Sulfide solid electrolyte
A technology of solid electrolytes and sulfides, applied in solid electrolytes, sulfide conductors, non-aqueous electrolytes, etc., can solve problems such as increased reaction resistance and lower battery characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0059] (1) Manufacture of sulfide solid electrolyte
[0060] to be Li 5.4 P.S. 4.4 Cl 0.8 Br 0.8 Weigh Li by way of composition 2 S powder, P 2 S 5 powder, LiCl powder, and LiBr powder so that the total amount becomes 75 g. These powders were pulverized and mixed using a ball mill to obtain a mixed powder. The mixed powder was fired to obtain a fired product having the above-mentioned composition. Firing was performed using a tubular electric furnace. During firing, hydrogen sulfide gas with a purity of 100% was circulated in the electric furnace at 1.0 L / min. The firing temperature was set to 500° C., and firing was performed for 4 hours. The fired product was pulverized with a mortar and pestle, and then pulverized with a wet bead mill to obtain a sulfide solid electrolyte. Expose the obtained sulfide solid electrolyte to the atmosphere at [dew point -30]°C for 24 hours to generate LiCl·H 2 O and LiBr·H 2 O, the target sulfide solid electrolyte was obtained. In...
Embodiment 7
[0110] to be Li 5.4 P.S. 4.4 Cl 0.8 Br 0.8 Weigh Li by way of composition 2 S powder, P 2 S 5 powder, LiCl powder, and LiBr powder so that the total amount becomes 75 g. These powders were pulverized and mixed using a ball mill to obtain a mixed powder. The mixed powder was fired to obtain a fired product having the above-mentioned composition. Firing was performed using a tubular electric furnace. During firing, hydrogen sulfide gas with a purity of 100% was circulated in the electric furnace at 1.0 L / min. The firing temperature was set to 500° C., and firing was performed for 4 hours. The fired product was pulverized with a mortar and pestle, and then pulverized with a wet bead mill to obtain a sulfide solid electrolyte.
[0111] Combine the obtained sulfide solid electrolyte with LiBr H 2 O was mixed under an Ar atmosphere to obtain the target sulfide solid electrolyte. LiBr H 2 The amount of O added relative to the sulfide solid electrolyte and LiBr·H 2 The t...
Embodiment 8 and comparative example 4 and 5
[0113] In addition to making relative to the sulfide solid electrolyte and LiBr·H 2 Total amount of O LiBr·H 2 A solid battery was fabricated in the same manner as in Example 7 except that the ratio of the amount of O added was as shown in Table 3.
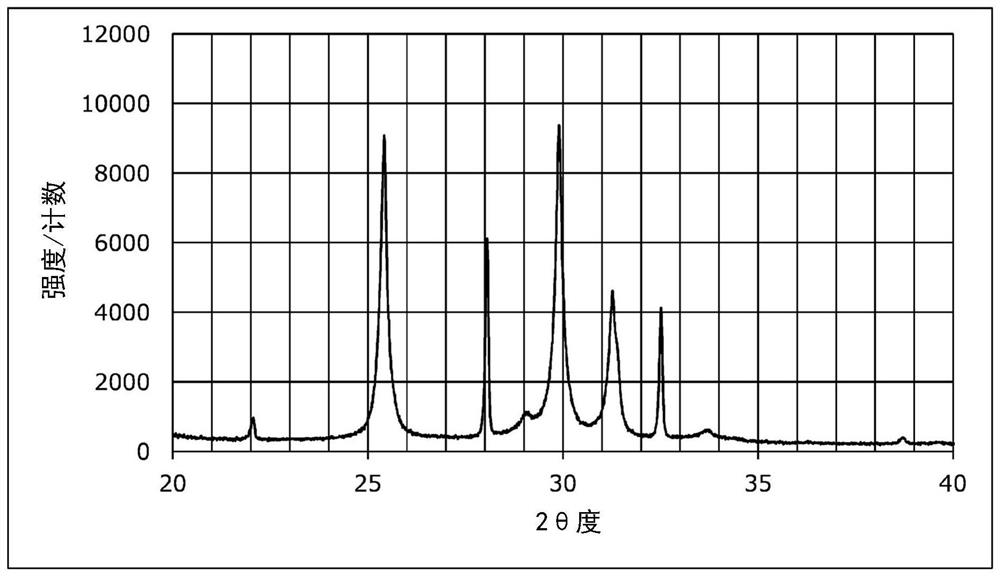
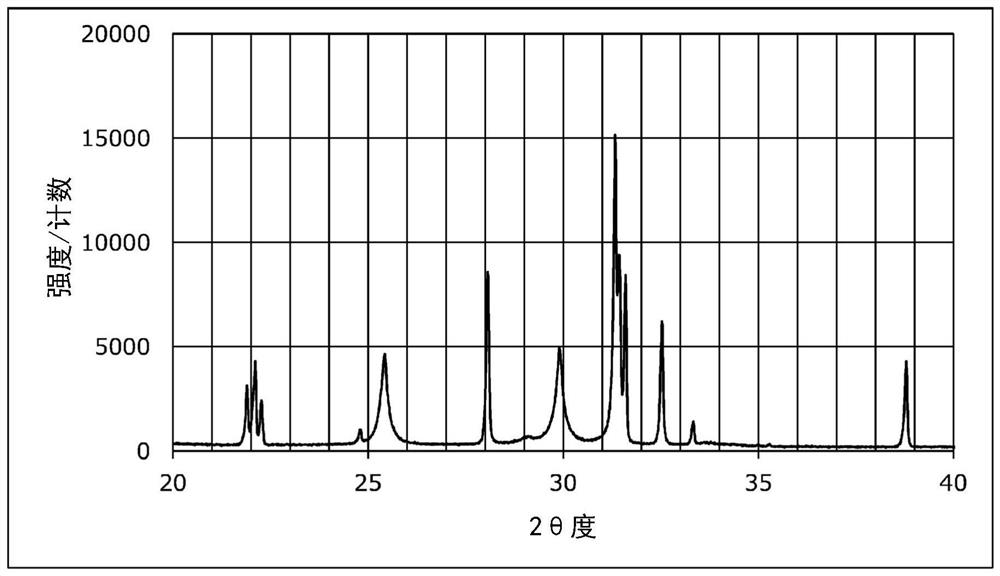
[0114] The XRD measurement results of the sulfide solid electrolytes obtained in Examples 7 and 8 and Comparative Examples 4 and 5 are shown in Figures 2 to 5 . In addition, the 0.1C discharge capacities of the solid batteries obtained in Examples 7 and 8 and Comparative Examples 4 and 5 were measured. Furthermore, the 5C discharge capacity and discharge capacity retention rate of the solid state batteries obtained in Examples 7 and 8 and Comparative Examples 4 and 5 were measured. These results are shown in Table 3. The measurement method is the same as in Example 5 above.
[0115] table 3
[0116]
[0117] From the results shown in Table 3, it can be seen that the solid batteries using the sulfide solid electrolytes ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com