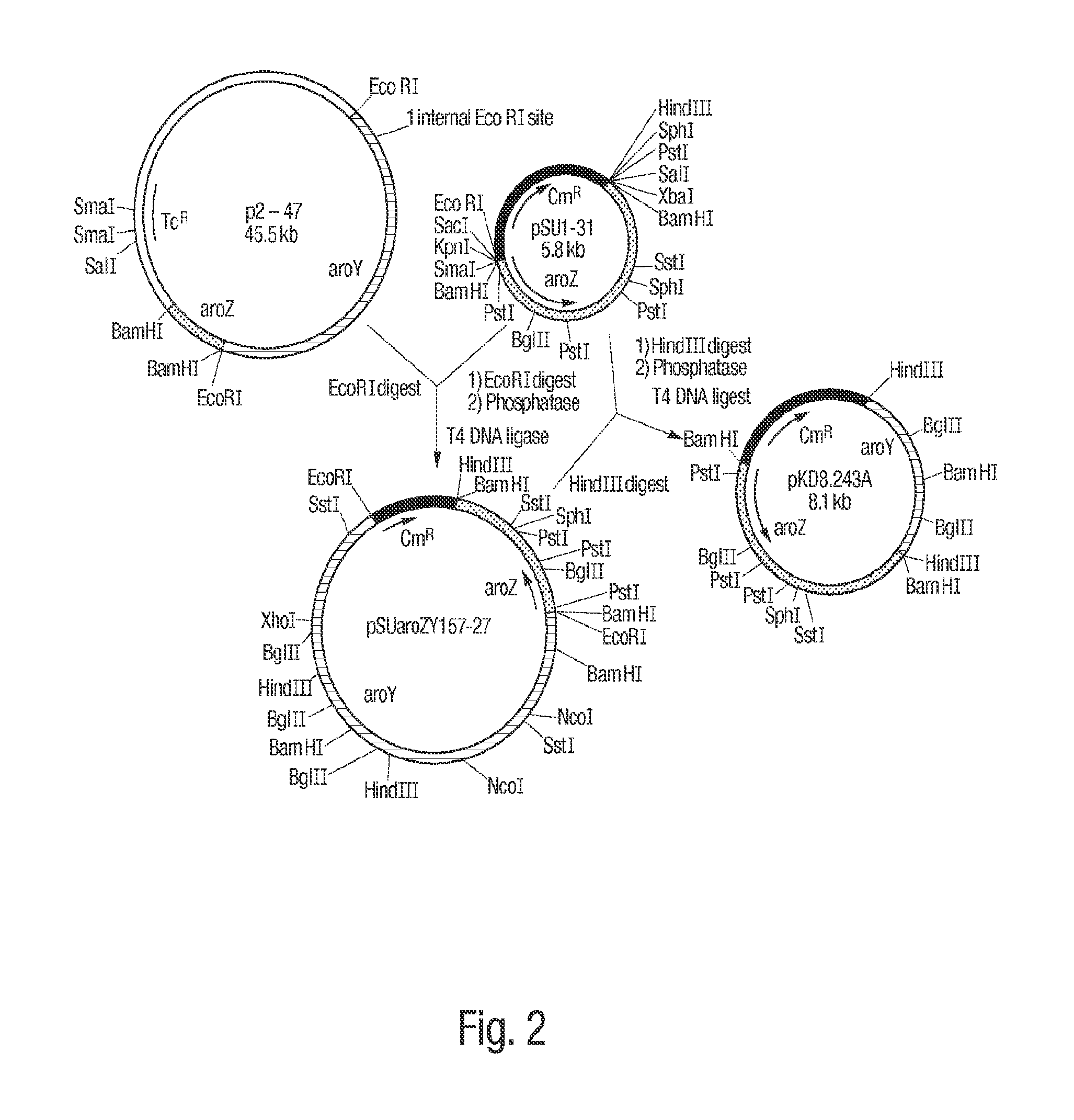
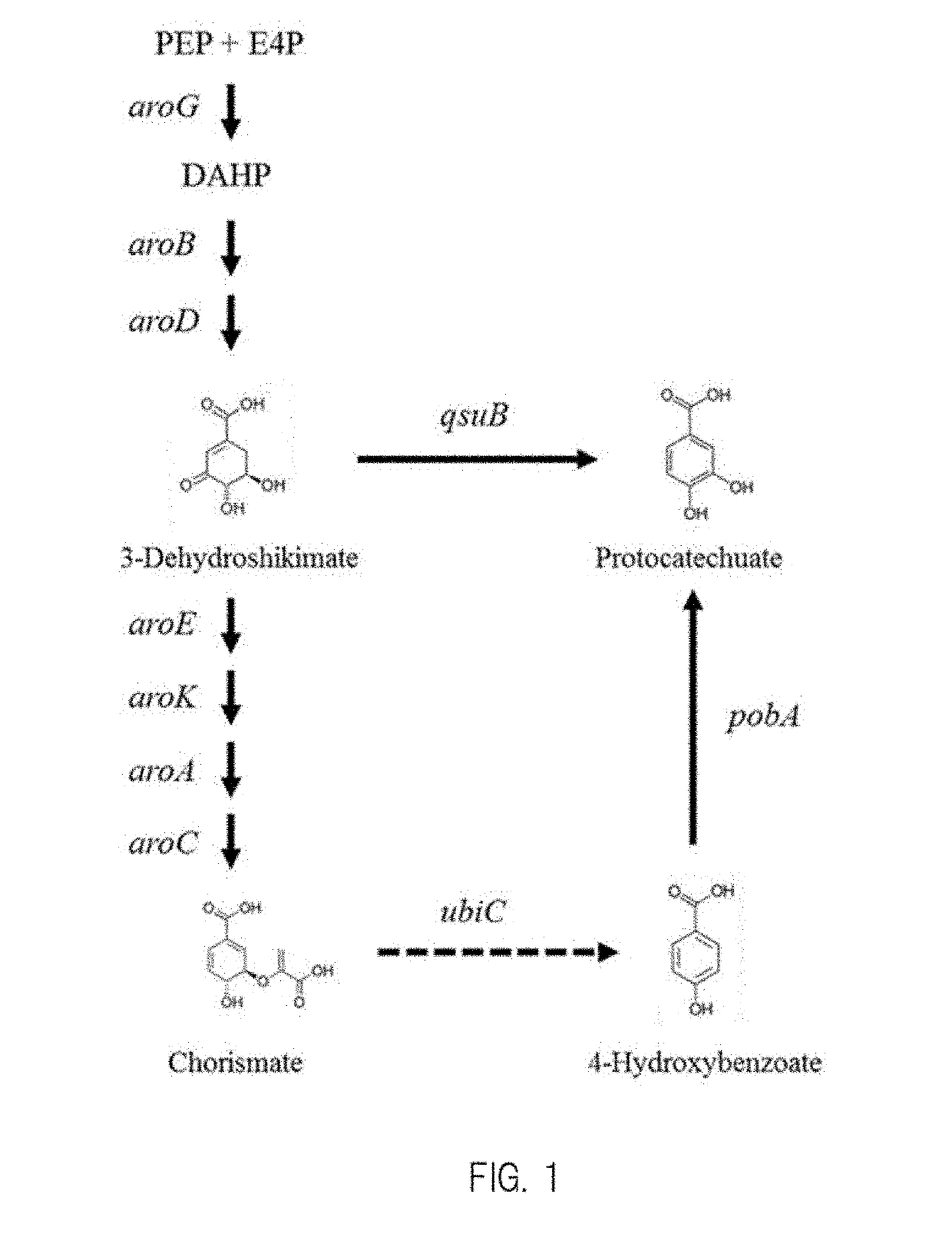
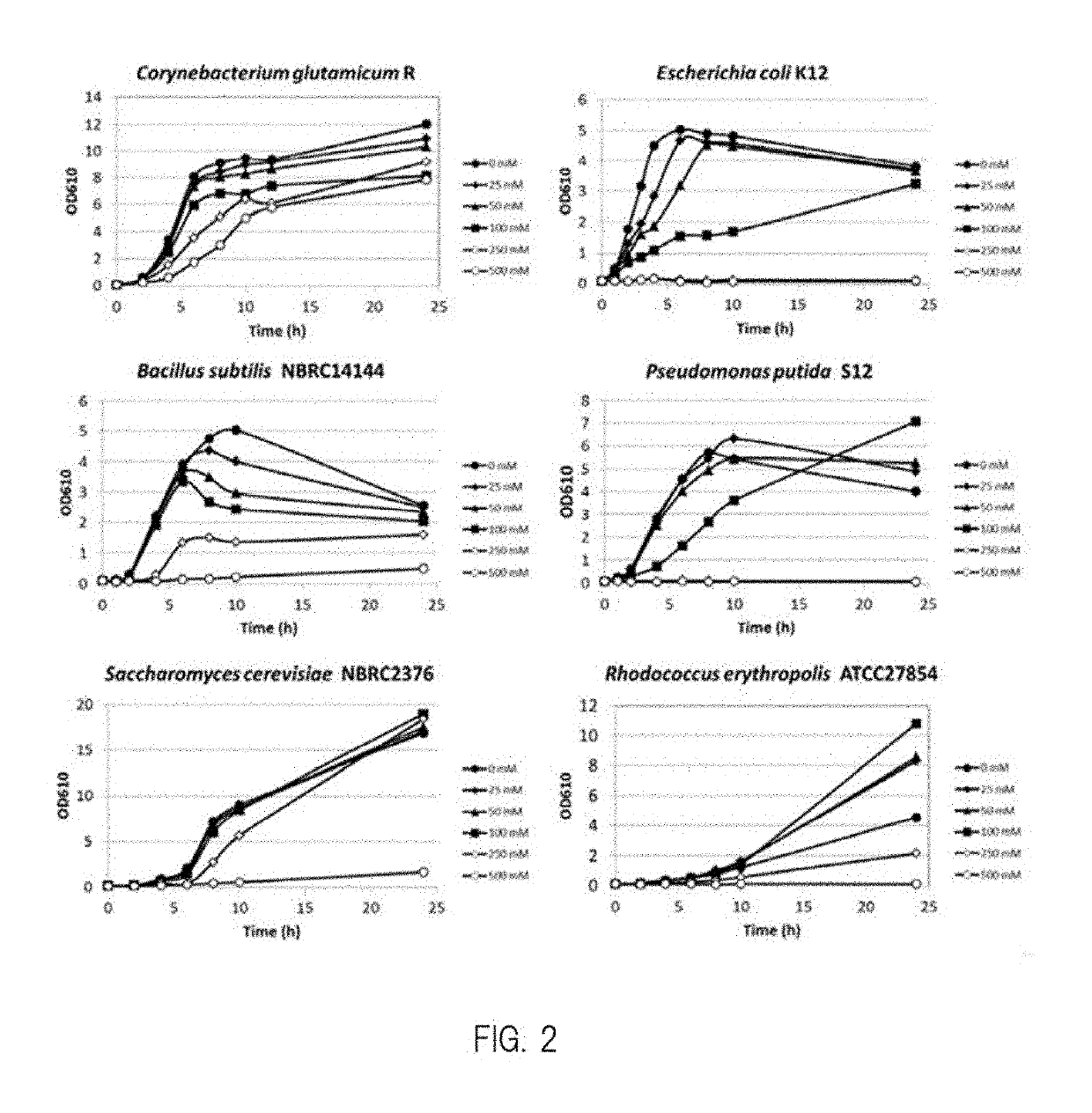
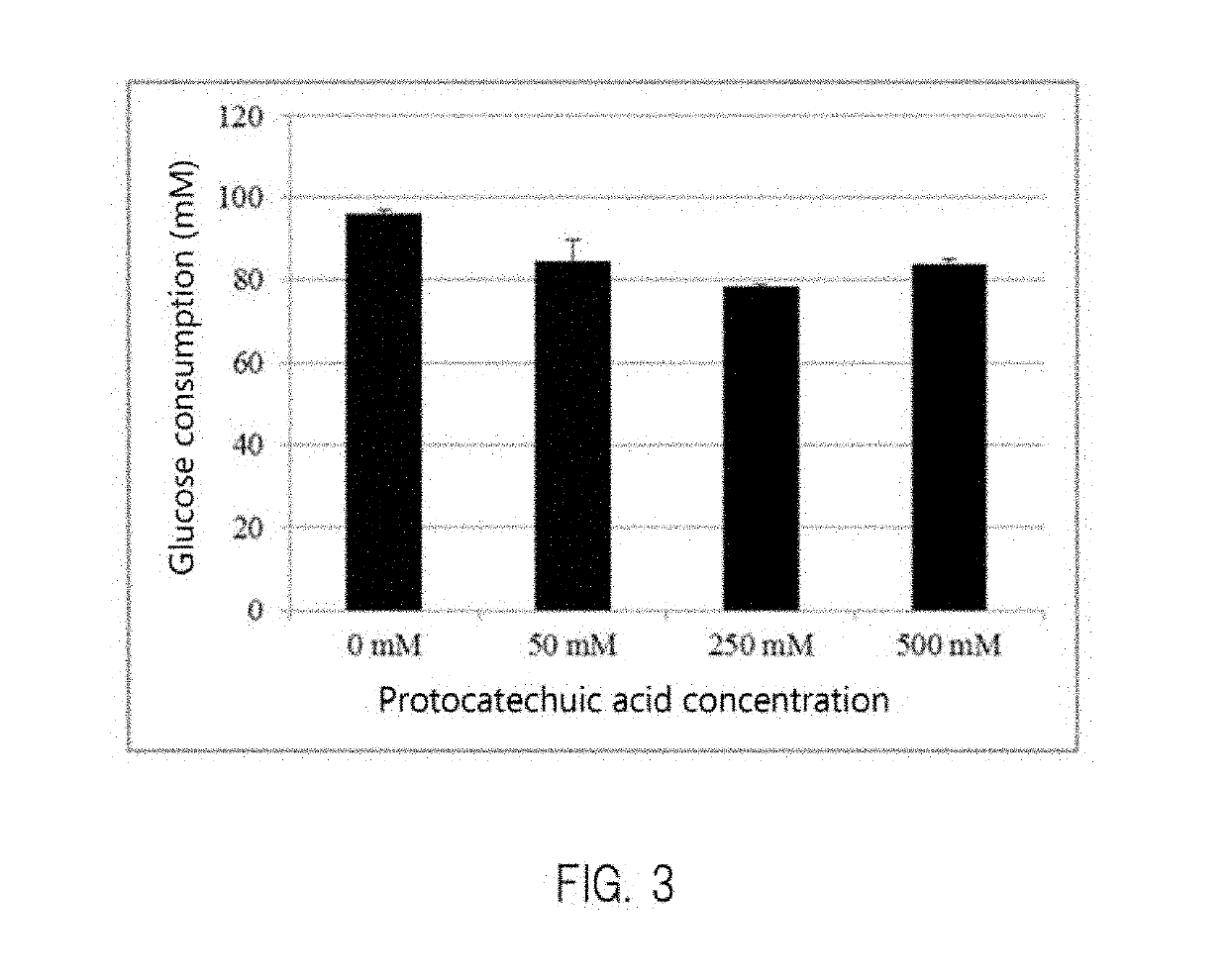
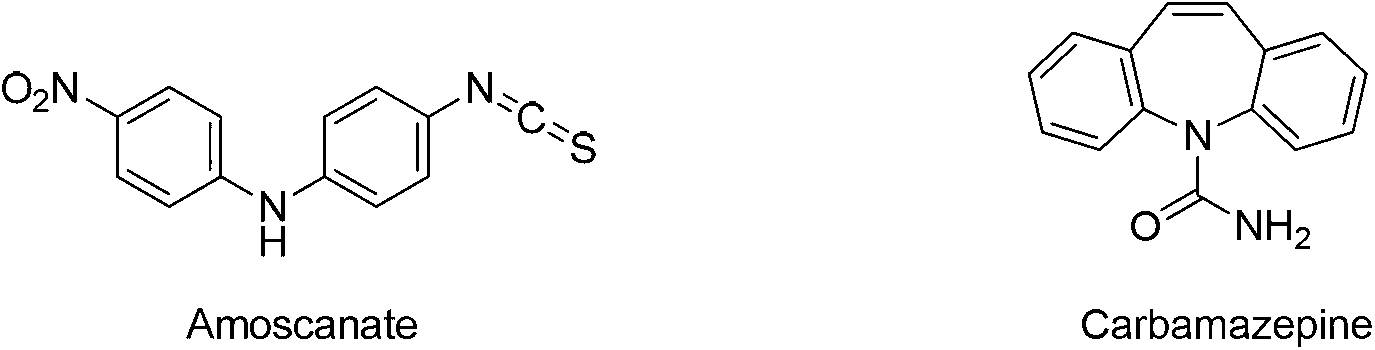