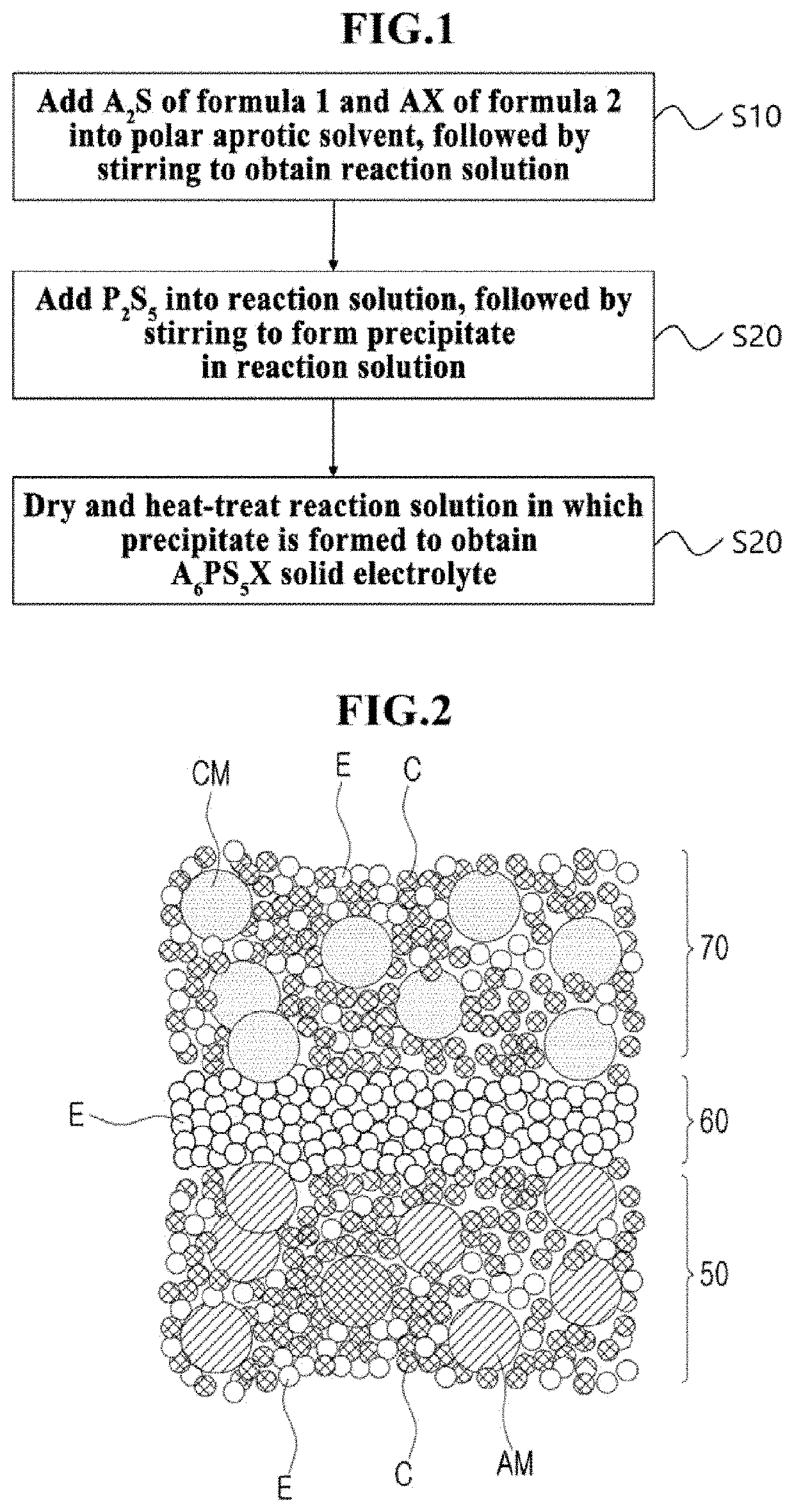
A method of manufacturing an argyrodite-type solid electrolyte, an argyrodite-type solid electrolyte, and an all-solid-state battery including the argyrodite-type solid electrolyte are provided. The method includes a first step of adding precursors represented by the following Formulas 1 and 2 into a polar aprotic solvent, followed by stirring to obtain a reaction solution; a second step of adding P2S5 into the stirred reaction solution, followed by further stirring to form a precipitate obtained as a result of the reaction in the reaction solution; and a third step of drying and heat-treating the reaction solution in which the precipitate is formed to obtain a solid electrolyte: [Formula 1] A2S [Formula 2] AX wherein A represents an alkali metal, and X represents an element of the halogen group.
