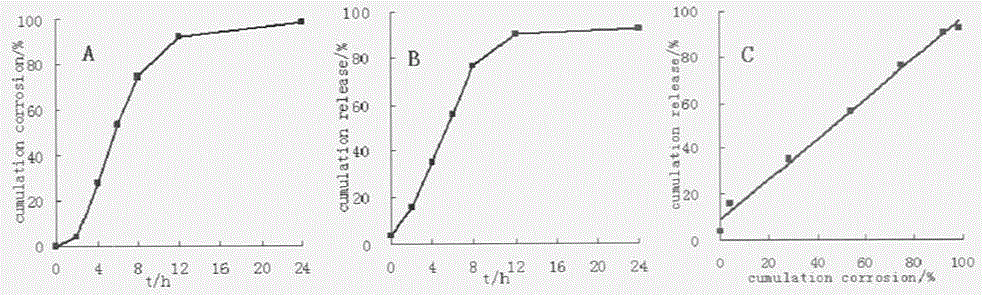
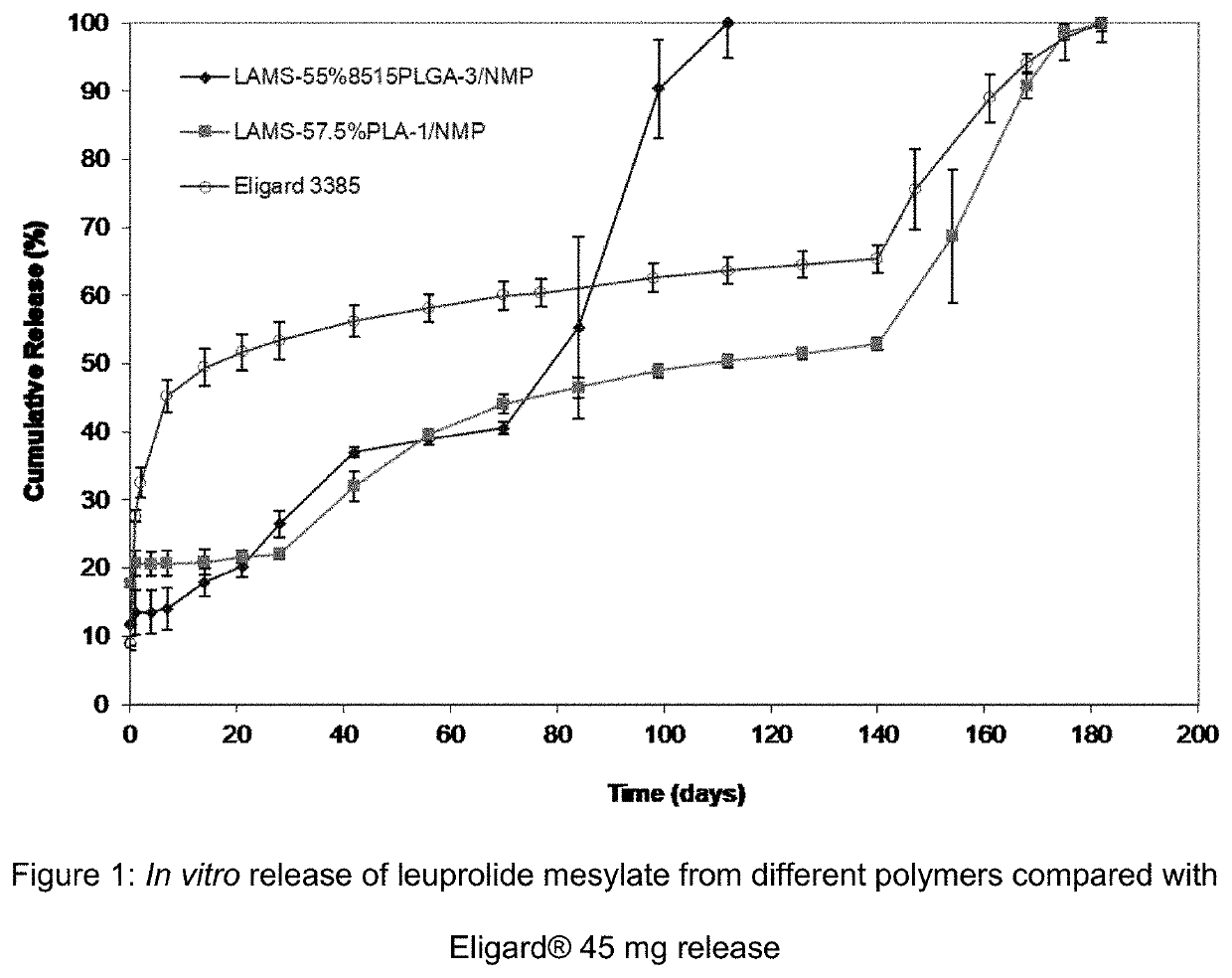
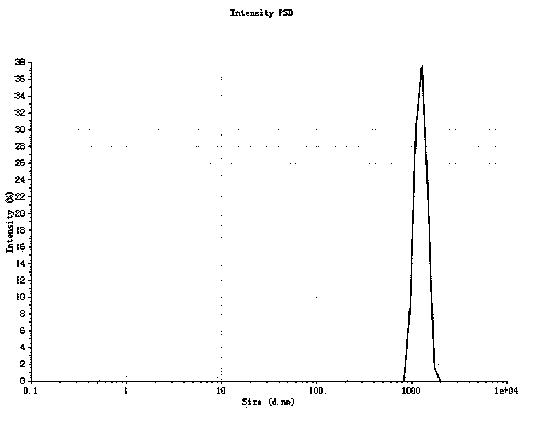
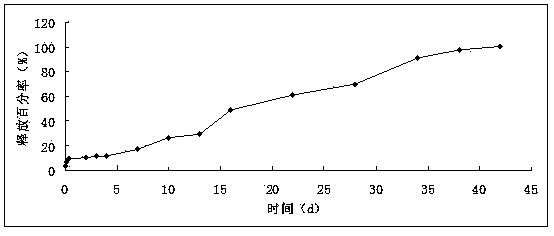
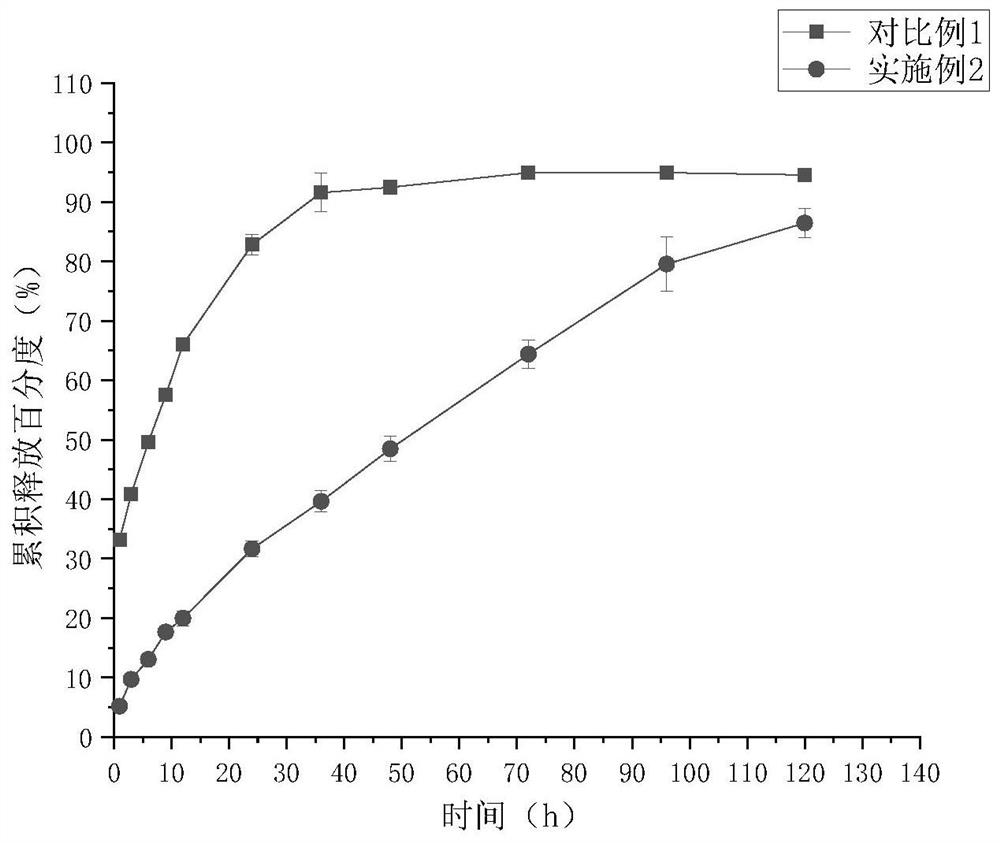
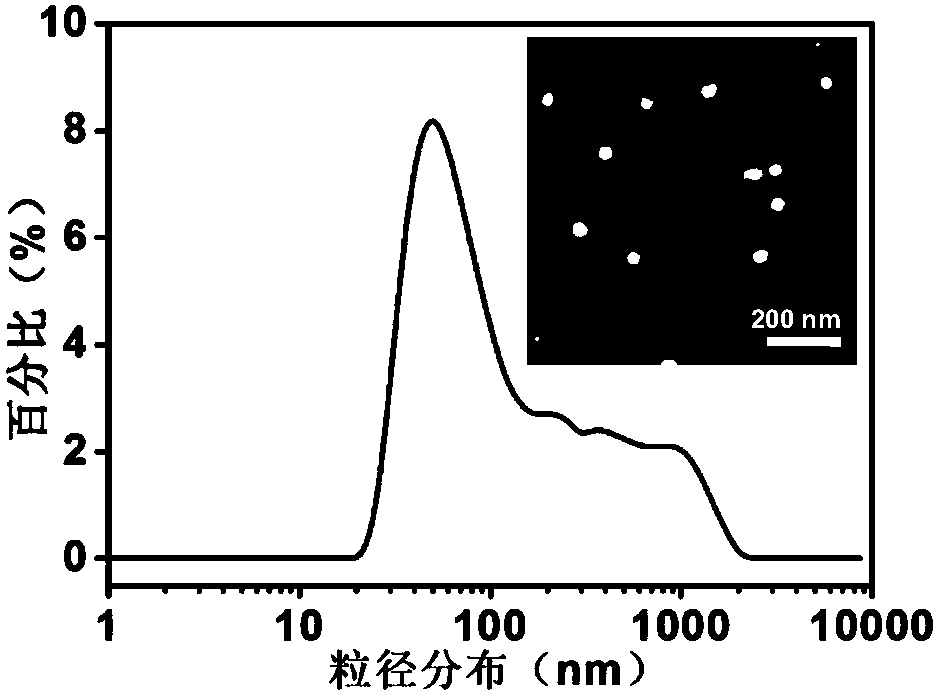
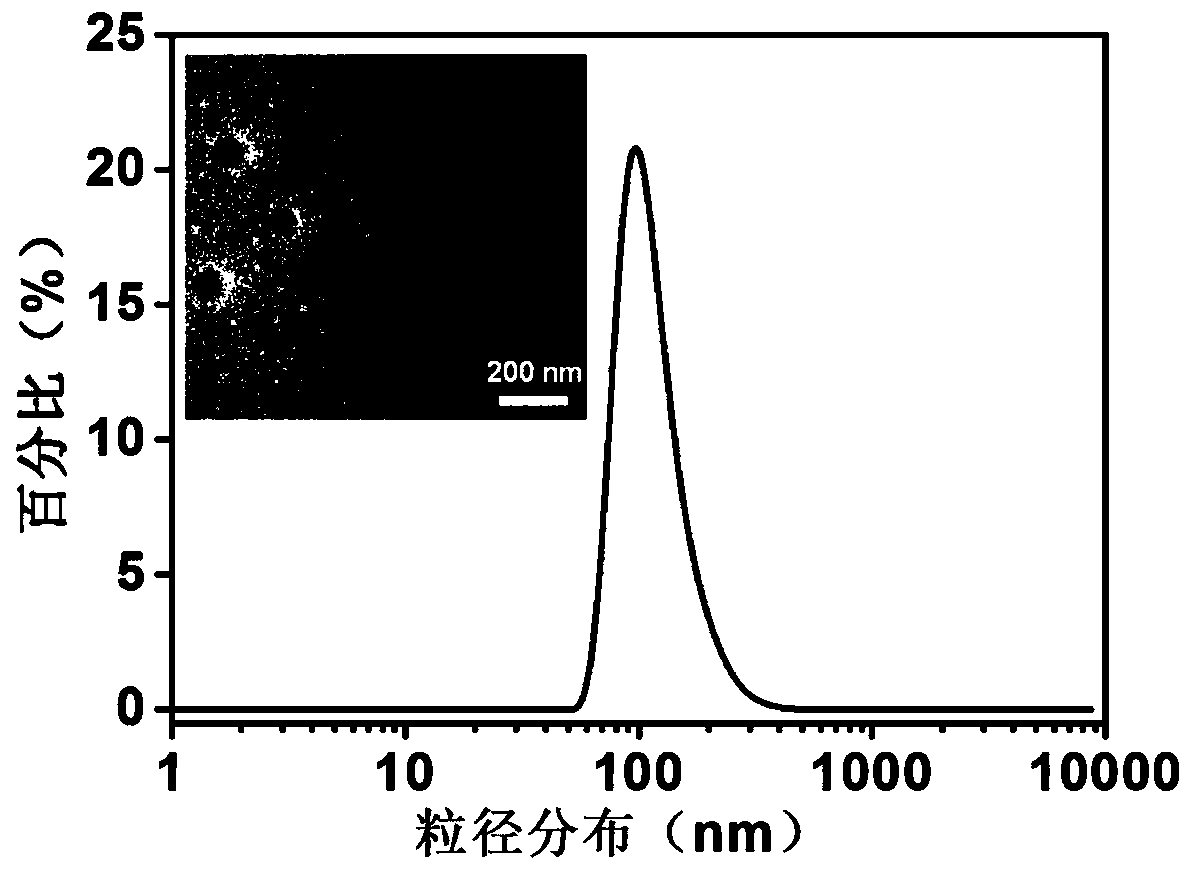
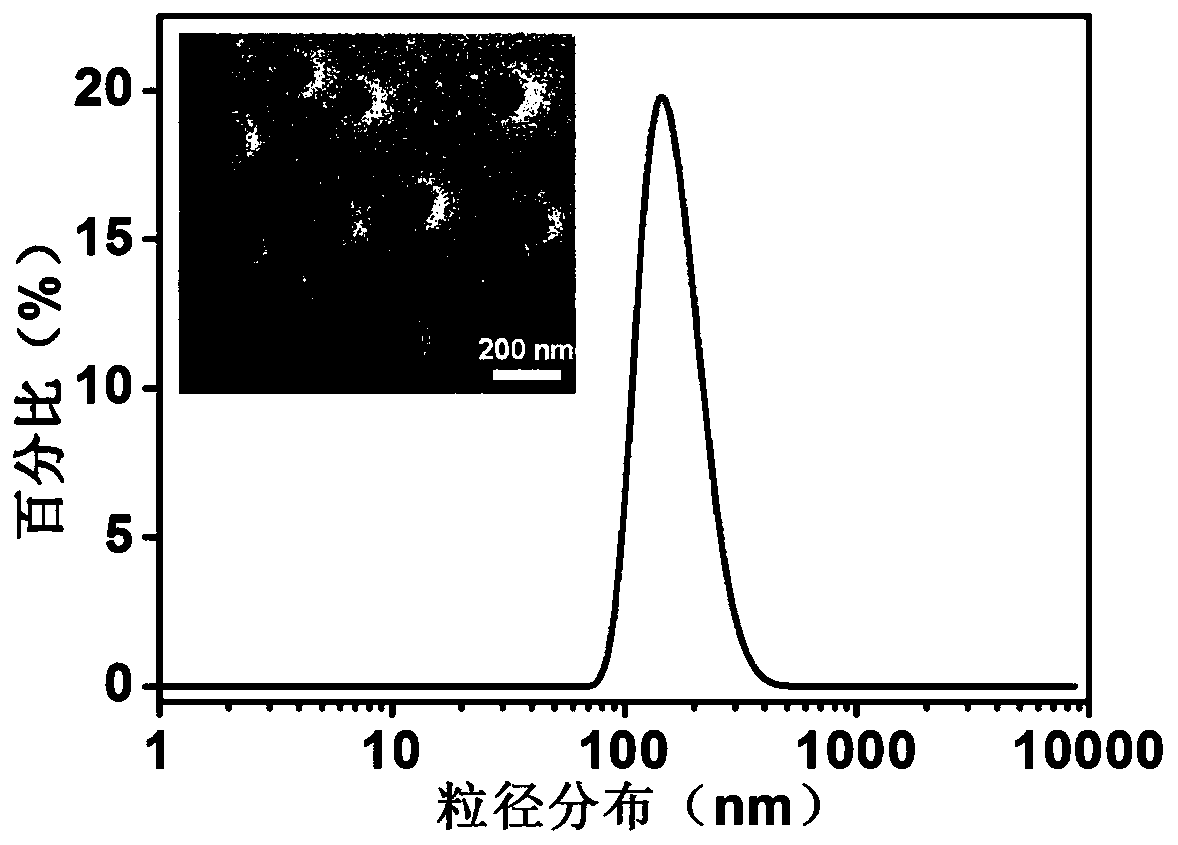
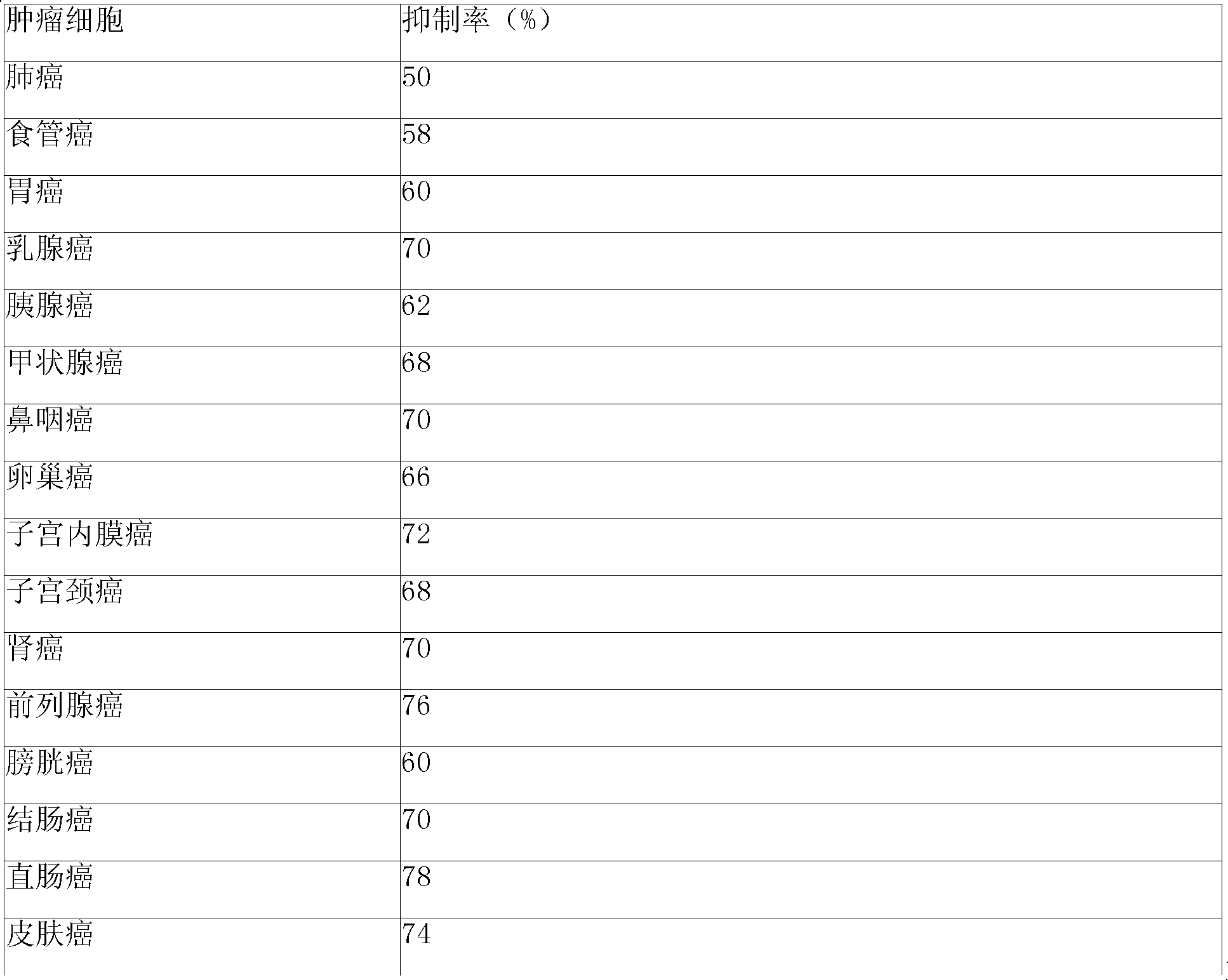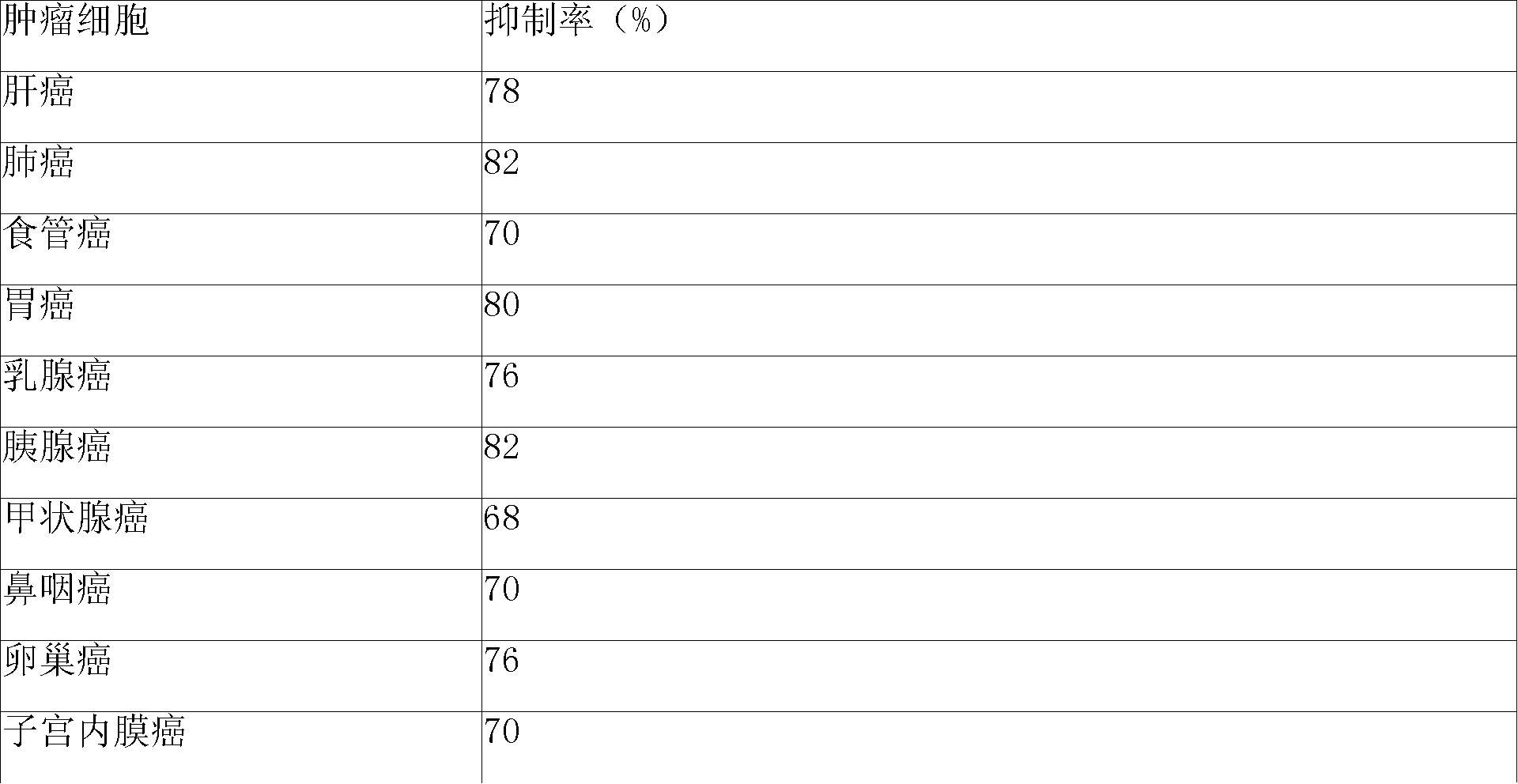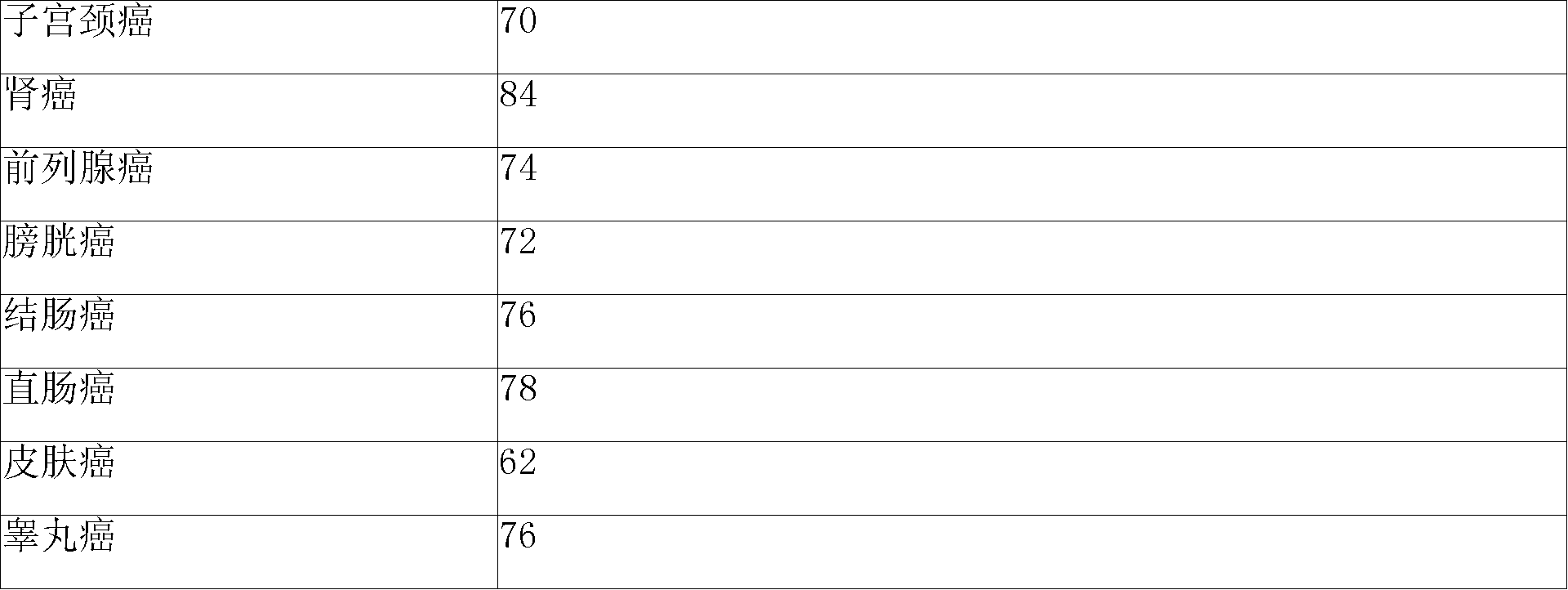Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Alsactide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alsactide (INN) (brand name Synchrodyn 1-17 or simply Synchrodyn, former development code name Hoechst 433), also known as alisactide, is a synthetic peptide and analogue of adrenocorticotropic hormone (ACTH) which is used in Italy as a diagnostic agent in kidney function for adrenal insufficiency. Like ACTH, alsactide is thought to act as a non-selective agonist of the melanocortin receptors, including the ACTH receptor (MC₂R). However, it appears to show a different profile of receptor selectivity relative to ACTH, as it apparently demonstrated no evidence of inhibition of endogenous ACTH in Addison's disease patients.
