Gefitinib sustained-release implantation agent for curing entity tumour
A slow-release implant, gefitinib technology, applied in the field of medicine, can solve the problems of systemic toxicity and side effects limiting clinical application, limiting clinical application, cardio-liver-pulmonary system toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
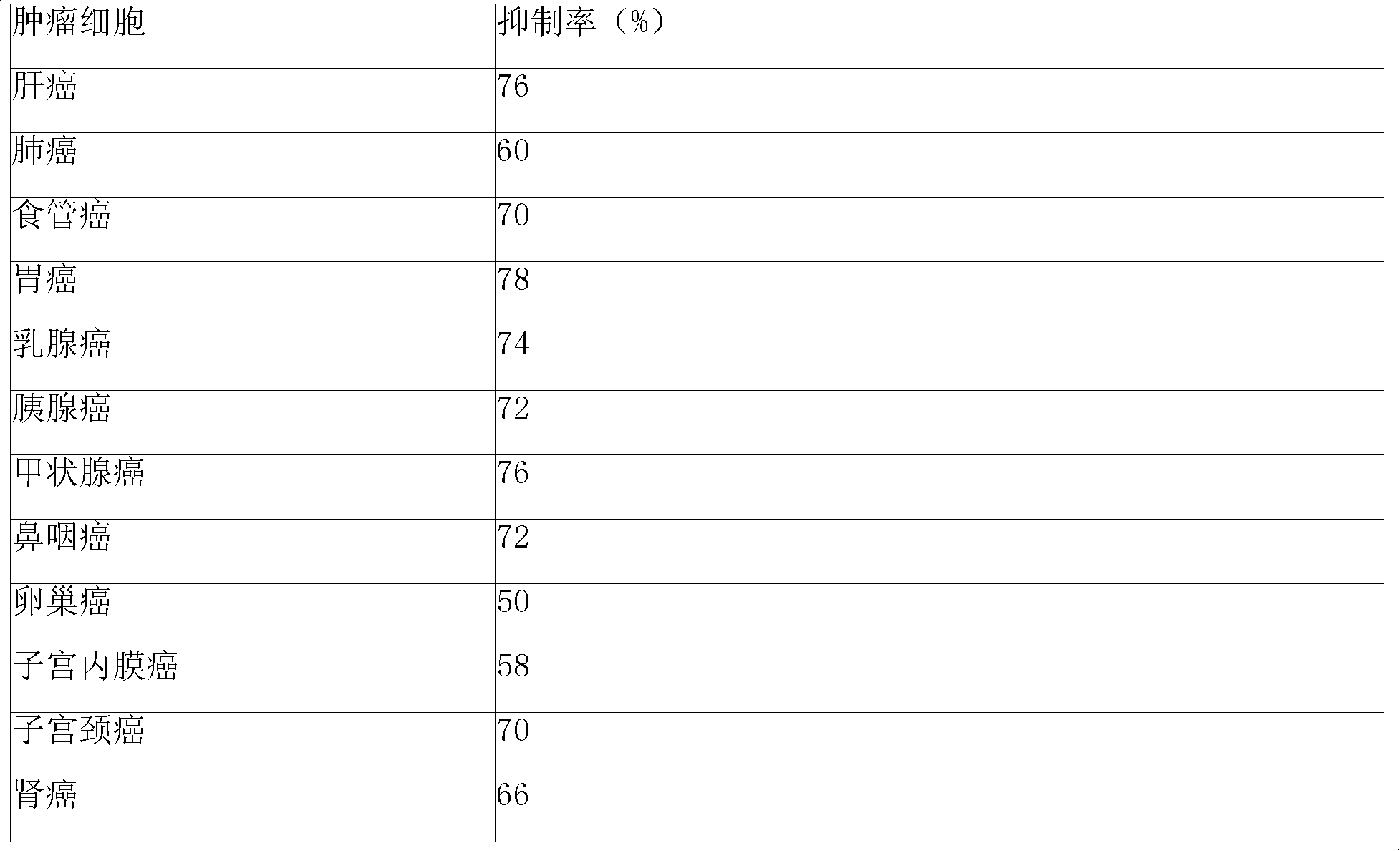
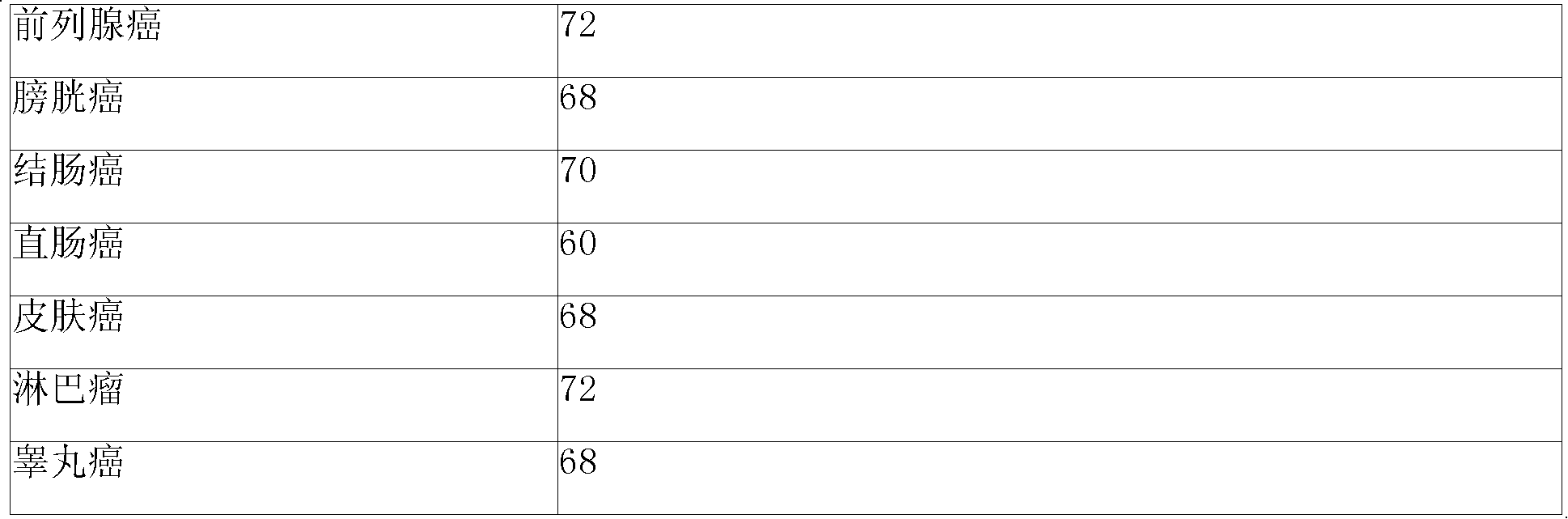
Image
Examples
Embodiment 1
[0085] Put the weighed (90mg) sustained-release excipient (polylactic acid (PLA) with a molecular weight of 15000-30000) into the container, add a certain amount of organic solvent to dissolve and mix (subject to full dissolution), then add 10mg For Fitinib, re-shake well and dry in vacuo to remove organic solvents. The dried solid composition is shaped immediately, subpackaged and sterilized by radiation to obtain a slow-release implant containing 10% gefitinib. The release time of the sustained-release implant in physiological saline in vitro is 20-25 days, and the release time in mouse subcutaneous is 22-26 days.
Embodiment 2
[0087] Sustained-release implants were made according to the method described in Example 1, but the anti-cancer active ingredients contained were one of the following:
[0088] (A) 1% gefitinib and 99% polylactic acid;
[0089] (B) 5% gefitinib and 95% polylactic acid;
[0090] (C) 10% gefitinib and 90% polylactic acid;
[0091] (D) 15% gefitinib and 85% polylactic acid;
[0092] (E) 20% gefitinib and 80% polylactic acid.
Embodiment 3
[0094]Put the weighed (80mg) sustained-release excipient (PLGA with a molecular weight of 15000-25000, 50:50) into the container, add a certain amount of organic solvent to dissolve and mix (subject to full dissolution), then add 20mg of Gefid Tini, re-shake well and dry in vacuo to remove organic solvents. The dried solid composition is shaped immediately, subpackaged and sterilized by radiation to obtain a slow-release implant containing 20% gefitinib. The release time of the slow-release implant in physiological saline in vitro is 24-30 days, and the release time in mouse subcutaneous is 25-34 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com