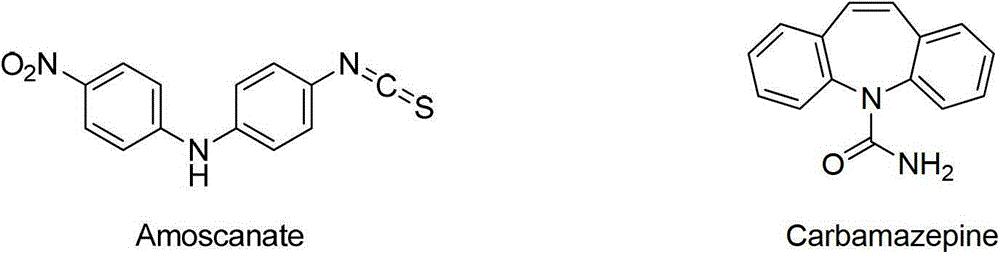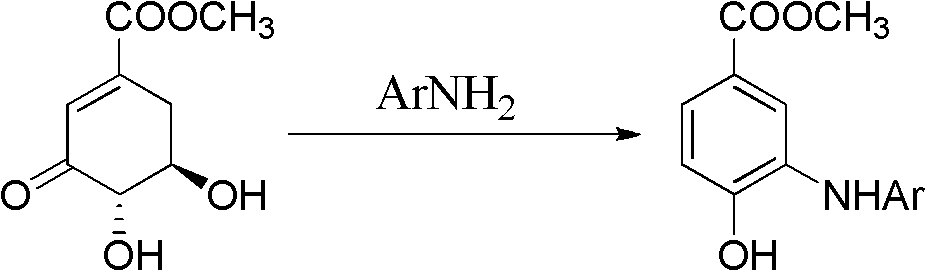Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "3-Dehydroshikimic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
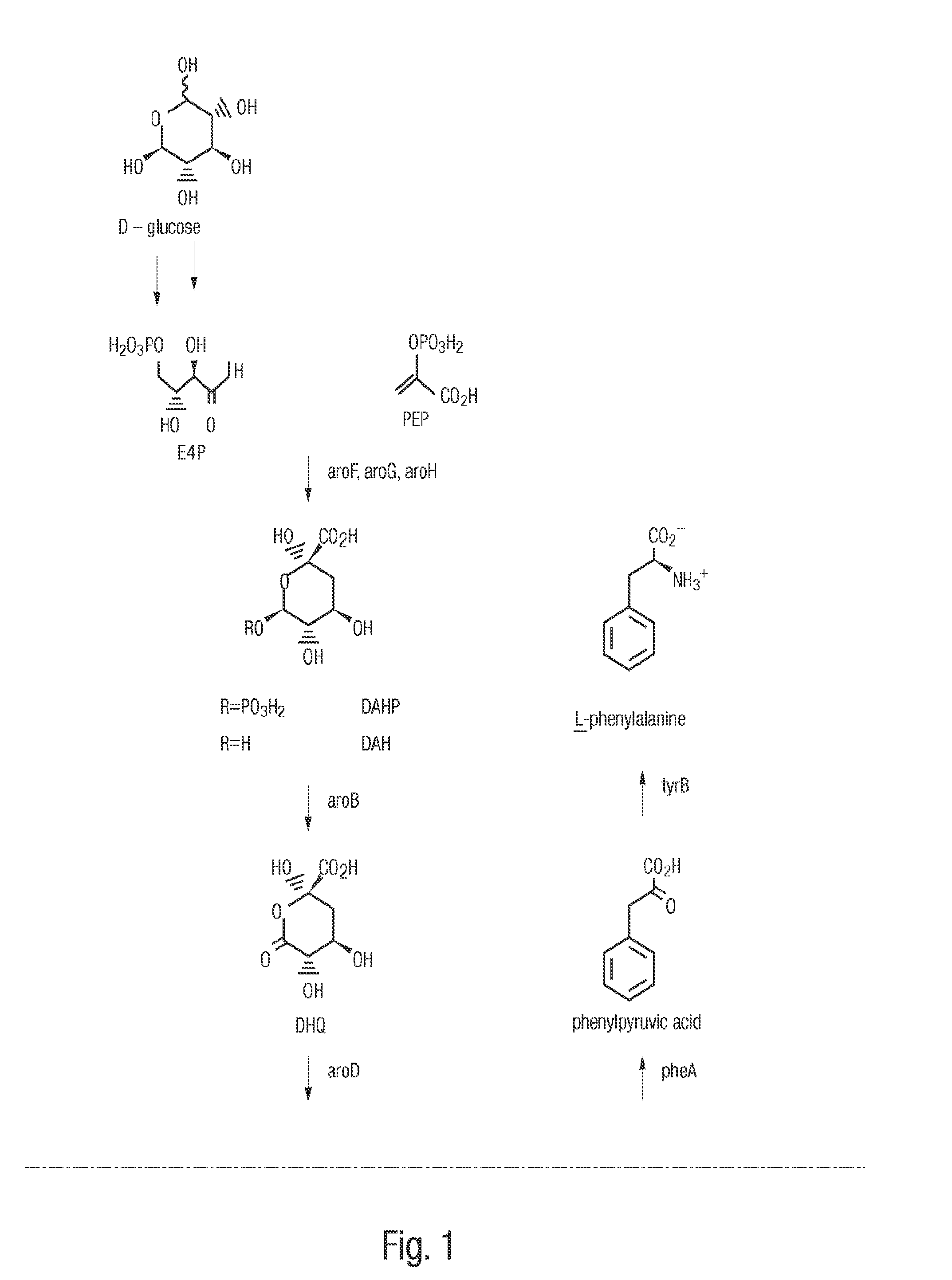