Preparation and application of intracellular triggering reduction sensitive drug linked gene targeted co-carrier
InactiveCN103566379AIncrease concentrationLow toxicityGenetic material ingredientsNanomedicineCross-linkCross-linked polyethylene
The invention relates to an intracellular triggering reduction sensitive drug linked gene targeted co-carrier which is a gene and drug double-loaded system and comprises disulfide bond cross-linked polyethylene imine, a poly-lactide glycolide copolymer and a surface modified targeted micromolecule. According to the invention, the surface modified active targeted group of a carrier can target drugs to a tumor part and enrich the drugs; a disulfide bond cross-linked in a polymer can be broken in a cell under the condition of high reduction, and the structure of the carrier is disassembled so that the drugs are released. The intracellular triggering reduction sensitive drug linked gene targeted co-carrier disclosed by the invention can effectively load the gene and the hydrophobic micromolecular drug, ensure the stability of the gene and is degraded and releases the gene and the micromolecular drug under the condition of reduction. An in-vivo experiment shows that the intracellular triggering reduction sensitive drug linked gene targeted co-carrier disclosed by the invention has outstanding targeting on tumors.
Owner:CHINA PHARM UNIV
Organism degradable star-type structure poly (glycolide-lactide) medicine carrier microsphere and preparation method thereof
InactiveCN1927906AImprove poor wrapping abilityImprove drug loading and embedding efficiencyPharmaceutical non-active ingredientsLong actingDrug
The present invention relates to one kind of biodegradable medicine-carrying polyglycolide-lactide microsphere in star structure and its preparation process. The biodegradable medicine-carrying polyglycolide-lactide microsphere has coating material of star polyglycolide-lactide, coated protein polypeptide medicine of bovine serum albumin or leuteinizing hormone-releasing homone (LH-RH) analog, medicine carrying amount of 17.56-67.51 mcg / mg microsphere and embedding rate of 28.68-78.39 wt%. It is prepared through a W / O / W composite emulsifying process to coat protein polypeptide medicine into star polyglycolide-lactide to form microsphere. Compared with linear medicine-carrying polyglycolide-lactide microsphere, the present invention has obviously increased medicine carrying amount and embedding rate and obvious long-acting and slowly releasing effect.
Owner:NANKAI UNIV
Anticancer sustained-release gel injection containing stines medicine
The invention relates to an anticancer sustained release gel injection with stine drugs, comprising sustained release microspheres with stine drugs, a amphiphilic block copolymer, solvent and releasing moderator; wherein, the mixture of the amphiphilic block copolymer and the solvent possesses sensitive gelation property; after in vivo injection, the injection can be transformed into nonflowing, biodegradable gel insoluble in water; the insoluble gel can release the contained drugs in local tumor for weeks, even months. Intra-tumor injection or local injection can be used to treat different tumors and unresectable tumors, control late complications and the postoperative residual tumor cell recurrent, reinforce the effect of radiotherapy and chemotherapy and the effect of radiotherapy particles; nimustine and carmustine and other stines; the amphiphilic block copolymer is a PLGA-PEG-PLGA copolymer with the molecular weight of PEG 1200-1600 accounting for 20% of the amphiphilic block copolymer weight; in the poly lactide coglycolide copolymer, the molar ratio of glycolide and lactide is 6:1.
Owner:济南基福医药科技有限公司
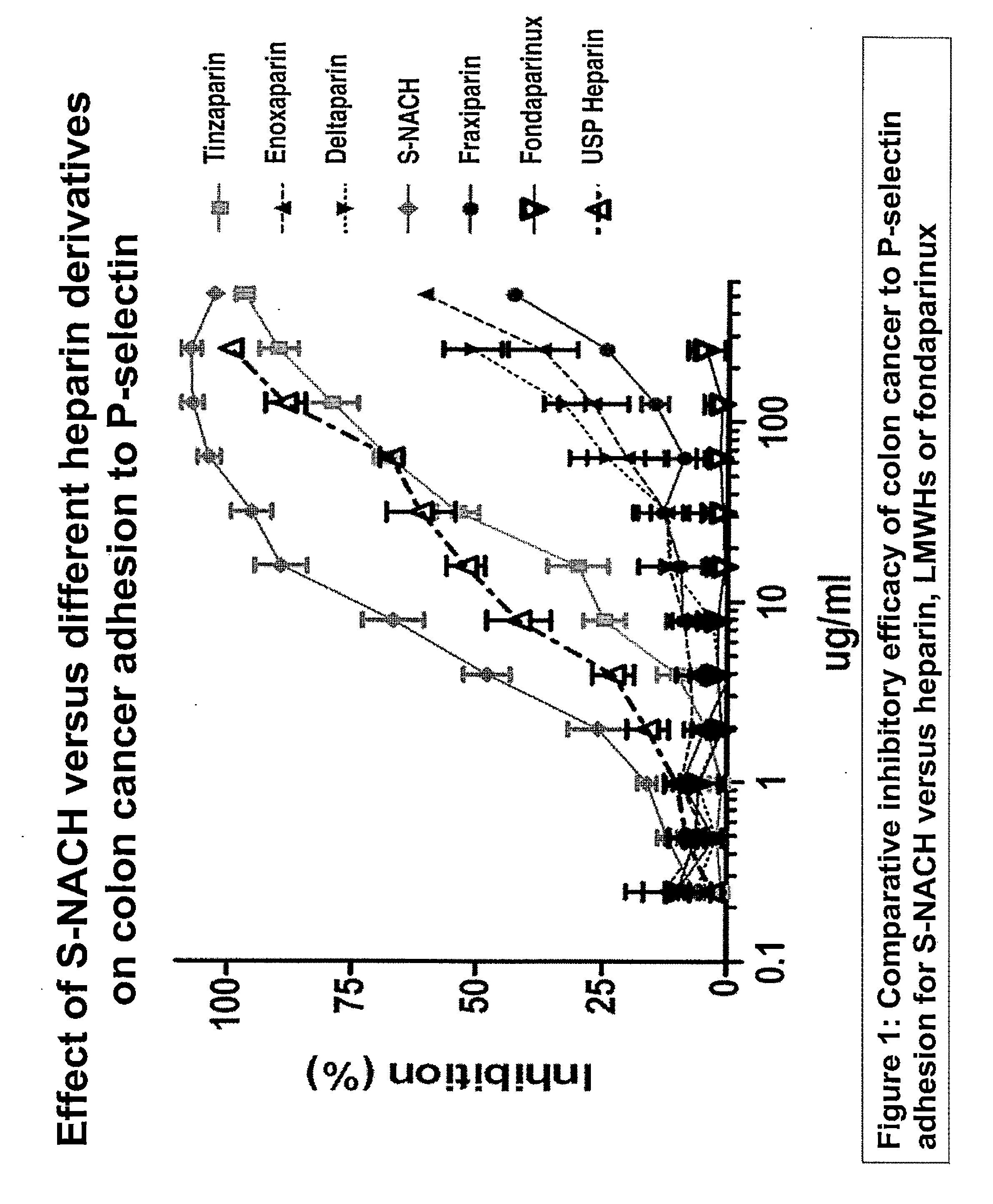
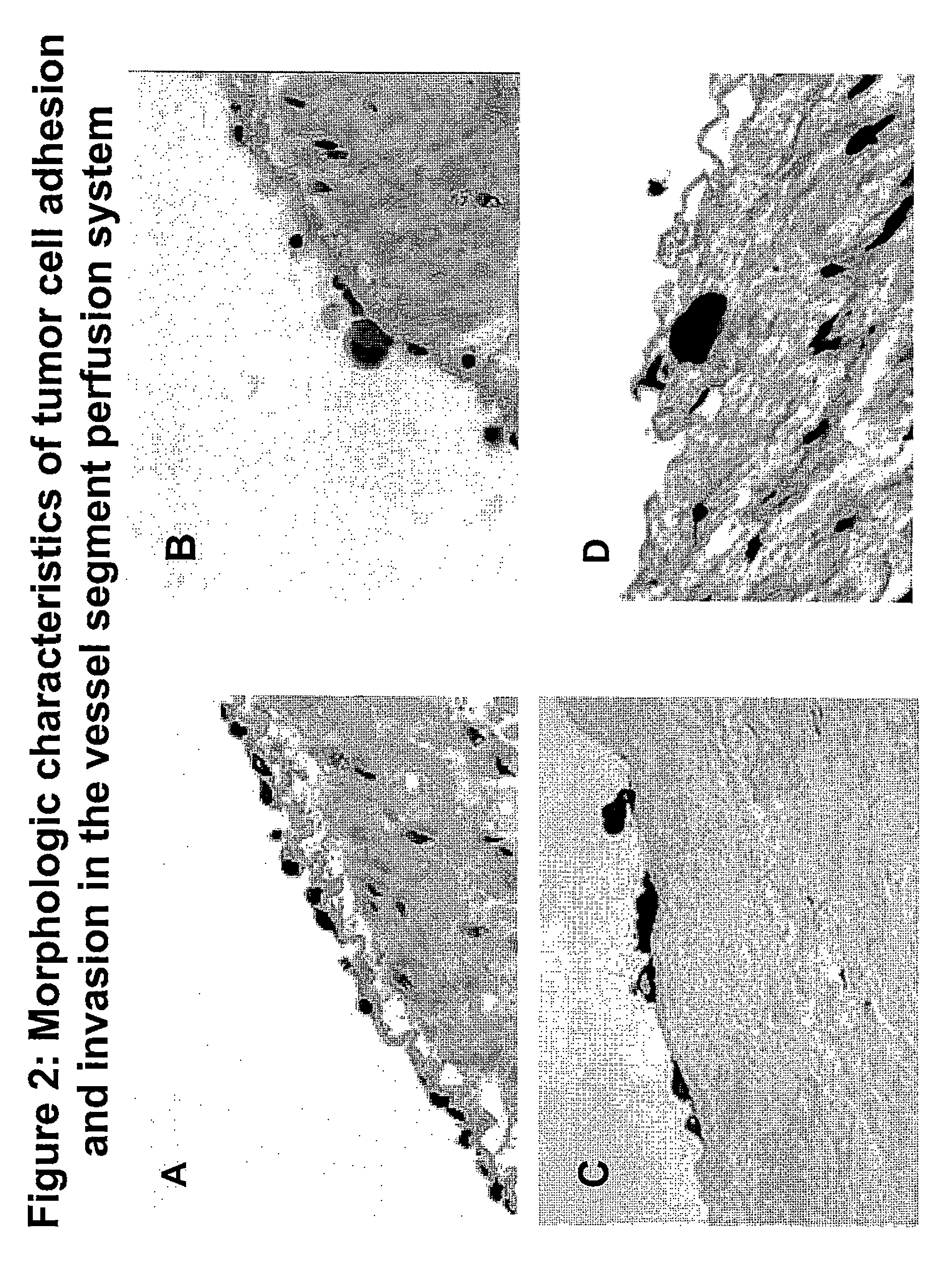
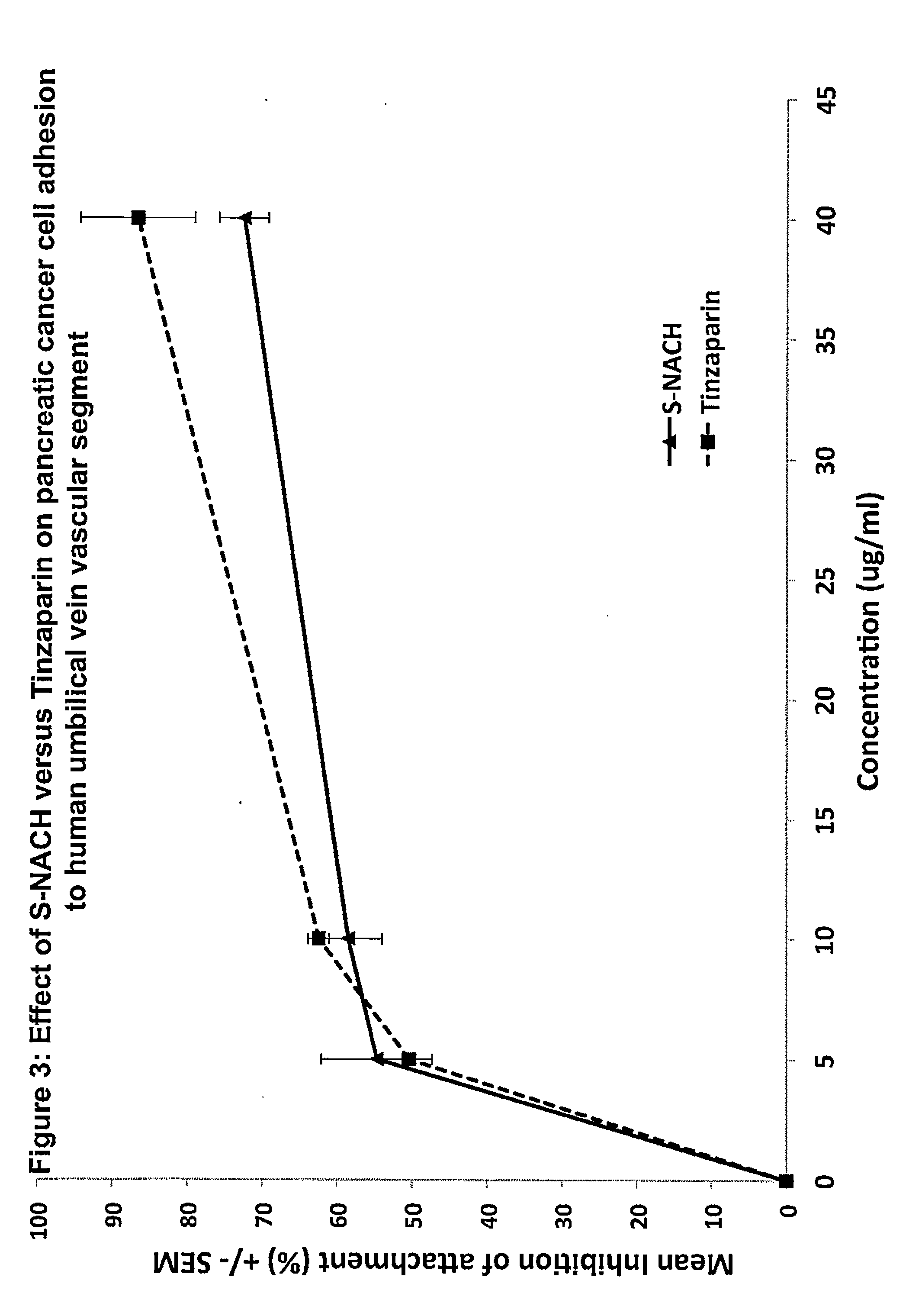
Composition and method for sulfated non-anticoagulant low molecular weight heparins in cancer and tumor metastasis
A nanoformulation that includes nanoparticles. Each nanoparticle includes a shell in which a glycosaminoglycan (GAG is encapsulated. The GAG is ionically or covalently bonded to the shell. The GAG is selected from the group consisting of sulfated non-anticoagulant heparin (SNACH), super-sulfated non-anticoagulant heparin (S-SNACH), and a combination thereof. The shell includes Poly (lactic-co-glycolic acid) (PLGA), Polyethylene Glycol (PEG)-PLGA, maleimide-PEG-PLGA, chitosan, chitosan-PLGA, methoxy-polyethyleneglycol-poly (lactide-co-glycolide) (MPEG-PLGA)-(maleimide-PEG-PLGA), PLGA-Polycaprolate, or calcium alginate. A method of using the nanoformulation to treat a cancer in a subject includes administering to the patient a therapeutically effective amount of the nanoformulation for treating the cancer.
Owner:MOUSA SHAKER A
Lapatinib sustained-release implantation agent for treating solid tumors
InactiveCN101219102AOrganic active ingredientsPharmaceutical delivery mechanismEthyl phosphateToxic reaction