Owner:SOLIVIS INC
Preparation method of argyrodite
PendingCN114728804AFast preparationEasy to manufactureSolid electrolytesLithium compoundsLithiumBiochemical engineering
The invention relates to a novel method for producing lithium argyrodite, as well as to the products obtainable by said method, and to the use thereof, in particular as solid electrolytes.
Owner:SOLVAY SA
Solid electrolyte material for lithium battery and preparation method of solid electrolyte material
PendingCN114852980AImprove conductivityImprove dynamic performanceSolid electrolytesPhosphorus halides/oxyhalidesSolid state electrolyteCrystal system
The invention provides a solid electrolyte for a lithium battery, the solid electrolyte comprises a sulfide with a cubic system argyrodite type crystal structure, and the sulfide has the following molecular formula: Li < 5 + x > PS < 4 + x > Cl < 2-x-y-z > Br < y > I < z >, wherein x, y and z satisfy 0 < x < 1, 0 < y < 1, 0.09 < z < = 0.11, x + y + z = 2, and the ratio (y / x) of the molar ratio of Br to the molar ratio of Cl is 0.02-8, and the solid electrolyte has high ionic conductivity and extremely high stability to lithium metal.
Owner:WUHAN UNIV OF TECH +1
Solid electrolyte with excellent ion conductivity
PendingUS20220181676A1Improve ionic conductivityReduce deviationNon-metal conductorsSolid electrolytesChemical physicsArgyrodite
An argyrodite-based solid electrolyte may contain a compound having a novel composition calculated by simulation of Ab initio molecular dynamics (AIMD). The solid electrolyte containing a compound having a novel composition that satisfies a certain range of monoatomic disorder (è) and a certain range of standard deviation (STD) of the size of the area formed as migration paths of Li metals, which are calculated by AIMD simulation, has an advantage of excellent ion conductivity due to promoted diffusion of monoatoms in the area.
Owner:HYUNDAI MOTOR CO LTD +2
Sulfide solid electrolyte and preparation method and application thereof
PendingCN114551992AImprove stabilityDecreased ionic conductivitySolid electrolytesSecondary cellsSolid state electrolyteArgyrodite
The invention provides a sulfide solid electrolyte and a preparation method and application thereof, the chemical formula of the sulfide solid electrolyte is LiaSibSbcSdI (1-x) Brx, a is more than or equal to 6 and less than or equal to 7, b is more than or equal to 0.5 and less than or equal to 0.7, c is more than or equal to 0.3 and less than or equal to 0.5, d is more than or equal to 4.5 and less than or equal to 5.5, and 1lt is 0; xlt; the phase of the sulfide solid electrolyte comprises an argyrodite phase and a LiI crystal phase, the stability of the sulfide solid electrolyte in an organic solvent and air can be improved by doping antimony and bromine in the electrolyte, and meanwhile, the conductivity of the sulfide solid electrolyte is improved.
Owner:SVOLT ENERGY TECH (WUXI) CO LTD
Sulfide solid electrolyte and preparation method and application thereof
PendingCN114597483AImprove stabilityDecreased ionic conductivitySolid electrolytesSilicon halogen compoundsSolid state electrolyteOrganic solvent
The invention provides a sulfide solid electrolyte and a preparation method and application thereof, the chemical formula of the sulfide solid electrolyte is Li Si Sb < c > S < 5-x > O < x > I, a is greater than or equal to 6 and less than or equal to 7, b is greater than or equal to 0.5 and less than or equal to 0.7, c is greater than or equal to 0.3 and less than or equal to 0.5, and 1lt is 0; xlt; 1, the phase of the sulfide solid electrolyte is an argyrodite phase, the stability of the sulfide solid electrolyte in an organic solvent and air can be improved by doping antimony and oxygen elements in the electrolyte, and meanwhile, the conductivity of the sulfide solid electrolyte is improved;
Owner:SVOLT ENERGY TECH (WUXI) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com