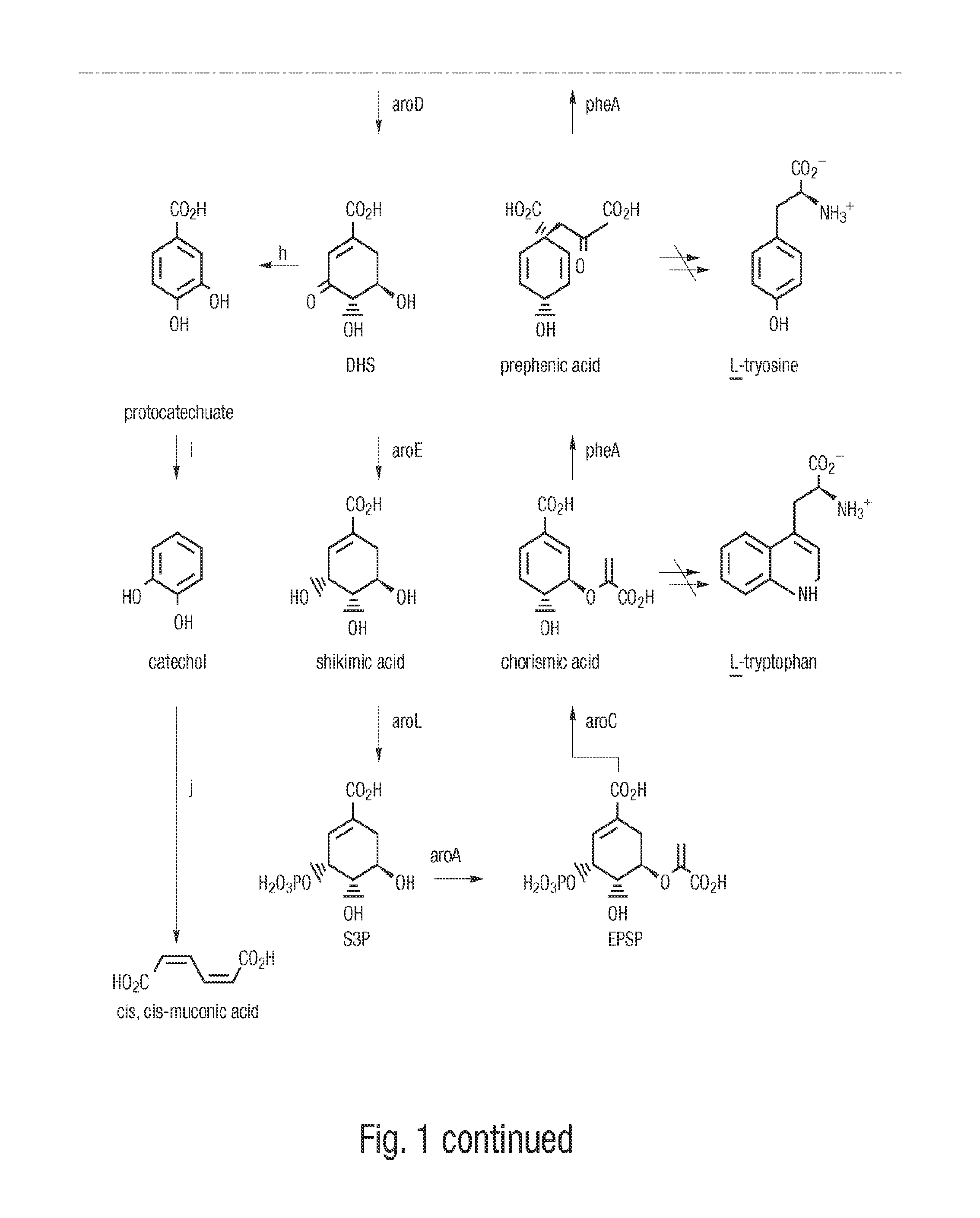
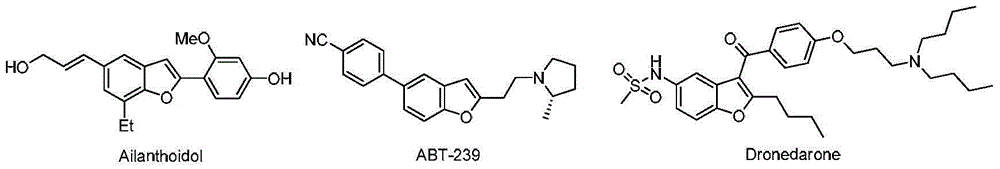
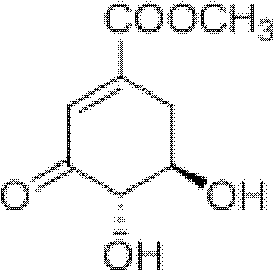
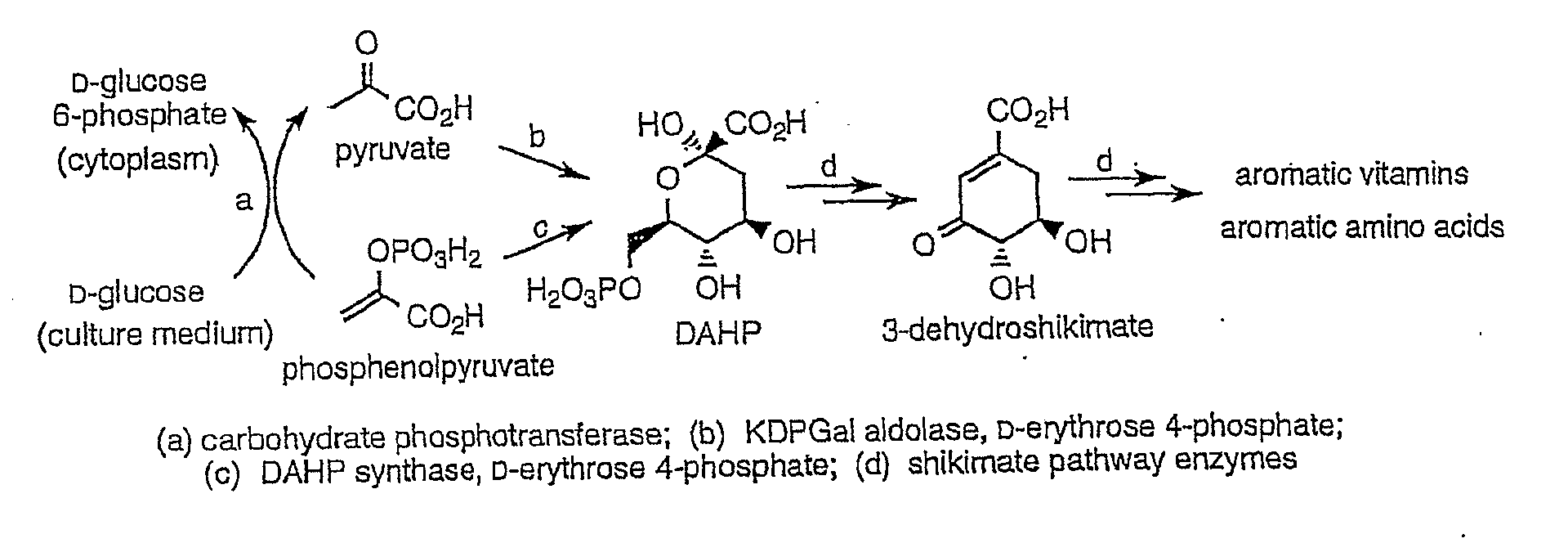
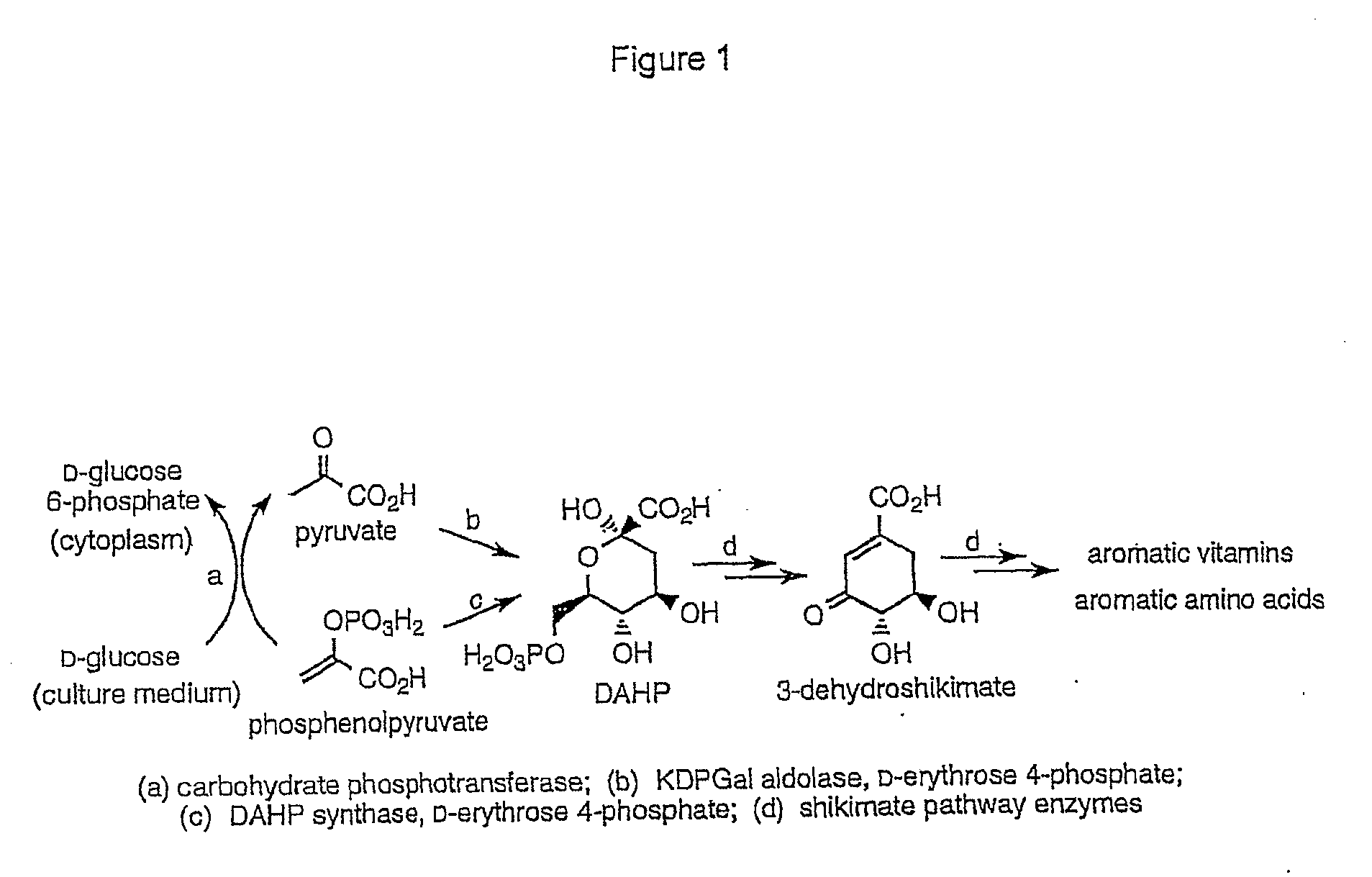
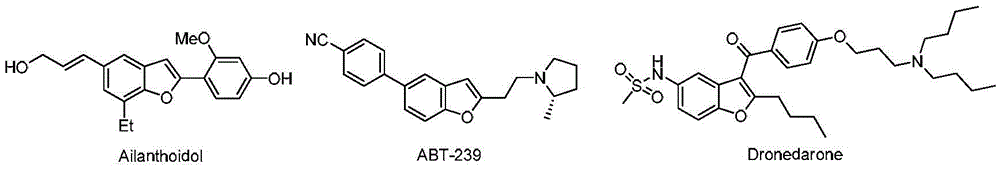
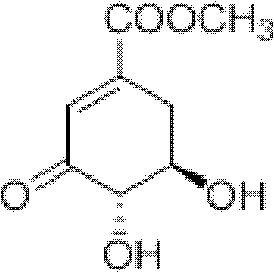
3-Dehydroshikimic acid is a chemical compound related to shikimic acid. 3-DHS is available in large quantity through engineering of the shikimic acid pathway.