The invention relates to a solid tumors-curing Lapatinib sustained-release implant, which is characterized in that the sustained-release implant contains effective anti-tumor dose of Lapatinib, sustained-release auxiliary material and a certain amount of sustained-release regulator. Solid tumors consist of lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer and colorectal cancer. The sustained-release auxiliary materials are mainly one or mixture of copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate). When the sustained-release auxiliary materials are degrading and absorbed, Lapatinib is slowly released into tumor part, thus while reducing toxic reaction of the whole body, the sustained-release auxiliary materials can sustain concentration of effective drug at the tumor part. Placing the anti-tumor sustained-release implant at the tumor part can not only reduce toxic reaction of the whole body of Lapatinib, but also improve drug concentration at the tumor part and enhance treatment effects of nonspecific treatments, such as chemotherapeutics, radiotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Estramustine sustained-release implantation agent for curing entity tumour
InactiveCN101181230AIncreased sensitivityHigh clinical application valueOrganic active ingredientsPharmaceutical delivery mechanismPhosphateStomach cancer
The invention relates to an estramustine sustained-release implant for the treatment of solid tumors, which is characterized in that the sustained-release implant contains anti-cancer effective amount of estramustine, sustained-release excipient and certain amount of sustained-release regulator. The solid tumors include lung cancer, esophageal cancer, stomach cancer, liver cancer, breast cancer, ovarian cancer, prostate cancer, bladder cancer and colorectal cancer. The sustained-release excipient mainly comprises one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate). The invention can slowly release the estramustine in the local part of the tumor during the degradation and absorption process, so the invention can maintain effective drug concentration at the local part of the tumor at the same time of significantly reducing systemic toxic reaction. The sustained-release implant is arranged at the local part of the tumor, which can not only reduce the systemic toxic reaction of estramustine, but can also selectively improve the drug concentration at the local part of the tumor and strengthen the treatment effects of chemotherapy drugs, radiation therapy and other non-surgical therapies.
Owner:SHANDONG LANJIN PHARMA +1
Easily degradable responsive core-crosslinkable amphiphilic block polymer, preparation method thereof and application of amphiphilic block polymer as drug carrier
PendingCN113105614AEasy to operateMild reaction conditionsPharmaceutical non-active ingredientsEmulsion deliveryPolymer scienceSide effect
The invention discloses an easily degradable response type core-crosslinkable amphiphilic block polymer, a preparation method thereof and an application of the amphiphilic block polymer as a drug carrier. According to the amphiphilic block polymer, methoxypolyethylene glycol-b-polylactide serves as a main chain, and a side chain is modified with carboxyl and a dithiopyridine group; the amphiphilic block polymer and a water-insoluble drug can easily form a response type core cross-linked micelle, and the micelle is stable in a normal physiological environment, and is easy to be stimulated by environment after reaching tumour cells, and then, the cross-linked structure is damaged, so that the controlled release of the medicine is realized, the utilization rate of the medicine is improved, and the toxic and side effects of the loaded medicine are reduced; theamphiphilic block polymer is easy to degrade, so thatlong-term toxicity caused by accumulation of micelles in human tissues and organs is avoided; the amphiphilic block polymer has goodbiocompatibility, and has wide potential application prospects in the field of cancer chemotherapy; and the synthetic method of the amphiphilic block polymer is simple, mild in condition, few in side reaction and beneficial to expanded production.
Owner:XIANGTAN UNIV
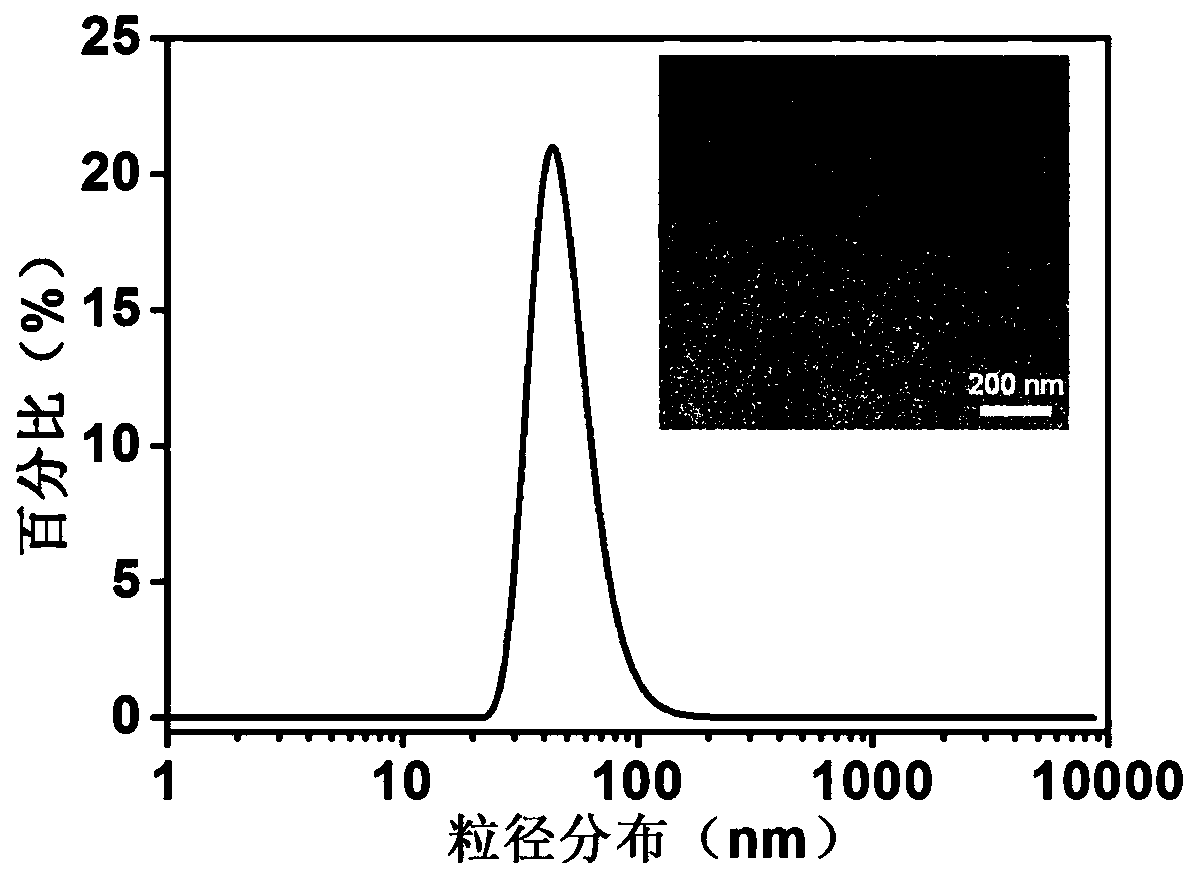
Nano-particle with excellent blood stabilizing performance, and preparation method thereof
ActiveCN108542893ASimple ingredientsGood blood stabilityPharmaceutical non-active ingredientsAntineoplastic agentsDiseaseHalf-life
The invention belongs to the technical field of nano-medicines, and provides a nano-particle with an excellent blood stabilizing performance, and a preparation method thereof. Poly(glycolide-co-lactide) (PLGA), a medicine / probe molecule, distearoyl phosphoethanolamine-polyethylene glycol-maleimide (DSPE-PEG) and pluronic F127 (PF127) which are used as raw materials undergo a rapid nanoprecipitation technique to prepare the nano-particle having the advantages of controlled particle size, good monodispersity, and excellent stability and performances in various solutions. A large number of hydroxyl groups and negative charges on the surface of the particle have a protection effect, so the nano-particle do not bind to proteins in serum, thereby the protein is avoided from being absorbed and metabolized by the reticuloendothelial system; and the hydrophobic medicine and the hydrophobic or amphipathic probe are encapsulated, so the particle can significantly prolong the blood circulation half-life of the medicine and the probe, and provides a good medicine delivery platform for disease detection and treatment.
