Nano-particle with excellent blood stabilizing performance, and preparation method thereof
A nanoparticle and performance-stabilizing technology, applied in the field of nanoparticles and their preparation, can solve the problems of limiting the use of nano-drugs and the inability to significantly improve the bioavailability of nano-drugs, achieving good blood stability and improving blood circulation stability. and highly controllable effect of cycle time and particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of nanoparticles loaded with indocyanine green (ICG) by rapid nanoprecipitation technique
[0036] In this example, ICG-loaded nanoparticles with three particle sizes were prepared by adjusting the volume ratio of the aqueous phase / organic phase and the liquid flow rate of the channel. The specific implementation steps are as follows:
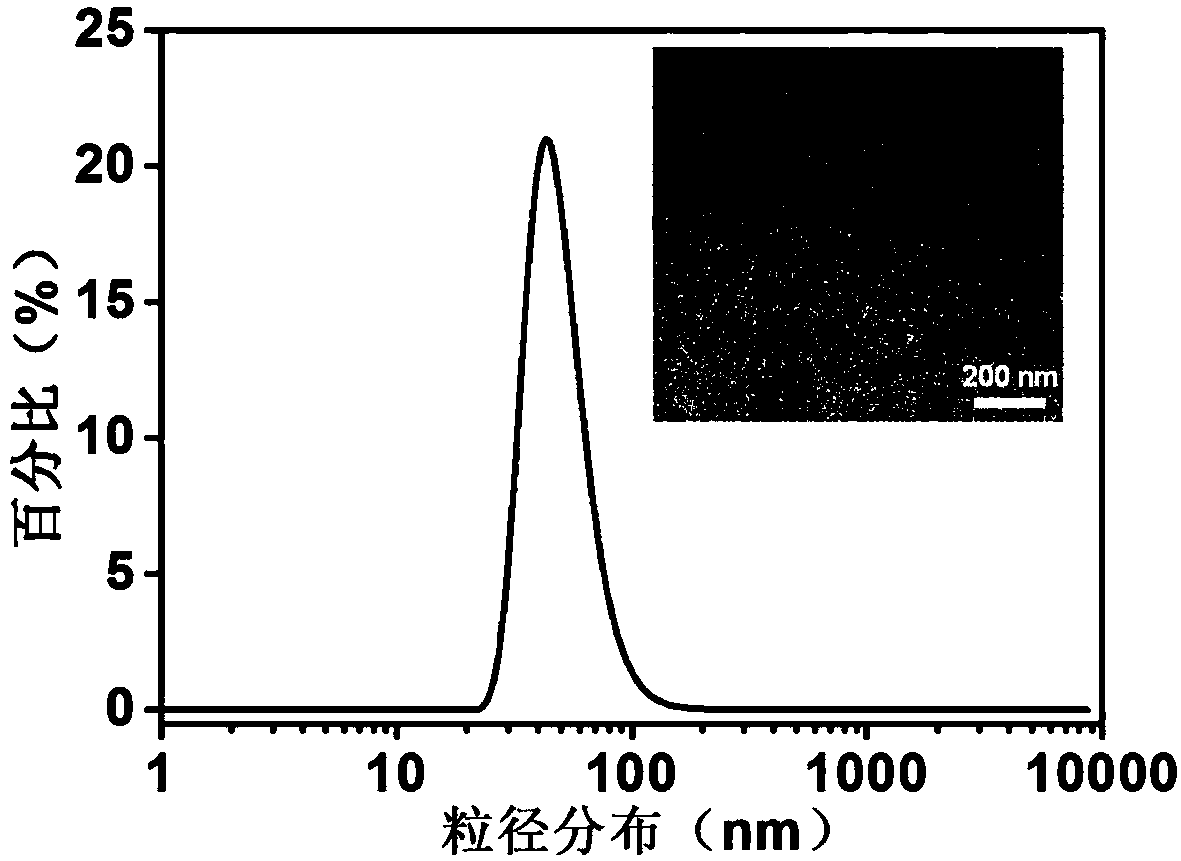
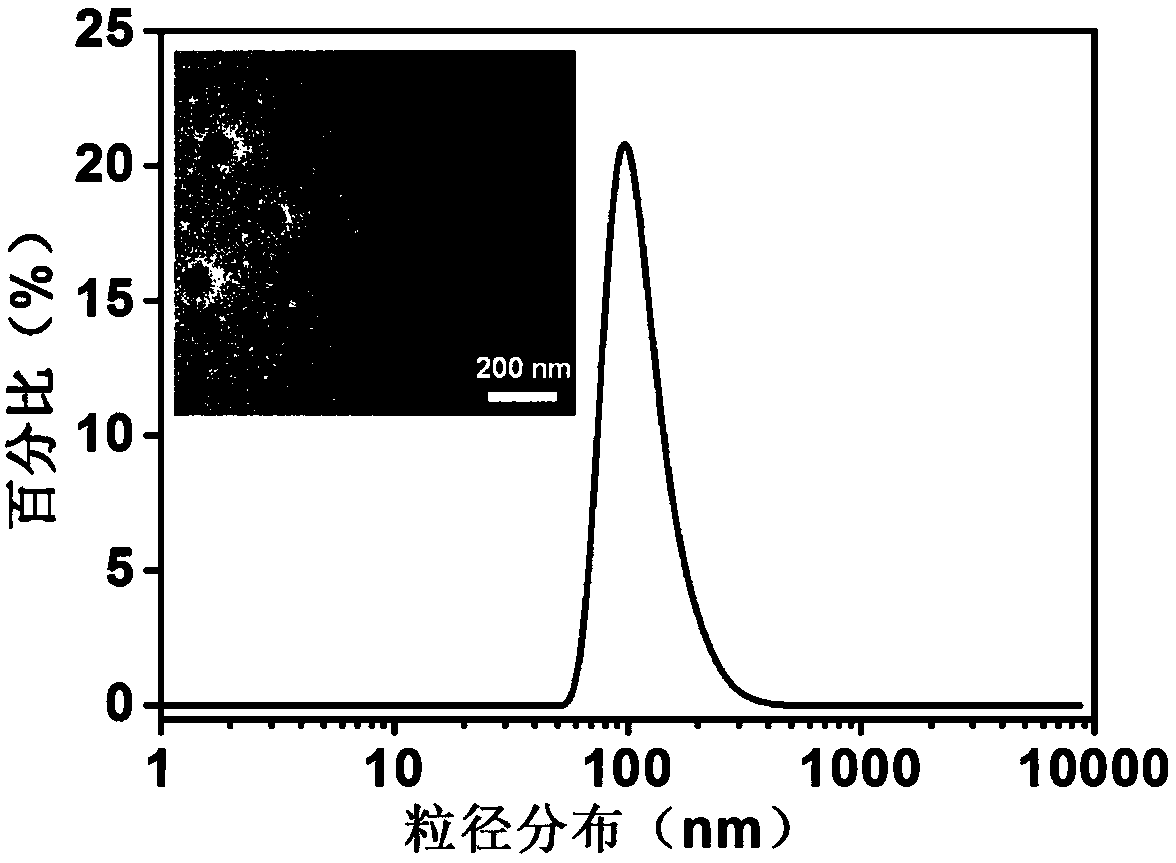
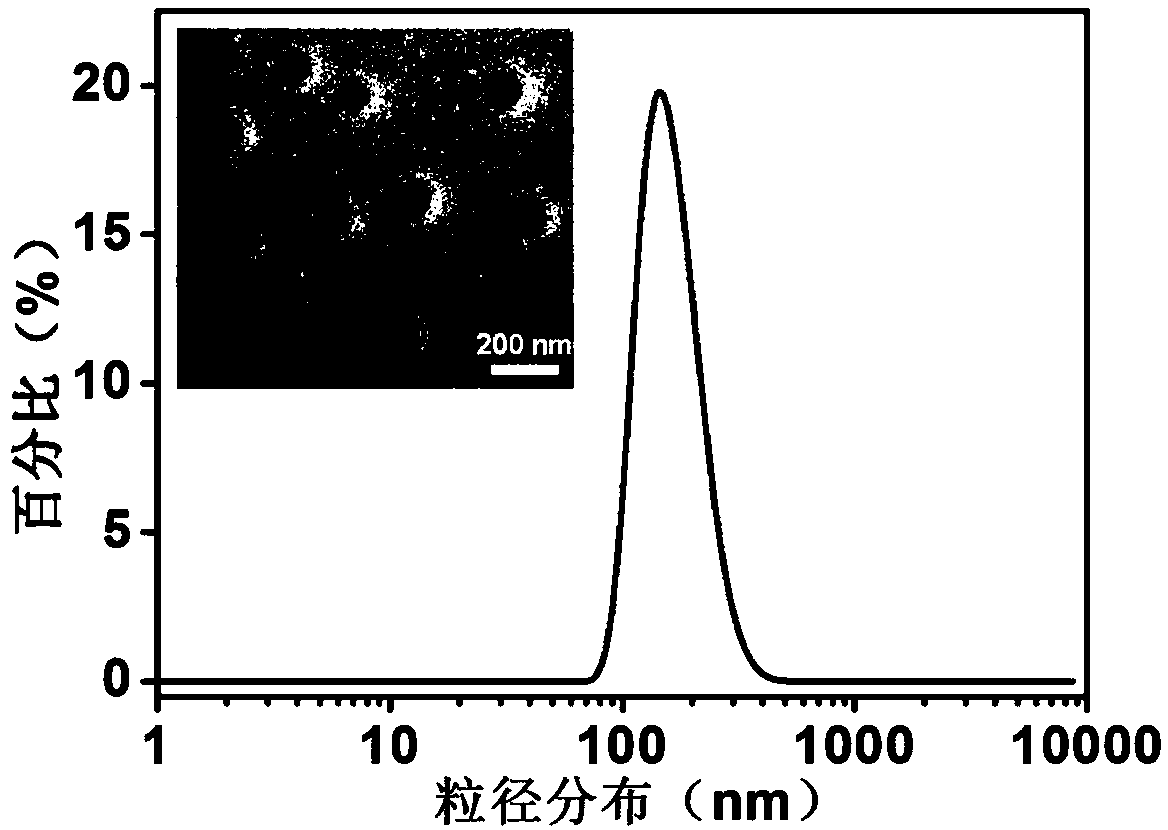
[0037]1. The specific implementation steps of nanoparticle 1: ① Dissolve 6 mg PLGA and 0.6 mg ICG in 3 mL organic solvent acetonitrile, vortex and mix well; dissolve 15 mg PF127 and 1 mg DSPE-PEG in 3 mL ultrapure water, Shake and