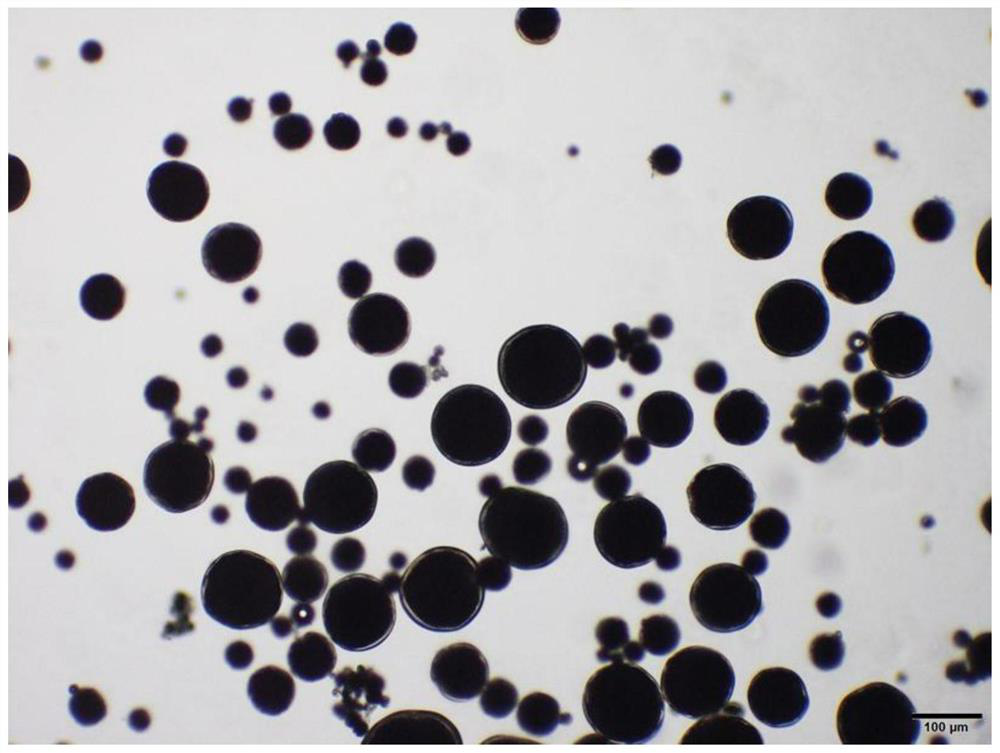
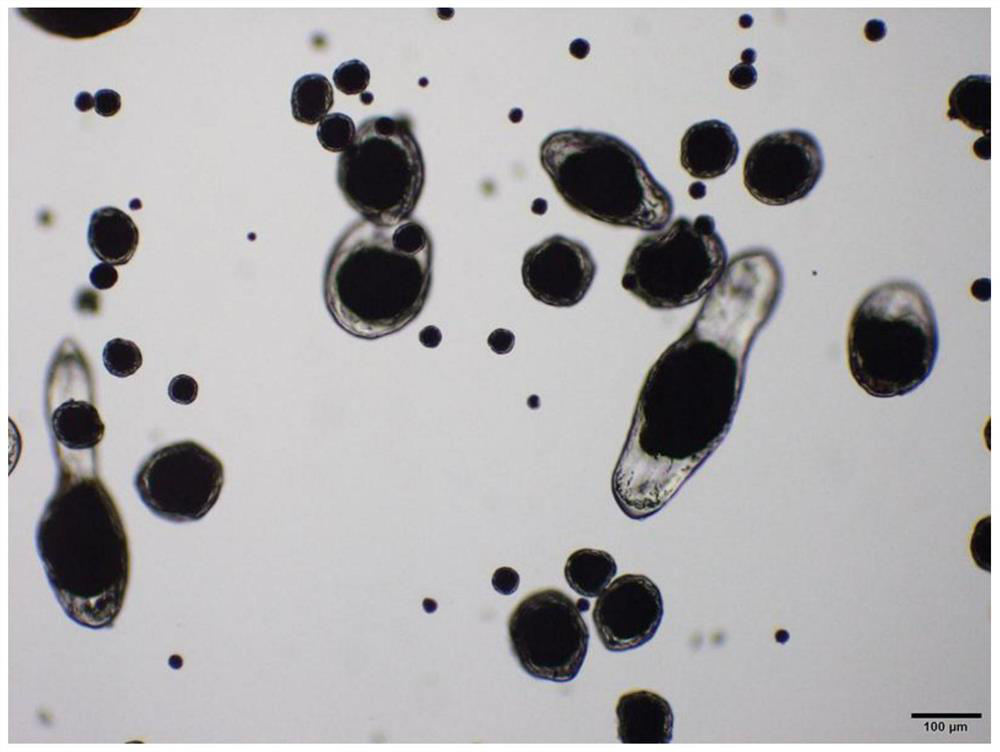
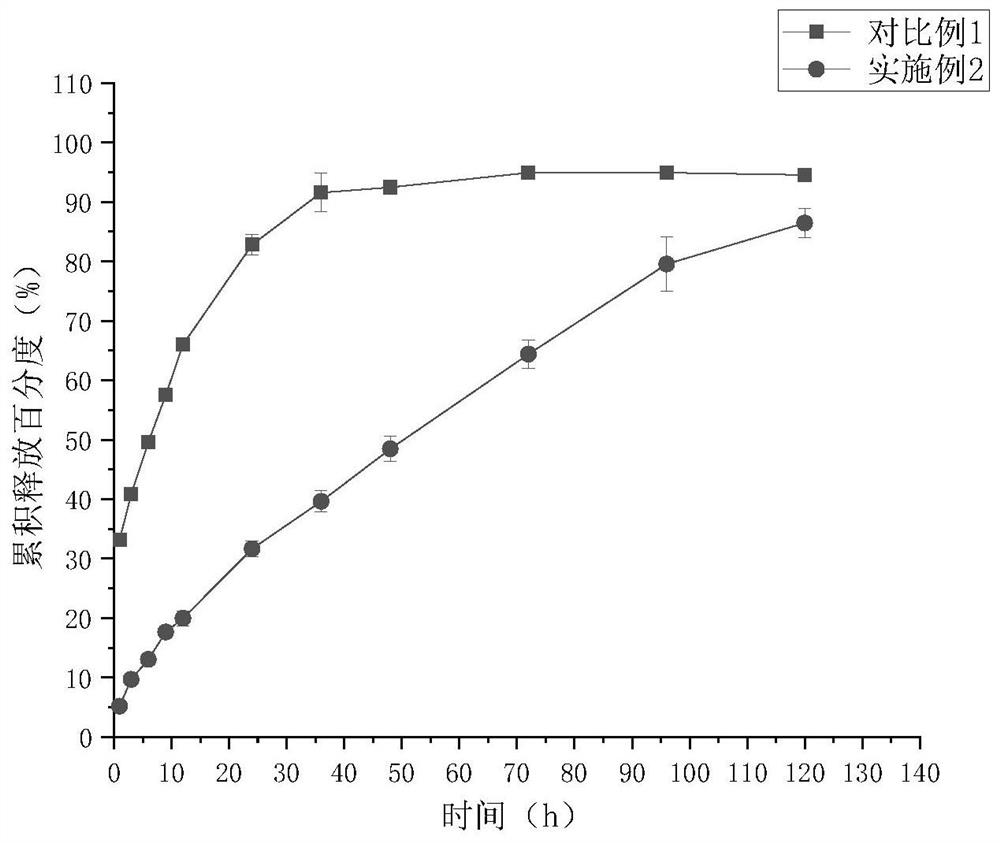
Rasagiline mesylate microsphere preparation and preparation method thereof
A technology of rasagiline mesylate and microspheres, which can be used in pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc. To solve problems such as poor stability of colostrum, achieve the effect of reducing initial release, increasing stability, and increasing viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 15 mg of rasagiline mesylate in 0.3 mL of 5% tannic acid solution to obtain an inner aqueous phase. 270 mg of PLGA (50 / 50, 0.36 dl / g, carboxyl-terminated) was dissolved in 3 ml of dichloromethane to obtain an oily phase. Under 12000rpm, the inner water phase and the oil phase were shear mixed to obtain the primary emulsion. Add the primary emulsion to the continuous external aqueous phase containing 0.8% sodium chloride and 1% PVA through a peristaltic pump, and homogeneously emulsify for 10 minutes under stirring at 900 rpm to form double emulsion. Subsequently, the stirring speed was reduced to 300 rpm for 6 hours, and the organic solvent was volatilized and removed. The microspheres were collected by filtration, washed three times with deionized water, and freeze-dried to obtain microsphere powder.
Embodiment 2
[0031] The microsphere preparation steps and basic prescription composition of this example are the same as those in Example 1, but the mass percent concentration of the tannic acid solution that dissolves the drug to form the inner water phase is 2%.
Embodiment 3
[0033] The microsphere preparation steps and basic prescription composition of this example are the same as Example 1, but the mass percent concentration of the tannic acid solution that dissolves the drug to form the inner water phase is 1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com