Owner:SUN YAT SEN UNIV
Axitinib sustained-release implplant treating for solid tumor
InactiveCN101204367AOrganic active ingredientsPharmaceutical delivery mechanismWhole bodyProstate cancer
The invention relates to an Axitinib sustained-release implant for treating a solid tumor, which is characterized in that: the sustained-release implant contains an effective anticancer amount of Axitinib and sustained-release excipients and a certain amount of sustained-release regulator. The solid tumors include the liver cancer, the lung cancer, the esophageal canner, the gastric canner, the breast canner, the ovarian canner, the prostate canner, the bladder canner and the rectum canner. The sustained-release excipients are mainly a copolymer of polylactic acid, glycollic acid and hydroxyacetic acid and one of the three materials, polifeprosan, poly-(L-lactide-co-ethyl phosphonate) and Poly(L-lactide-co-propyl phosphonate) or a combination of the three materials, in the degradation and absorption process of which the Axitinib is sustainedly released to part of the tumor, thus the entire toxicity of the Axitinib is significantly reduced while an effective medicine consistency is maintained on part of the tumor. That the sustained-release implant is implanted inside part of the tumor can not only reduce the entire toxicity of the Axitinib, but also enhance the medicine consistency on part of the tumor, thereby increasing the curing effect of non-operative therapeutics such as chemotherapeutic drugs and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Bosutinib sustained release implant for treating solid tumors
InactiveCN101224185AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a bertschtinib sustained-release implant for treating solid tumors, which is characterized in the sustained-release implant contains anti-cancer effective dose of bertschtinib, sustained-released auxiliary materials and a certain amount of sustained-release regulator. Solid tumors comprise lung cancer, esophageal cancer, gastric cancer, liver cancer, mammary cancer, oophoroma, prostatic cancer, carcinoma of urinary bladder and colorectal cancer. The sustained-released auxiliary materials are mainly one or composition of the copolymer of glycolic acid and glycollic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co- propyl phosphate), which can slowly release bertschtinib to the tumor local during degeneration and absorption, thus keeping concentration of effective medicine in the part of the tumor while distinctively reducing overall toxic reaction. Putting the sustained-release implant in the tumor can not only reduce overall toxic reaction of bertschtinib, but also selectively improve drug concentration in the part of the tumor, thereby promoting treatment effect of non-operative treatment, such as chemotherapeutics, radiotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Nilotinib sustained-release implant for treating solid tumor
InactiveCN101185627AOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerTherapeutic effect
A sustained release implant includes 0.1%-50% (w / w) nilotinib, 50-99% sustained release excipients and 0-15% sustained release moderator. Sustained release excipients are one or the combination of poly (L-lactide-co-ethyl phosphate), poly (L-lactide-co- phosphoric acid propyl), glycolic acid and copolymer of glycolic acid and hydroxyacetic acid, and polifeprosan; sustained release moderator is one or combination of mannitol, sorbic alcohol and chondroitin; sustained release implant applied in local tumor can slowly release nilotinib onto local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction, thus the invention not only reduces overall toxic reaction of nilotinib, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy. The implant can be used for treating solid tumors including lung cancer, esophageal carcinoma, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic carcinoma, pancreatic cancer, bladder carcinoma, cerebroma, and colorectal cancer.
Owner:SHANDONG LANJIN PHARMA +1
Particle formulation with polycation complex
ActiveUS10765638B2Reduce, alleviate, or prevent one or more symptomsReduce the burden onPowder deliveryOrganic active ingredientsPolyesterCancer cell
Compositions and methods for efficient delivery of therapeutic agents in vivo are provided. Typically, the compositions are in the form of polymeric particles formed from one or more therapeutic agent complexed with a polycationic polymer which is further encapsulated in one or more amphiphilic polymers, preferably diblock copolymer of a polyalkylene oxide and a polyester such as poly(D,L-lactide)-poly(ethylene glycol) (PLA-PEG). In the preferred embodiments, the chemotherapeutic agent reduces, or inhibits N-glycosylation of one or more receptor tyrosine kinases of cancer cells. Methods of using the particles to treat cancer are also provided.
Owner:YALE UNIV
Mitoxantrone sustained-release implantation agent for curing entity tumour
InactiveCN101176710AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a mitoxantrone sustained-release implant capability of curing solid tumors, such as lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer, colon cancer and rectum cancer. The invention is characterized in that: the sustained-release implant comprises mitoxantrone, sustained-release excipient and a certain amount of sustained-release regulator; the amount of the mitoxantrone and sustained-release excipient is sufficient to control cancer; the sustained-release excipient is mainly one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate); the mitoxantrone can be released slowly into part of the tumor during the degradation and adsorption, significantly reducing the systemic toxicity and sustaining the effective medicine concentration simultaneously. The invention has the advantages that: the systemic toxicity of mitoxantrone can be significantly reduced; the effective medicine concentration can be improved selectively at part of the tumor, and the therapeutic effects of non-operative treatments such as chemotherapeutic drugs and radiotherapy can be reinforced.
Owner:JINAN SHUAIHUA PHARMA TECH
Injectable temperature-sensitive gel preparation for treating acute pancreatitis
InactiveCN105342984ASmall systemic side effectsSimple preparation processOrganic active ingredientsPeptide/protein ingredientsAcute pancreatitisPolyvinyl alcohol
Critical conditions of acute pancreatitis have high mortality. Trypsinization Theory is one of important pathogeneses of acute pancreatitis. Pancreatin inhibitor drugs, such as gabexate mesylate, ulinastatin and aprotinin, are selectively clinically applied against the mechanism. The routine administration mode of the drugs is intravenous injection, and has certain disadvantages. The invention provides an injectable temperature-sensitive gel preparation for treating acute pancreatitis. The dosage form of the above gel comprises a poloxamer polymer, a polylactide-co-glycolide / polyethylene glycol block copolymer, a polycaprolactam / polyethylene glycol block copolymer, a chitosan / beta-sodium glycerophosphate system, a chitosan / polyvinyl alcohol system or a chitosan-sodium bicarbonate system, or an arbitrary combination thereof. The gel has a reverse gelling characteristic, is a liquid at a low temperature, and converts to a semisolid state at a human body temperature. The invention also provides a preparation method of the injectable temperature-sensitive gel preparation for treating acute pancreatitis.