mix; ② Acetonitrile solution containing PLGA and ICG passes through channel 1 (flow rate 10 mL / min), aqueous solution containing PF127 and DSPE-PEG passes through channel 2 (flow rate 10 mL / min), 12 mL ultrapure water passes through channel respectively 3 and 4 (both flow rates are 40 mL / min), transported to a multi-channel vortex mixer for high-speed mixing and solvent exchange to obtain ...
Embodiment 2
[0041] Example 2 Verification of the system stability of nanoparticles in phosphate buffered saline (PBS) and DMEM medium (containing 10% fetal bovine serum)
[0042] In this example, taking the nanoparticles 1, 2, and 3 prepared in Example 1 as examples, the particle size stability and entrapped fluorescence of the three nanoparticles in PBS and DMEM medium (containing 10% fetal bovine serum) were explored. The fluorescence stability of the probe, the specific implementation steps are as follows:
[0043] Take the solutions of NP-1, NP-2 and NP-3 loaded with ICG, add the three solutions into PBS (pH=7.4) and DMEM medium containing 10% fetal bovine serum) respectively, and incubate at 37°C For 5 days, track and test the particle size of the nanoparticles and the fluorescence intensity value of the particles at 810nm every day, the results are as follows Figure 4~5 shown.
Embodiment 3
[0044] Example 3 Detection of long-term circulation stability of nanoparticles in blood
[0045] This example aims to detect the half-life of nanoparticles in blood, and provide basic research evidence for the biological application of nanoparticles to prolong the effective delivery of drugs or molecular probes. The specific implementation steps are as follows:
[0046] Twelve BALB / c mice were randomly divided into 4 groups, 3 mice in each group; ②Inject free ICG, NP-1, NP-2 and NP-3 into the mice through tail vein injection; ③In the Collect 0.1 mL of mouse blood at 5 / 15 / 30 minutes and 1 / 2 / 4 / 8 / 16 / 24 / 48 hours, and mix it well with 0.1 mL PBS (containing 1 mg / mL heparin sodium) for detection; ④ according to For the absorption peak of ICG at 780 nm, use a multifunctional microplate reader to detect the absorption value of the sample at 780 nm; ⑤ Prepare ICG concentrations of 10 -5 mg / mL, 5×10 -5 mg / mL, 2×10 -4 mg / mL, 10 -3 mg / mL and 5×10 -3 mg / mL PBS solution with 5 gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface potential | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com