Methods for Producing Isomers of Muconic Acid and Muconate Salts
ActiveUS20130030215A1Improve solubilityUnique utilityOrganic compound preparationCarboxylic compound preparationProtocatechuate decarboxylaseGluconic acid
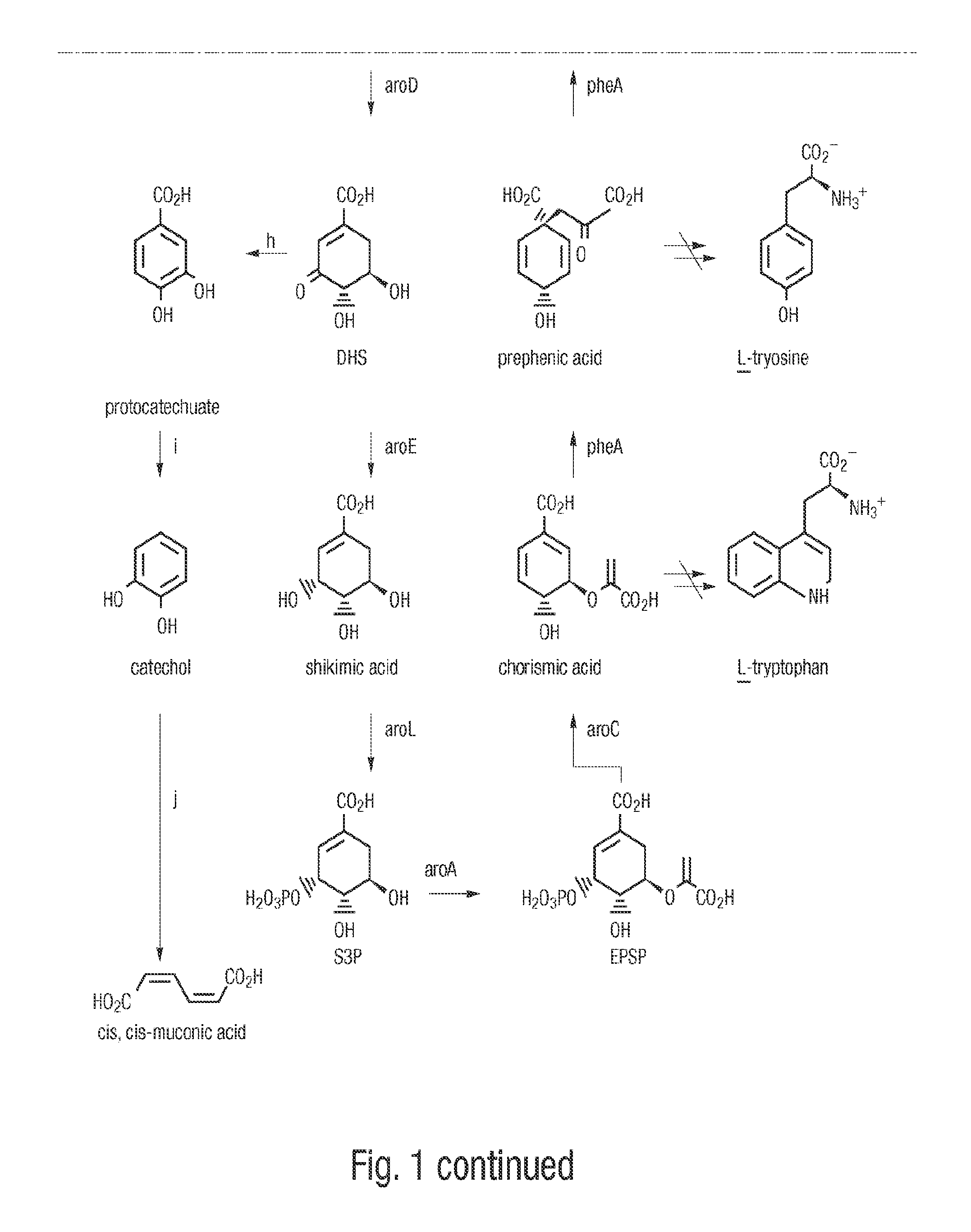
A method for producing cis,trans- and trans,trans-isomers of muconate by providing cis,cis-muconate produced from a renewable carbon source through biocatalytic conversion; isomerizing cis,cis-muconate to cis,trans-muconate under reaction conditions in which substantially all of the cis,cis-muconate is isomerized to cis,trans-muconate; separating the cis,trans-muconate; and crystallizing the cis,trans-muconate. The cis,trans-isomer can be further isomerized to the trans,trans-isomer. In one example, the method includes culturing recombinant cells that express 3-dehydroshikimate dehydratase, protocatechuate decarboxylase and catechol 1,2-dioxygenase in a medium comprising the renewable carbon source and under conditions in which the renewable carbon source is converted to 3-dehydroshikimate by enzymes in the common pathway of aromatic amino acid biosynthesis of the cell, and the 3-dehydroshikimate is biocatalytically converted to cis,cis-muconate.