Owner:GENERAL HOSPITAL OF PLA
Pharmaceutical Compositions having a Selected Release Duration
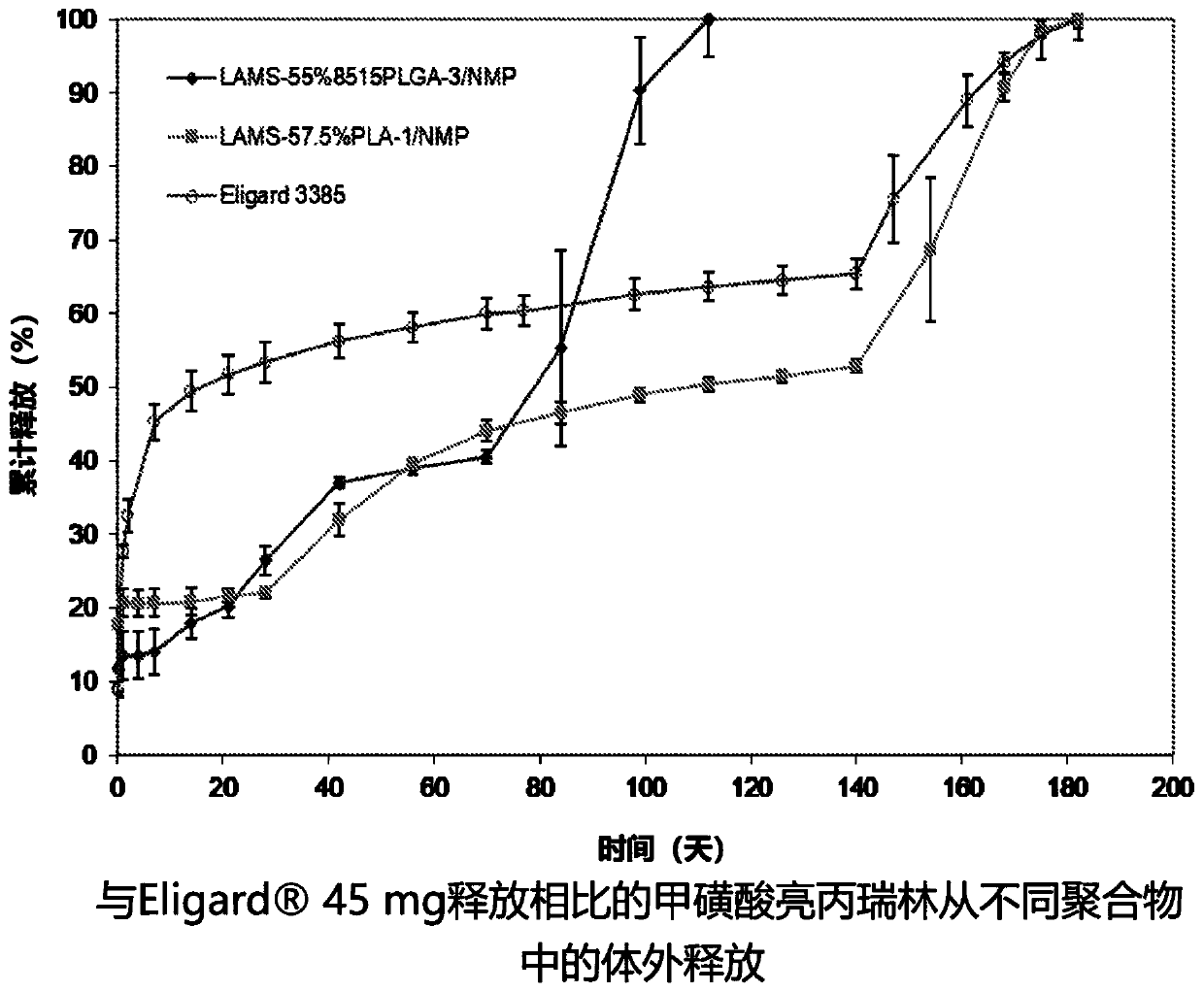
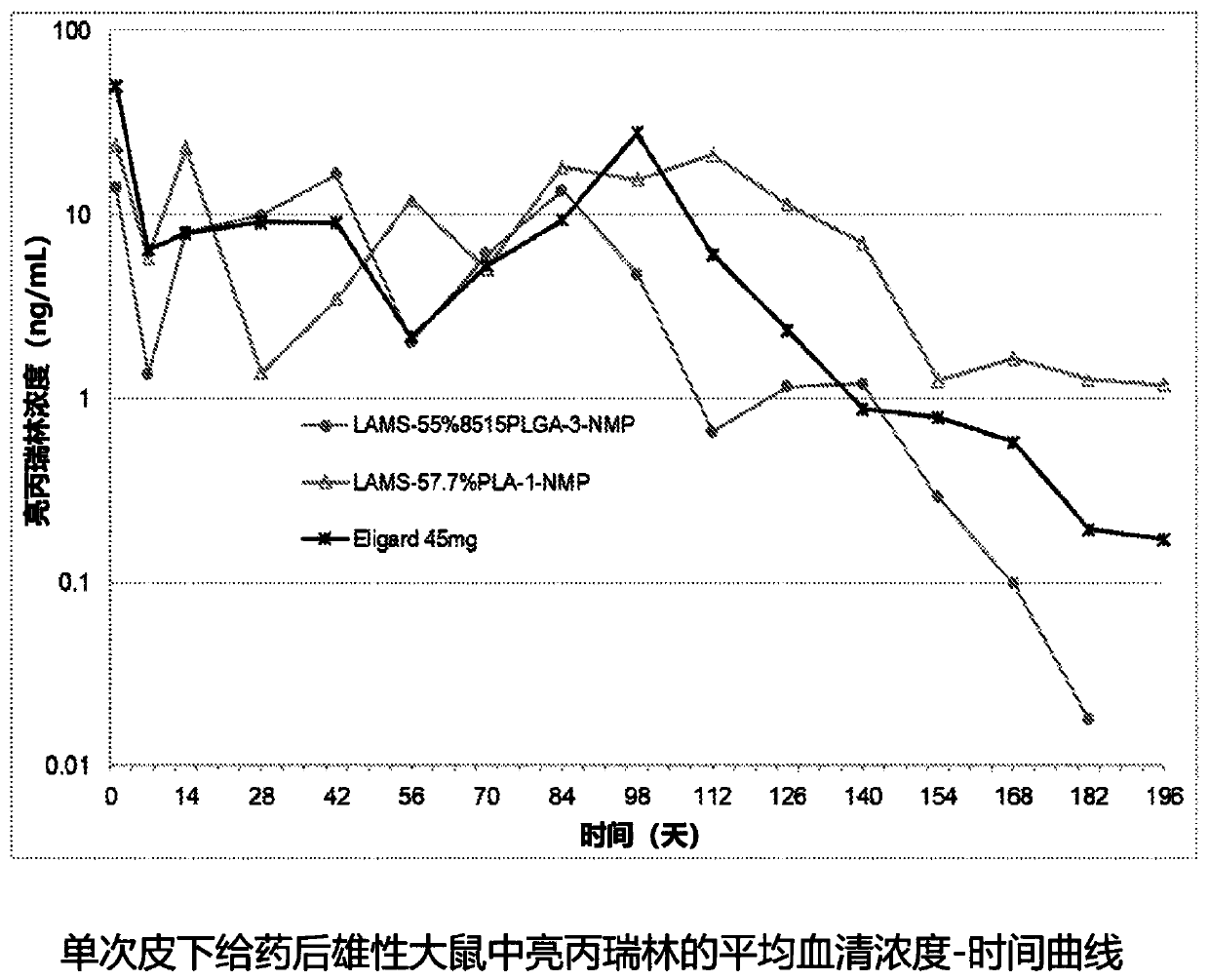
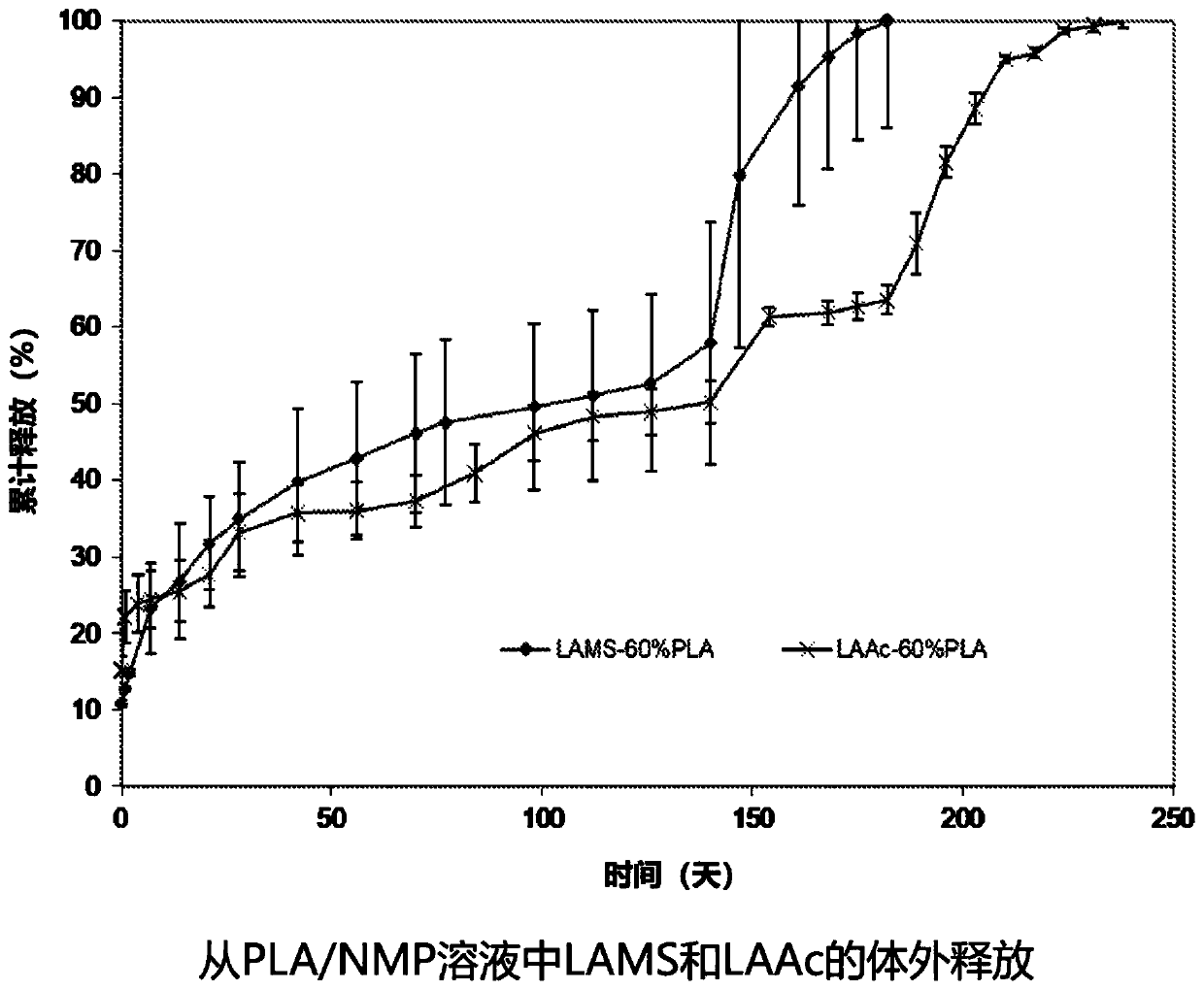
ActiveUS20200390849A1Improve stabilitySatisfied with stabilityPeptide/protein ingredientsPharmaceutical delivery mechanismLeuprorelinLactide
The present invention provides for a stabilized biodegradable polymeric composition useful as a controlled release delivery system for peptide agents. The compositions of the present invention comprise a) a strong acid salt of a LHRH agonist or antagonist; b) a biodegradable polymer of poly(lactide-co-glycolide), wherein the ratio of lactide:glycolide of the copolymer is from 50:50 to about 100:0; and c) N-methyl-2-pyrrolidone (NMP), wherein the composition does not contain excess strong acid in addition to the strong acid used to form the salt of the LHRH agonist or antagonist. The composition, when injected, can provide a controlled release of leuprolide for a period of up to 6 months.
Owner:FORESEE PHARMA CO LTD
Iloperidone slow releasing microsphere and preparation method thereof
InactiveCN104352443AIncrease concentrationOrganic active ingredientsNervous disorderLactideMicrosphere
The invention provides an iloperidone slow releasing microsphere and a preparation method thereof. The microsphere contains an iloperidone and lactide-glycolide copolymer. The iloperidone slow releasing microsphere provided by the invention has the benefits that the drug loading capacity is higher, the slow releasing period is above 5 weeks, and an in vitro release curve is smooth and flat.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Gefitinib sustained-release implantation agent for curing entity tumour
InactiveCN101181233AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a gimatecan sustained-release implant for the treatment of solid tumors, which is characterized in that the sustained-release implant contains anti-cancer effective amount of gimatecan, sustained-release excipient and certain amount of sustained-release regulator. The solid tumors include lung cancer, esophageal cancer, stomach cancer, liver cancer, breast cancer, ovarian cancer, prostate cancer, bladder cancer and colorectal cancer. The sustained-release excipient mainly comprises one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate). The invention can slowly release the gimatecan in the local part of the tumor during the degradation and absorption process, so the invention can maintain the effective drug concentration at the local part of the tumor at the same time of significantly reducing systemic toxic reaction. The sustained-release implant is arranged at the local part of the tumor, which can not only reduce the systemic toxic reaction of gimatecan, but can also selectively improve the drug concentration at the local part of the tumor and strengthen the treatment effects of chemotherapy drugs, radiation therapy and other non-surgical therapies.
Owner:SHANDONG LANJIN PHARMA +1
Rasagiline mesylate microsphere preparation and preparation method thereof
ActiveCN112190553AImprove stabilityHigh viscosityPowder deliveryOrganic active ingredientsMicrosphereCombinatorial chemistry
The invention discloses a rasagiline sustained-release microsphere preparation. The rasagiline sustained-release microsphere preparation contains rasagiline mesylate, a lactide-glycolide copolymer anda release regulator. The invention also discloses rasagiline mesylate microspheres prepared by adopting a multiple emulsion solvent evaporation method. A proper amount of release regulator is added into an inner water phase, so that the burst release amount of the drug-loaded microspheres can be remarkably reduced; the stability of the colostrum is improved, and the inter-batch difference causedby poor stability of the colostrum can be avoided; and the micromorphology of the drug-loaded microspheres is improved. The drug-loaded microspheres prepared by the improved method have high encapsulation efficiency and low burst release amount, and can realize uniform and slow release of drugs.
Owner:CHINA PHARM UNIV
Marseilledinun sustained-release implantation agent for curing entity tumour
InactiveCN101181232AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a masitinib sustained-release implant for the treatment of solid tumors, which is characterized in that the sustained-release implant contains anti-cancer effective amount of masitinib, sustained-release excipient and certain amount of sustained-release regulator. The solid tumors include lung cancer, esophageal cancer, stomach cancer, liver cancer, breast cancer, ovariancancer, prostate cancer, bladder cancer and colorectal cancer. The sustained-release excipient mainly comprises one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate). The invention can slowly release the masitinib in the local part of the tumor during the degradation and absorption process, so the invention can maintain the effective drug concentration at the local part of the tumor at the same time of significantly reducing systemic toxic reaction. The sustained-release implant is arranged at the local part of the tumor, which can not only reduce the systemic toxic reaction of masitinib, but can also selectively improve the drug concentration at the local part of the tumor and strengthen the treatment effects of chemotherapy drugs, radiation therapy and other non-surgical therapies.
Owner:SHANDONG LANJIN PHARMA +1
Sunitinib sustained-release implantation agent for curing entity tumour
InactiveCN101176716AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a sunitinib sustained-release implant capability for curing solid tumors, such as lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer, colon cancer and rectum cancer. The invention is characterized in that: the sustained-release implant comprises sunitinib, sustained-release excipient and a certain amount of sustained-release regulator; the sustained-release excipient is mainly one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate); the sunitinib can be released slowly into part of the tumor during the degradation and adsorption, significantly reducing the systemic toxicity and sustaining the effective medicine concentration simultaneously. The invention has the advantages that: the systemic toxicity of sunitinib can be significantly reduced; the effective medicine concentration can be improved selectively at part of the tumor; the invention can be applied to the prevention of tumor recurrence after operation and chemotherapy and combined therapy of various solid tumors and metastatic tumors that are not suitable for operation.
