Solid electrolyte material for lithium battery and preparation method of solid electrolyte material
A solid electrolyte, lithium battery technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems such as lithium metal instability, to improve stability, overcome stability and high ion conductivity, increase kinetics Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] This embodiment also provides a method for preparing the solid electrolyte as described above, comprising: according to chemical formula (1) Li 5+x PS 4+x Cl 2-x-y-z Br y I z mixing a lithium source compound, a phosphorus source compound, a chlorine-containing compound, a bromine-containing compound, and an iodine-containing compound in a stoichiometric ratio to form a raw material mixture;
[0029] The raw material mixture is heat treated in an inert atmosphere or hydrogen sulfide atmosphere or under vacuum, and the heat treatment temperature is 350-570°C, most preferably 380-520°C. The compound for solid electrolyte has the advantages of high ionic conductivity and low production cost.
Embodiment approach
[0030] As an embodiment, the lithium source compound includes one or more of lithium sulfide, lithium polysulfide, lithium phosphide, lithium carbonate, and lithium metal element, preferably lithium sulfide.
[0031] As an embodiment, the phosphorus source compound includes at least one of phosphorus pentasulfide, phosphorus trisulfide, and elemental phosphorus, preferably phosphorus pentasulfide.
[0032] As an embodiment, the chlorine-containing compound includes PCl 3 , PCl 5 , P 2 Cl 4 , SCl 2 , S 2 Cl 2 At least one of them, preferably lithium chloride.
[0033] According to an embodiment of the present invention, the bromine-containing compound includes LiBr, PBr 3 , S 2 Br 2 At least one of them, preferably lithium bromide.
[0034] According to an embodiment of the present invention, the iodine-containing compound includes LiI, PI 3 , S 2 I 2 At least one of at least one, preferably lithium iodide.
[0035] In the present invention, through the co-doping ...
Embodiment 1
[0049] This example is Li 5.5 PS 4.5 Cl 0.8 Br 0.69 I 0.01 Electrolyte, the specific steps are as follows:
[0050] (1) Under the condition that the argon atmosphere, the water pressure and the oxygen partial pressure are all less than 1 ppm, the Li 2 S (Alfa 99.9%), P 2 S 5 (Alfa 99.9%), LiCl (Alfa 99.9%), LiBr (Alfa 99.9%), LiI (Alfa 99.9%) were weighed according to the molar ratio of 4:1:1.6:1.38:0.02, and mixed by low-speed ball milling at 100rpm for 1h to obtain mixture;
[0051] (2) The above mixture was then heat-treated at 520 °C for 10 h to obtain Li 5.5 PS 4.5 Cl 0.8 Br 0.69 I 0.01 electrolyte.
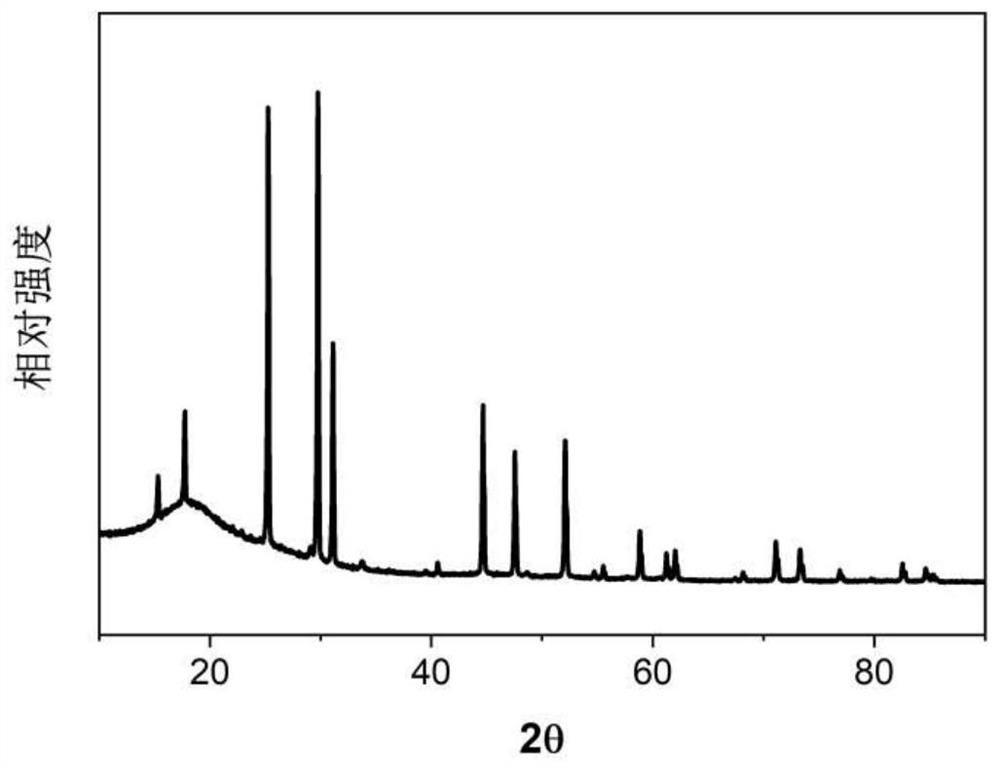
[0052] figure 1 SEM image of the prepared electrolyte powder. figure 2 Schematic diagram of solid electrolyte X-ray diffraction. The sulfide solid electrolyte powder prepared in this example has a characteristic peak between 17.4 and 17.9 degrees in the XRD diffraction pattern, and one characteristic peak between 25.0 and 25.4 degrees, and a characteristic p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| impedance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com