Owner:AMYRIS INC
Methods and materials for the production of shikimic acid
InactiveUS7790431B2BacteriaSugar derivatives3-dehydroquinate synthase3-deoxy-D-arabino-heptulosonate-7-phosphate
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Escherichia coli for producing adipic acid precursor namely cis,cis-muconic acid and application of escherichia coli
Cis,cis-muconic acid is an important chemical organic acid, has a wide range of application and can be used as a precursor of adipic acid. The cis,cis-muconic acid can be used for producing polymers including nylon 66 and the like after being converted into adipic acid through hydrogenation. The invention provides a recombinant escherichia coli TIB-LEc340 CGMCC No 6435 for producing cis,cis-muconic acid, wherein the recombinant escherichia coli is established by the steps of combining 3-dehydrogenated shikimic acid dehydrase which comes from different microbes and is optimized by codons, protocatechuic acid decarboxylase, catechol 1,2-dioxidase and artificially designed constitutive promoters and terminators into a heterologous expression module, and then introducing the heterologous expression module into shikimic acid dehydrogenase mutated escherichia coli. The escherichia coli TIB-LEc340 CGMCC No 6435 is fermented for 16-120 hours at 30-40 DEG C to obtain fermentation liquor, and the maximum content of cis,cis-muconic acid in the fermentation liquor can reach 52g / L, so that the escherichia coli has a wide application prospect.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Method for preparing epicatechol gallate and protocatechuic acid ester compounds from natural shikimic acid
InactiveCN102010338AIncrease productionStable sourceOrganic compound preparationCarboxylic acid esters preparationBenzoic acidAromatization
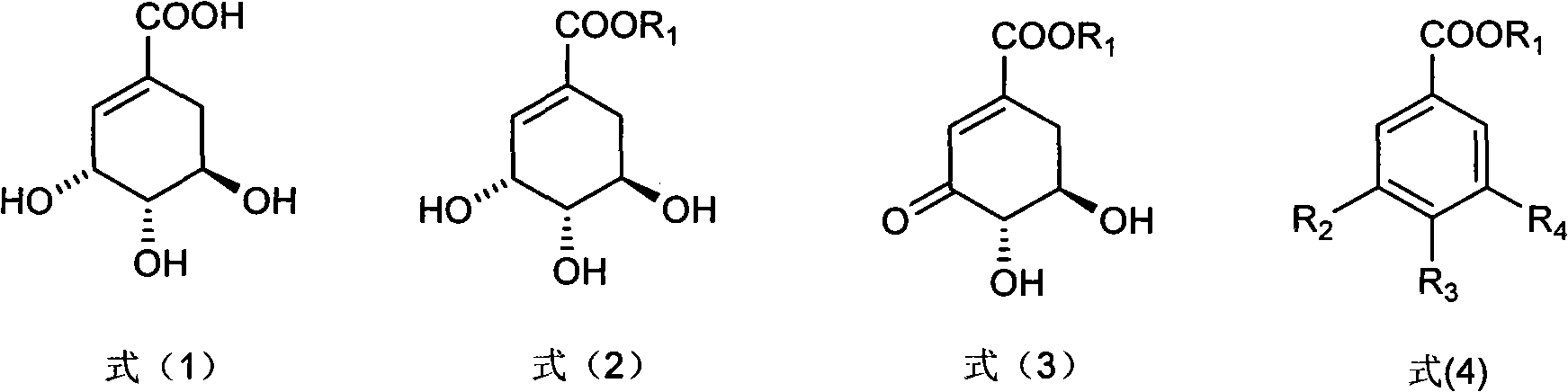
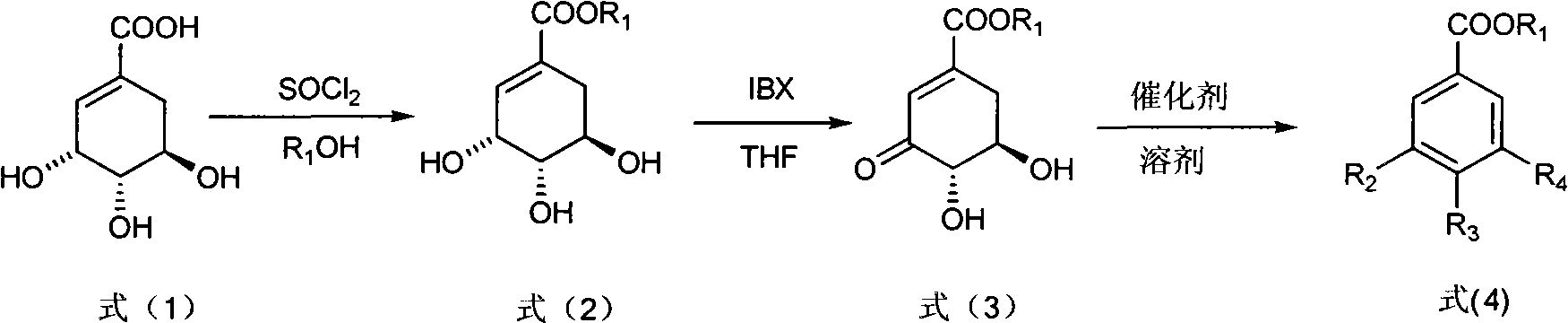
The invention discloses a method for preparing epicatechol gallate and protocatechuic acid ester compounds from a natural shikimic acid. The method comprises the following steps of: performing an esterification reaction of the shikimic acid serving as a raw material to obtain shikimic acid ester; obtaining 3-dehydro-shikimic acid ester under the action of 2-iodyl benzoic acid; and realizing aromatization under the action of a catalyst to obtain epicatechol gallate or protocatechuic acid ester compound. A renewable raw material serves as a starting material and a polyhydroxy substituted benzoic acid is formed by a brand new method, so the method has the advantages of simpleness in operation, mild reaction condition, high yield, capacity of realizing sustainable development and the like.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
Methods for producing isomers of muconic acid and muconate salts
ActiveUS8809583B2Improve solubilityUnique utilityOrganic compound preparationOxidoreductasesProtocatechuate decarboxylaseDioxygenase
A method for producing cis,trans- and trans,trans-isomers of muconate by providing cis,cis -muconate produced from a renewable carbon source through biocatalytic conversion; isomerizing cis,cis-muconate to cis,trans-muconate under reaction conditions in which substantially all of the cis,cis-muconate is isomerized to cis,trans -muconate; separating the cis,trans-muconate; and crystallizing the cis,trans-muconate. The cis,trans-isomer can be further isomerized to the trans,trans-isomer. In one example, the method includes culturing recombinant cells that express 3-dehydroshikimate dehydratase, protocatechuate decarboxylase and catechol 1,2-dioxygenase in a medium comprising the renewable carbon source and under conditions in which the renewable carbon source is converted to 3-dehydroshikimate by enzymes in the common pathway of aromatic amino acid biosynthesis of the cell, and the 3-dehydroshikimate is biocatalytically converted to cis,cis-muconate.
Owner:AMYRIS INC
Transformant, and method for producing protocatechuic acid or salt thereof using same
ActiveUS20190119664A1High activityIncrease productionMicroorganismsRecombinant DNA-technologyMicroorganismChorismate pyruvate lyase
Provided is a microorganism that is able to efficiently produce protocatechuic acid or a salt thereof by using a saccharide as a raw material, and a method of efficiently producing protocatechuic acid or a salt thereof by using the microorganism.Provided is a transformant having protocatechuic acid producing ability, subjected to modifications (A), (B), and (C) below:(A) enhancement of 3-dehydroshikimate dehydratase activity;(B) enhancement of chorismate pyruvate lyase activity; and(C) enhancement of 4-hydroxybenzoate hydroxylase activity.Also provided is a method of producing protocatechuic acid or a salt thereof, including the step of culturing the transformant in a reaction solution containing a saccharide so as to cause the transformant to produce protocatechuic acid or a salt thereof.