Owner:JINAN SHUAIHUA PHARMA TECH
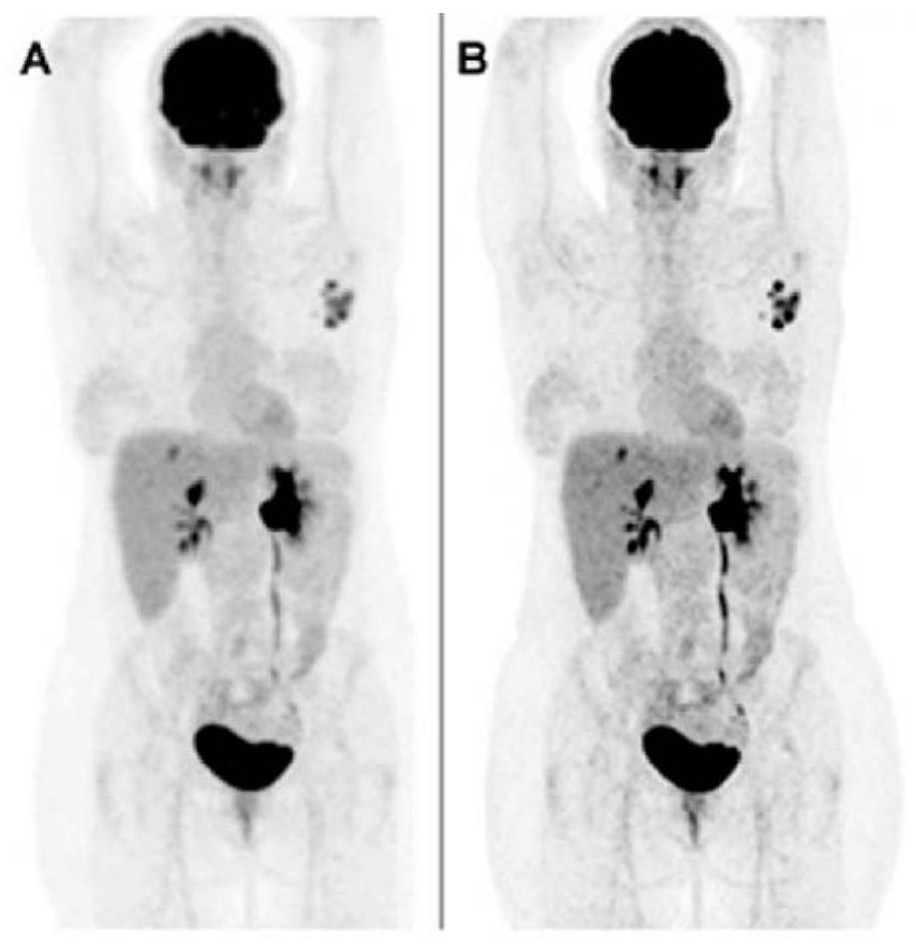
Method for tracing cervical deep lymph nodes and meningeal lymphatic vessels by using nanometer probes
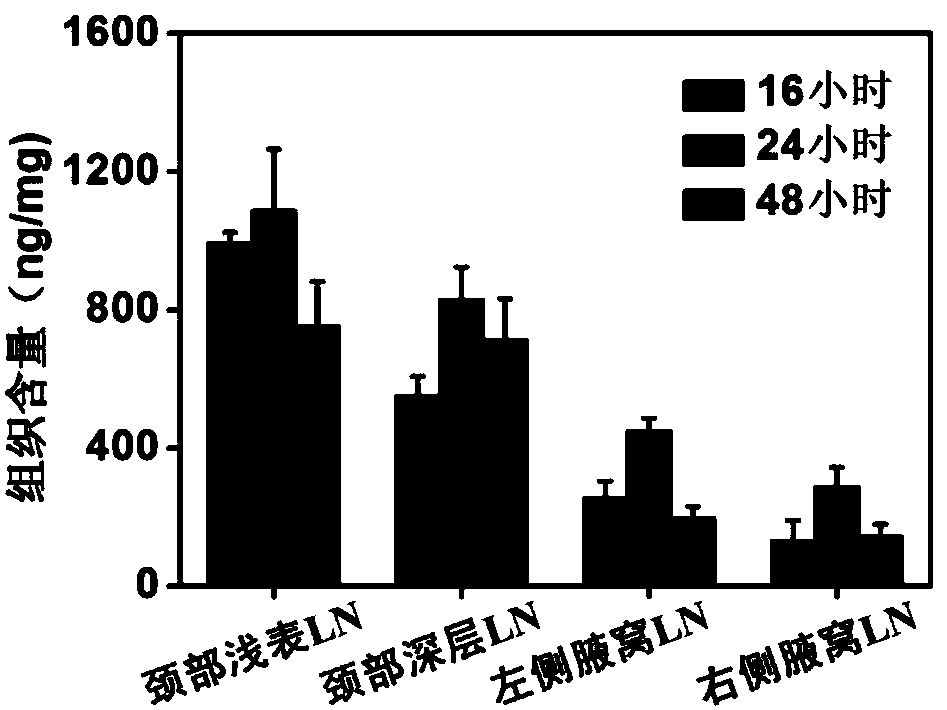
ActiveCN108434463ASimple and direct means of detectionPowder deliveryIn-vivo testing preparationsSubcutaneous injectionNeck lymph nodes
The invention belongs to the technical field of nanometer probes, and in particular relates to a method for tracing cervical deep lymph nodes and meningeal lymphatic vessels by using nanometer probes.According to the method, core-shell nanoparticles prepared by using polyglycolide lactide and pluronic F127 as main raw materials are used as a model nano drug-loading system (with a particle size of20-50nm), and are coated with a near-infrared fluorescent dye Cy5 so as to form the nanometer probes. After being subjected to subcutaneous injection in the right neck, the nanometer probes are enriched to the superficial lymph nodes, deep cervical lymph nodes and axillary lymph nodes by means of local lymphatic drainage of tissues, wherein the deep cervical lymph nodes and the meningeal lymphatic vessels are directly connected with each other. Therefore, the nanometer probes can be rapidly transmitted to the meningeal lymphatic vessels for real-time imaging tracing of the deep cervical lymphnodes and the meningeal lymphatic vessels, thus providing a simple and direct detection method for the lymphatic transport research of the nanoparticles in the neck and the physiological and pathological study of the brain lymphatic system.
Owner:SUN YAT SEN UNIV
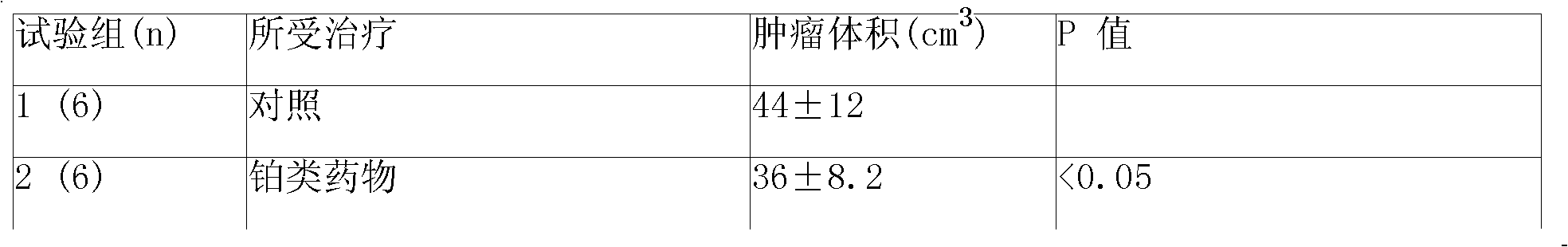
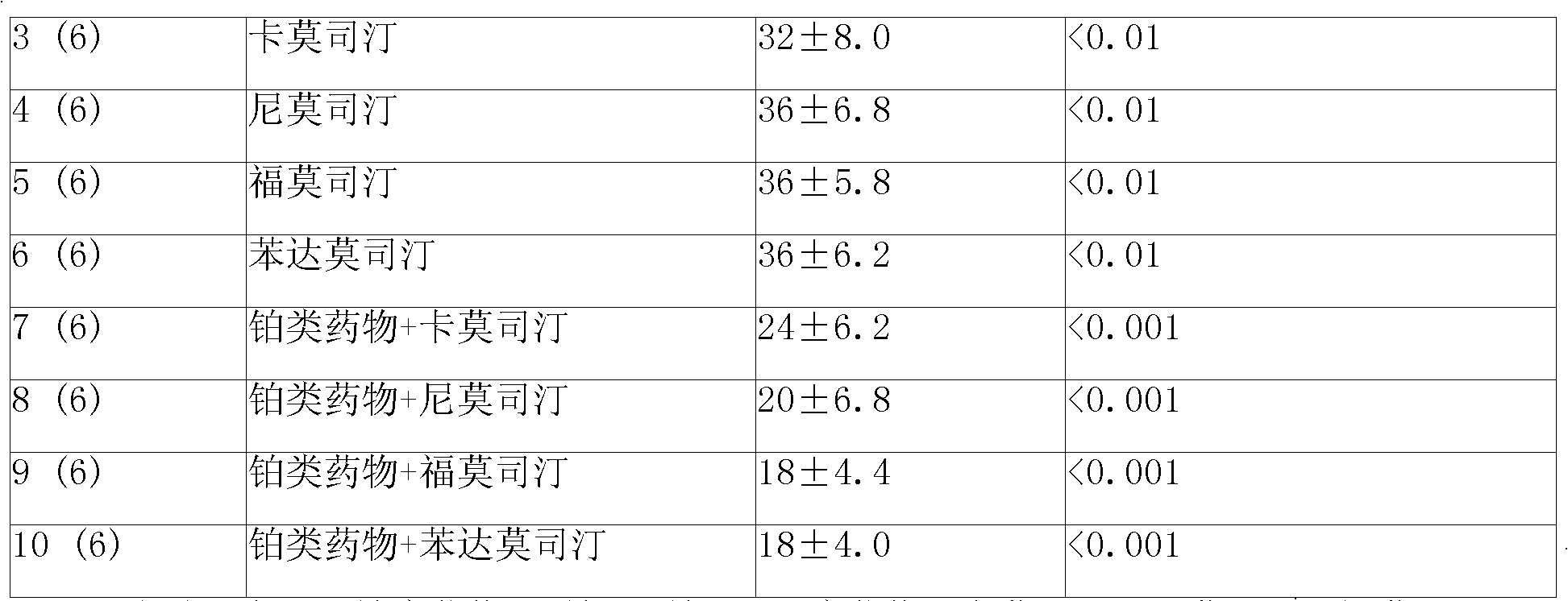
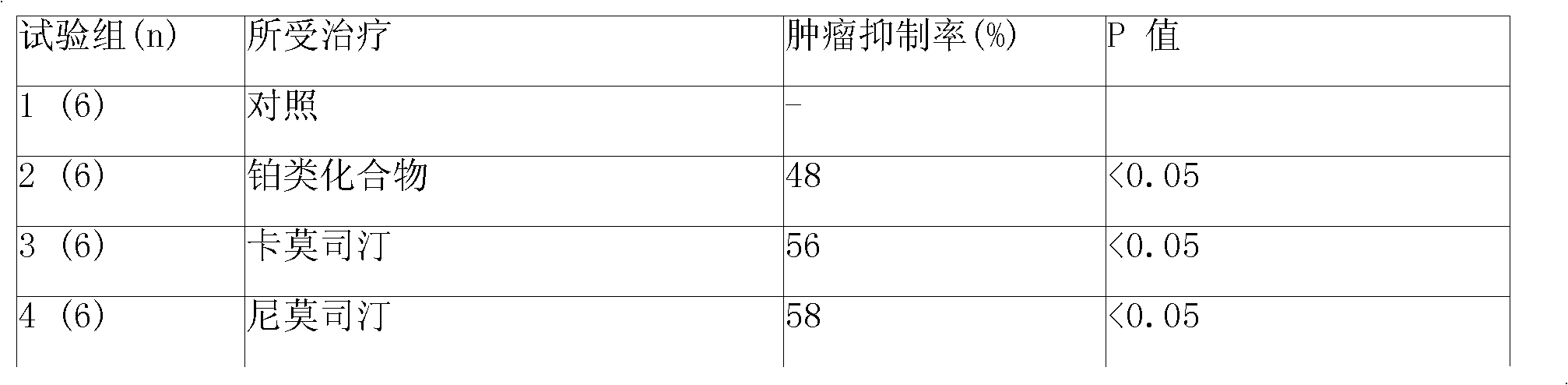
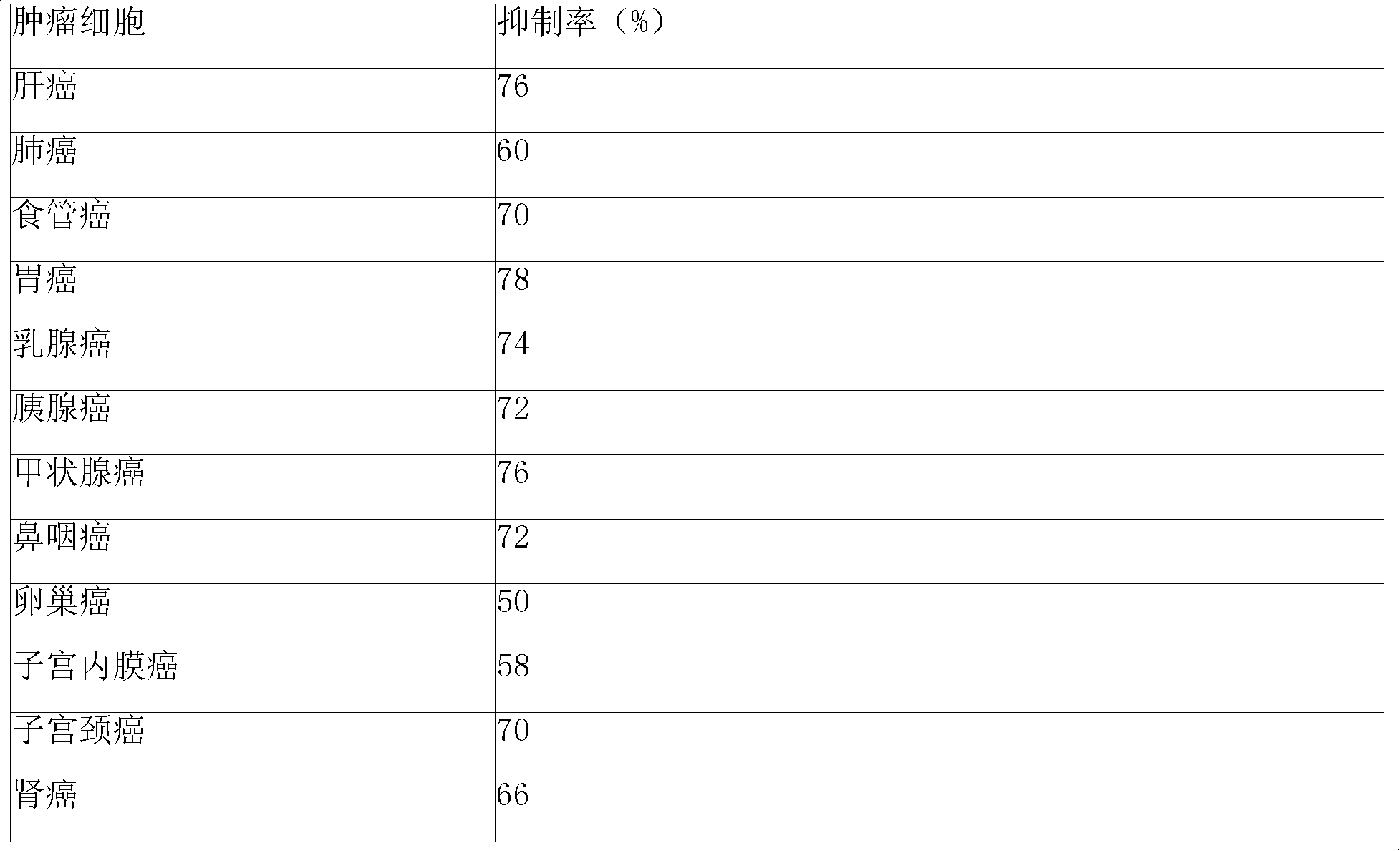
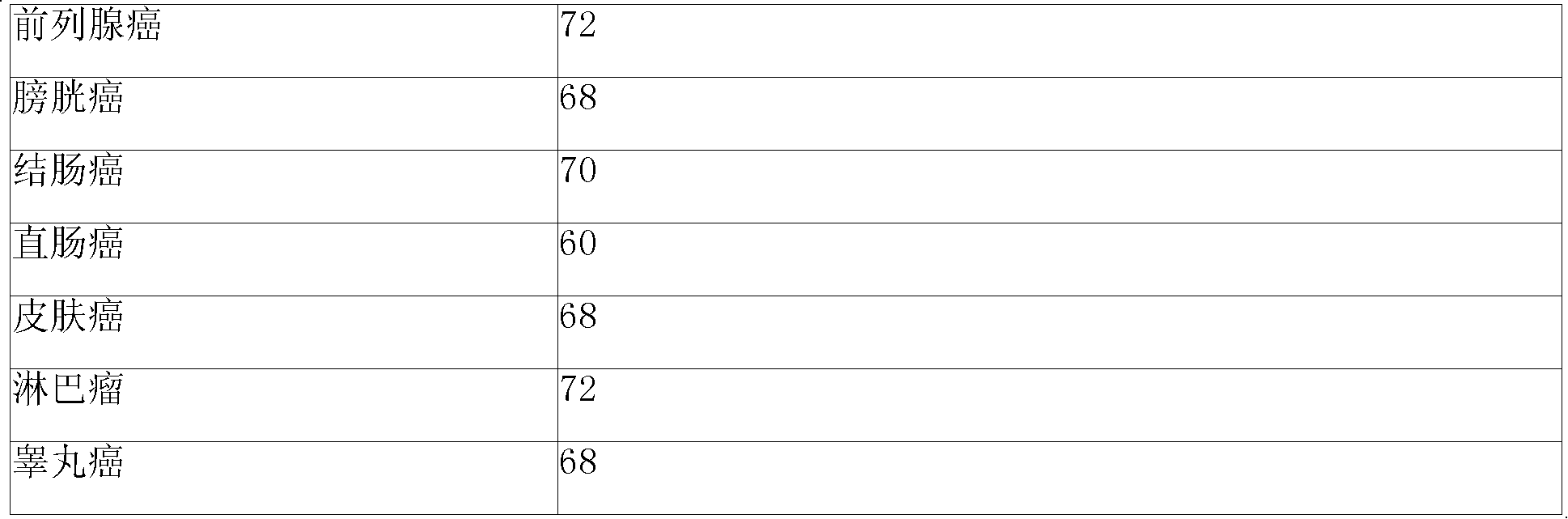
Sustained-release gel injection loaded with platinum compound and alkyl agent
InactiveCN101283977APharmaceutical delivery mechanismPharmaceutical non-active ingredientsChemistryRanimustine
A sustained-release gel injection comprises cisplatin, carboplatin, nedaplatin, oxaliplatin or satraplatin, stines, amphiphilic block copolymer and solvent. The stines are selected from carmustine, mimustine, ambnmustine, bendamustine, galamustine, fotemustine, estramustine, lomustine, semustine, SarCNU, ranimustine, tallimustine and spiromustine; the amphiphilic block copolymer is selected from glycolide-lactide copolymer and polyethylene glycol-glycolide-lactide copolymer; and the gel injection has a temperature sensitive gelatinization characteristic, isa fluid liquid at room temperature, and automatically becomes non-flowing biodegradable water insoluble gel in warm-blooded animals. The preparation can locally slowly release at tumor foci and maintain effective drug concentration for several weeks to several months; has effects on killing tumor cells, thereby effectively inhibiting tumor angiogenesis, reducing systemic toxicity of drug, and enhancing the treatment effect of chemotherapy and radiotherapy (particularly radioactive particles).
Owner:济南基福医药科技有限公司
Irinotecan sustained-release implant for treating solid tumor
InactiveCN101185628AIncreased sensitivityHigh clinical application valueOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerExcipient
An irinotecan sustained release implant includes effective dose of anticancer irinotecan, sustained release excipients and a certain quantity of sustained release moderator. Solid tumors include pancreatic cancer, lung cancer, liver cancer, breast cancer, cerebroma, ovarian cancer, prostatic carcinoma, esophageal carcinoma, lymphadenoma, osteosarcoma and colorectal cancer. Sustained release excipients are mainly one or combination of copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly(L-lactide-co-ethyl phosphate), and poly (L-lactide-co-phosphoric acid propyl); in the process of decompression, irinotecanpto can be slowly and steadily released to local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction. Being placed in local tumor, the sustained release implant not only reduces overall toxic reaction of irinotecan, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy.
Owner:SHANDONG LANJIN PHARMA +1
Gefitinib sustained-release implantation agent for curing entity tumour
InactiveCN101176715AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a gefitinib sustained-release implant capability for curing solid tumors, such as lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer, colon cancer and rectum cancer. The invention is characterized in that: the sustained-release implant comprises gefitinib, sustained-release excipient and a certain amount of sustained-release regulator; the amount of the gefitinib and sustained-release excipient is sufficient to control cancer; the sustained-release excipient is mainly one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate); the gefitinib can be released slowly into part of the tumor during the degradation and adsorption, significantly reducing the systemic toxicity and sustaining the effective medicine concentration simultaneously. The invention has the advantages that: the systemic toxicity of gefitinib can be significantly reduced; the effective medicine concentration can be improved selectively at part of the tumor, and the therapeutic effects of non-operative treatments such as chemotherapeutic drugs and radiotherapy can be reinforced.
Owner:JINAN SHUAIHUA PHARMA TECH
Lonafarnib sustained-release implant for treating solid tumor
InactiveCN101185632AOrganic active ingredientsPharmaceutical delivery mechanismEthyl phosphateToxic reaction
A ranophanib sustained release implant for treating solid tumors is characterized in that the sustained release implant includes effective dose of anticancer ranophanib, sustained release excipients and a certain quantity of sustained release moderator. Solid tumors include lung cancer, esophageal carcinoma, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic carcinoma, bladder carcinoma and colorectal cancer. Sustained release excipients are mainly one or combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly(L-lactide-co-ethyl phosphate) and poly (L-lactide-co- phosphoric acid propyl); in the process of decompression, ranophanib can be slowly released to local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction. Being placed in local tumor, the sustained release implant not only reduces overall toxic reaction of ranophanib, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy.
Owner:SHANDONG LANJIN PHARMA +1
188Re-labeled degradable nano-probe for breast cancer diagnosis and treatment and preparation method thereof
PendingCN113456841ARealize the integration of diagnosis and treatmentAchieve targeted trackingPowder deliveryRadioactive preparation carriersNode metastasisLactide
The invention discloses a 188Re-labeled degradable nano-probe for breast cancer diagnosis and treatment and a preparation method, the 188Re-labeled degradable nano-probe for breast cancer diagnosis and treatment comprises a nano-probe, and the nano-probe is formed by coating an IDO inhibitor with PLGA nano-particles, the nano-probe comprises the following components by molar mass: 4-6 mol of lactide, 1-2 mol of glycolide, an initiator, a surfactant, an IDO inhibitor and a dispersant, the nano-probe is connected with folic acid for targeting 4T1 breast cancer, and is connected with 188Re for targeted tracking of breast cancer and lymph node metastasis, SPECT imaging and internal irradiation sensitization treatment. The degradable nano-probe disclosed by the invention can realize targeted tracking, SPECT imaging and internal irradiation sensitization precise treatment of breast cancer and lymph node metastasis, and realizes diagnosis and treatment integration of breast cancer.
Owner:GENERAL HOSPITAL OF NUCLEAR IND
Tipifarnib sustained-release implantation agent for treating solid tumors
InactiveCN101219103AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a solid tumors-curing tipifarnib sustained-release implant, which is characterized in that the sustained-release implant contains effective anti-tumor dose of tipifarnib, sustained-release auxiliary material and a certain amount of sustained-release regulator. Solid tumors consist of lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer and colorectal cancer. The sustained-release auxiliary materials are mainly one or mixture of copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate). When the sustained-release auxiliary materials are degrading and absorbed, tipifarnib is slowly released into tumor part, thus while reducing toxic reaction of the whole body, the sustained-release auxiliary materials can sustain concentration of effective drug at the tumor part. Placing the anti-tumor sustained-release implant at the tumor part can not only reduce toxic reaction of the whole body of tipifarnib, but also improve drug concentration at the tumor part and enhance treatment effects of nonspecific treatments, such as chemotherapeutics, radiotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Pharmaceutical compositions having a selected release duration
The present invention provides for a stabilized biodegradable polymeric composition useful as a controlled release delivery system for peptide agents. The compositions of the present invention comprise a) a strong acid salt of a LHRH agonist or antagonist; b) a biodegradable polymer of poly(lactide-co-glycolide), wherein the ratio of lactide: glycolide of the copolymer is from 50:50 to about 100:0; and c) N-methyl-2-pyrrolidone (NMP), wherein the composition does not contain excess strong acid in addition to the strong acid used to form the salt of the LHRH agonist or antagonist. The composition, when injected, can provide a controlled release of leuprolide for a period of up to 6 months.
Owner:FORESEE PHARMA CO LTD
A nanoparticle with excellent blood stability and preparation method thereof
ActiveCN108542893BSimple ingredientsGood blood stabilityPharmaceutical non-active ingredientsAntineoplastic agentsDiseaseLactide
The invention belongs to the technical field of nano-medicines, and provides a nano-particle with an excellent blood stabilizing performance, and a preparation method thereof. Poly(glycolide-co-lactide) (PLGA), a medicine / probe molecule, distearoyl phosphoethanolamine-polyethylene glycol-maleimide (DSPE-PEG) and pluronic F127 (PF127) which are used as raw materials undergo a rapid nanoprecipitation technique to prepare the nano-particle having the advantages of controlled particle size, good monodispersity, and excellent stability and performances in various solutions. A large number of hydroxyl groups and negative charges on the surface of the particle have a protection effect, so the nano-particle do not bind to proteins in serum, thereby the protein is avoided from being absorbed and metabolized by the reticuloendothelial system; and the hydrophobic medicine and the hydrophobic or amphipathic probe are encapsulated, so the particle can significantly prolong the blood circulation half-life of the medicine and the probe, and provides a good medicine delivery platform for disease detection and treatment.
Owner:SUN YAT SEN UNIV
Erlotinib sustained-release implants for treating entity knub
InactiveCN101161234AIncreased sensitivityHigh clinical application valueOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
An erlotinib slow release implant for treating solid tumor is characterized in that the slow release implant contains anti-cancer effective amount erlotinib, slow release supplementary materials and some slow release regulator. The solid tumors comprise esophagus cancer, stomach cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer, liver cancer, lung cancer and colorectal cancer. The slow release supplementary materials mainly are one or their combination of co-polymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate), and poly (L-lactide-co-propyl phosphate), during its degradation and absorption, slow releasing erlotinib to tumor local, so it greatly decreases its whole body toxicity reaction, at the same time it also maintains effective medicine concentration of tumor local. The tumor local is arranged with the slow release implant, which can not only decrease whole body toxicity reaction of erlotinib, but also selectively promote medicine concentration of the tumor local, reinforce therapeutic effect of non-operation therapy such as chemotherapeutic drugs, radiotherapy and etc.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com