Owner:RES INST OF INNOVATIVE TECH FOR THE EARTH +1
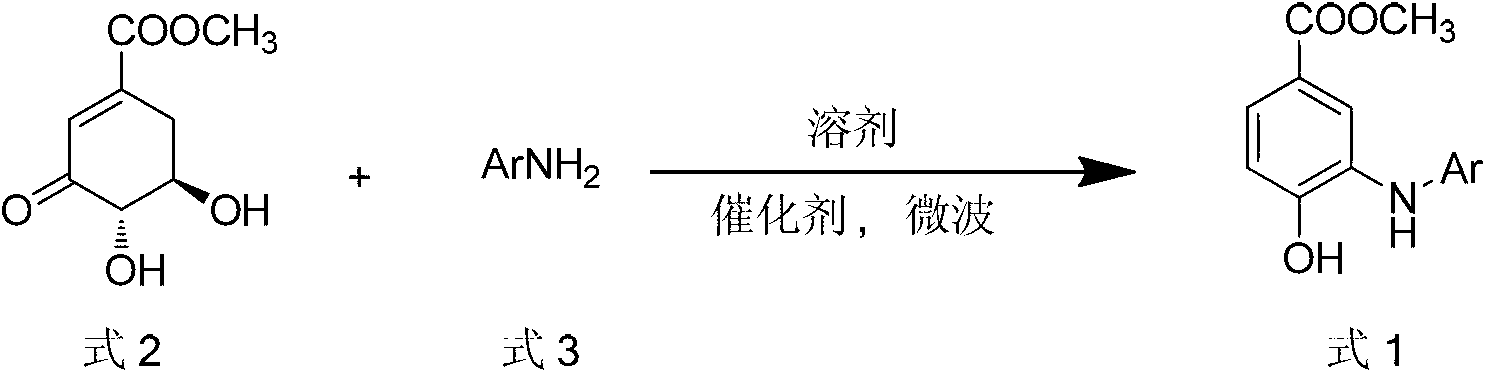
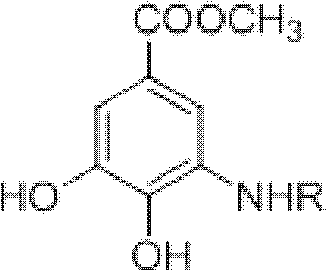
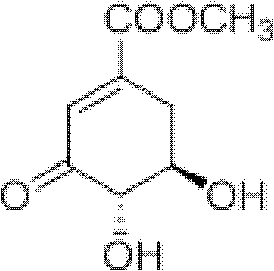
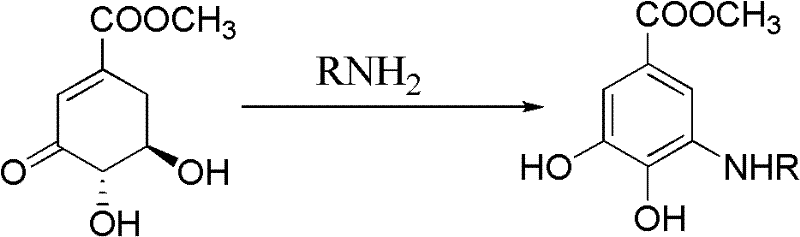
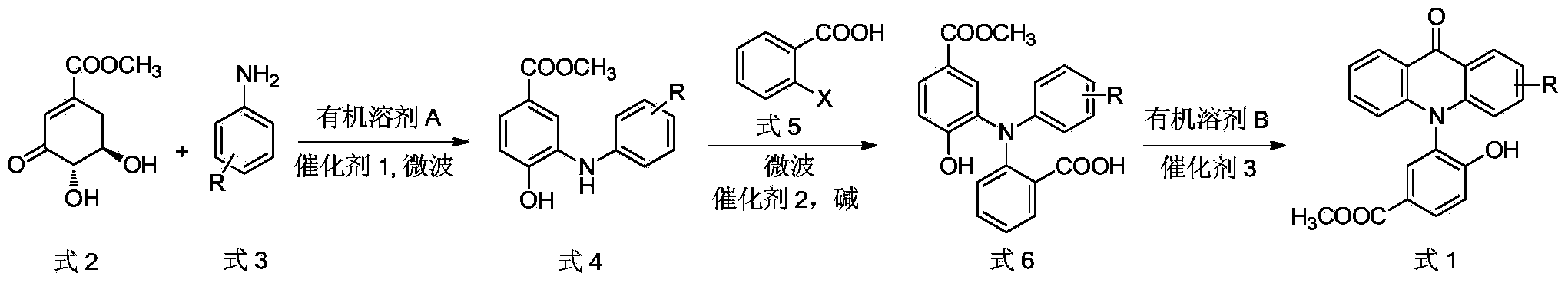
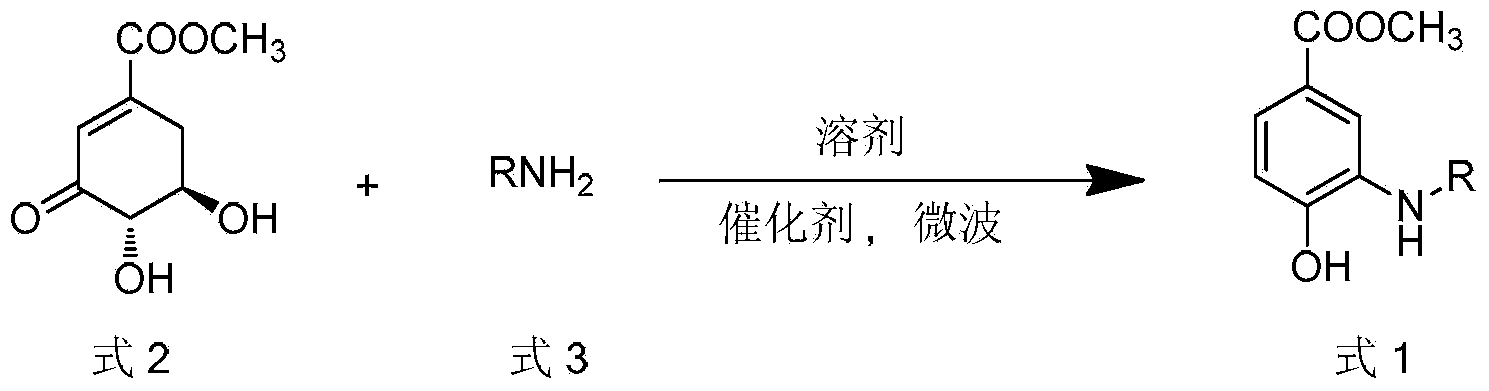
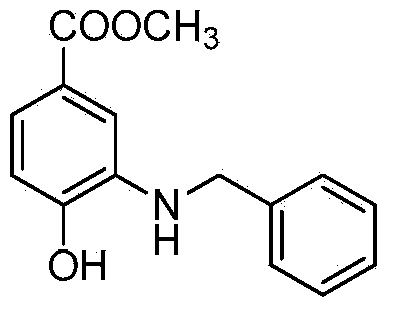
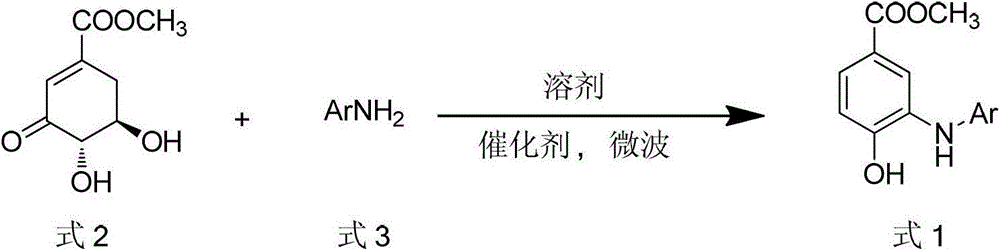
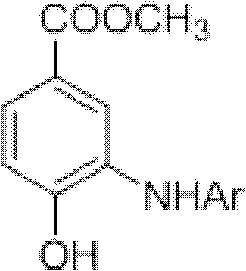
Microwave synthesis method for diarylamine compound
InactiveCN103214385ARealize sustainable development and utilizationShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationMicrowave methodIsomerization
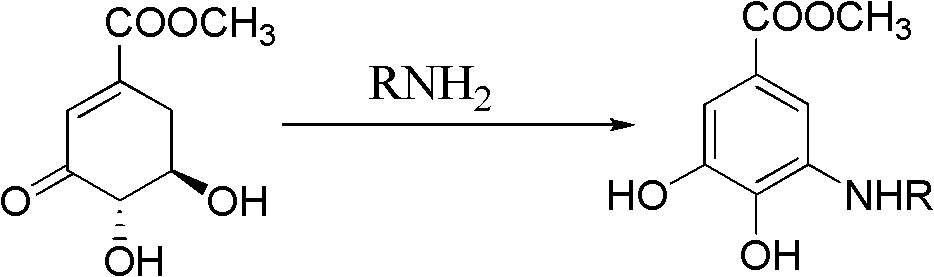
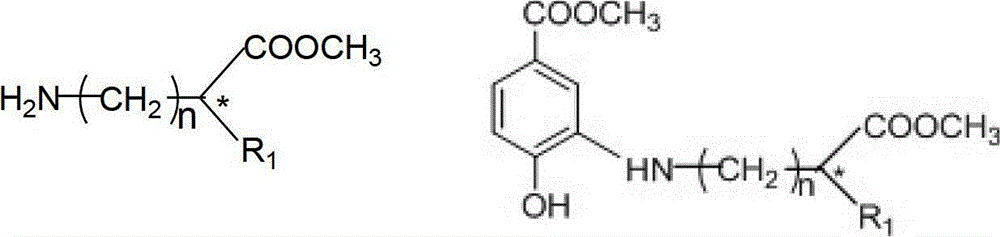
The invention discloses a microwave synthesis method for a diarylamine compound. The method comprises the following steps of: subjecting 3-methyl dehydroshikimic acid and a primary arylamine compound to a condensation reaction, an isomerization reaction and a dehydration reaction under the action of an organic solvent, a catalyst and microwaves to enable a hexatomic ring framework to be aromatized; and then, cooling a reaction liquid, pouring the reaction liquid into saturated brine, rapidly stirring the solution, separating out a solid, and carrying out suction filtration, drying and recrystallization to obtain a 3-amido-4-hydroxybenzoic acid methyl ester compound. The raw material 3-methyl dehydroshikimic acid adopted in the method is a non-aromatic compound and can be prepared from shikimic acid by using a simple and convenient method without depending on fossil resources; sustainable exploitation and utilization can be realized; in addition, the microwave synthesis method adopts a microwave method which is short in reaction time, simple and convenient in operation, convenient in after-treatment and high in yield; and the microwave synthesis method is clean in reaction, friendly to the environment and low in energy consumption.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
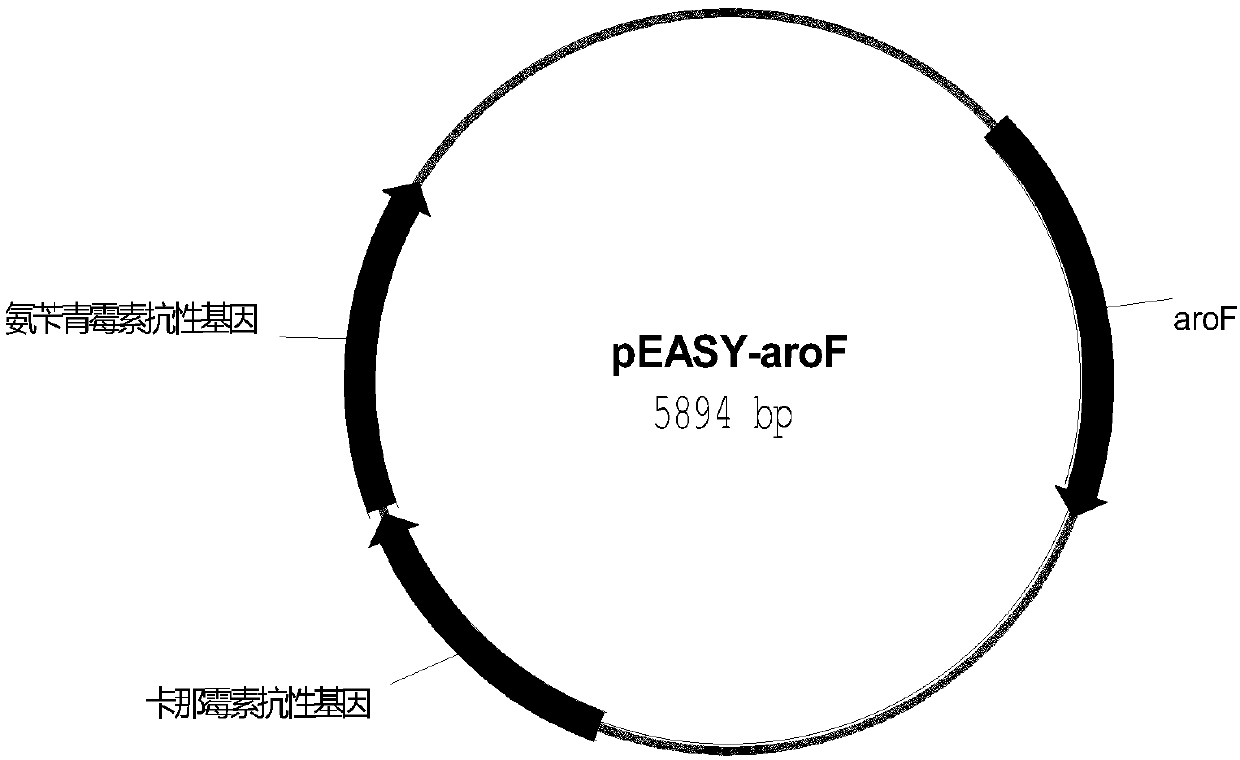
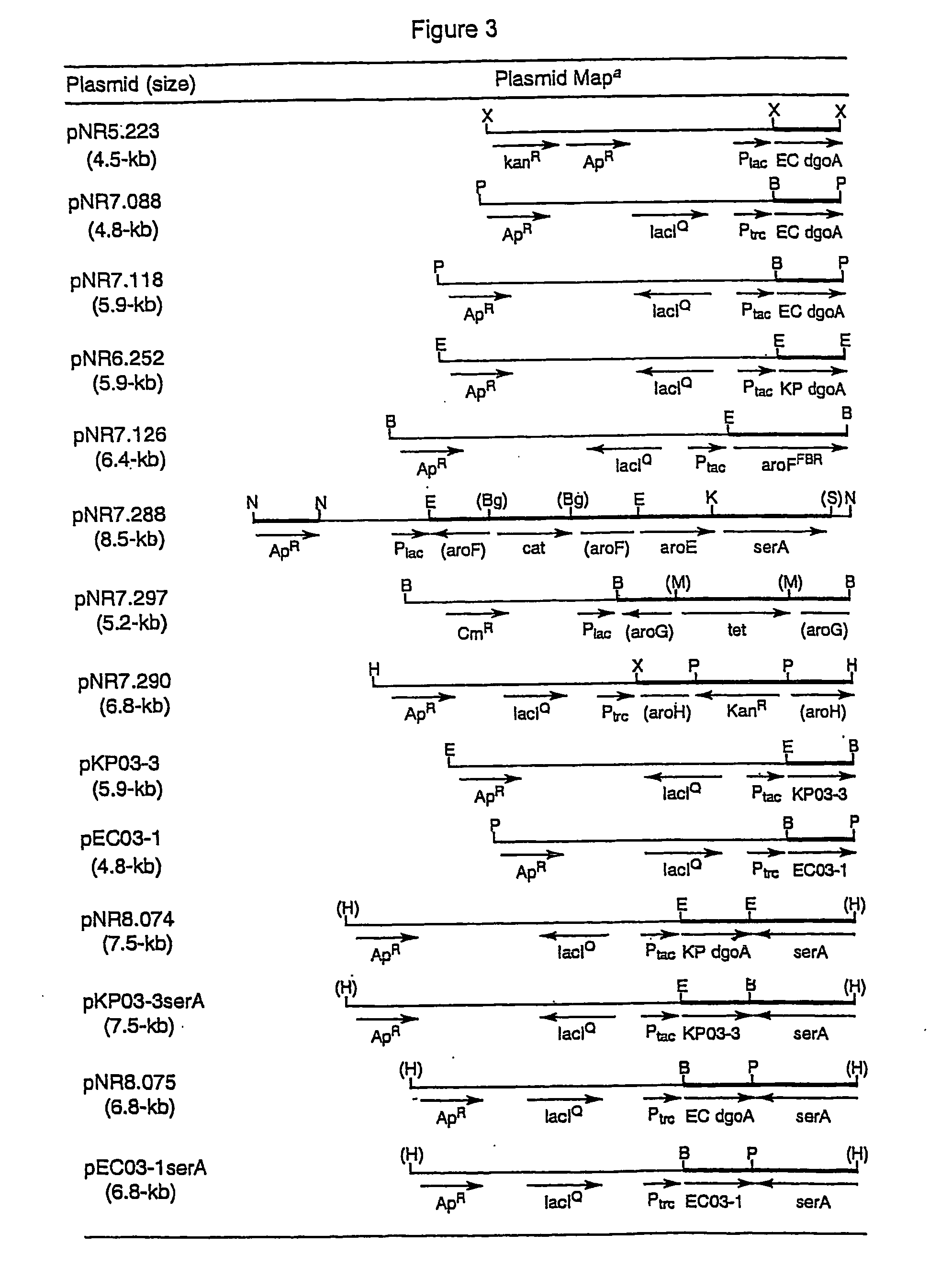
Escherichia coli recombinant bacterial strain for producing 3-dehydroshikimic acid as well as establishment method and application thereof
ActiveCN107619817ALow costReduce manufacturing costBacteriaMicroorganism based processesEscherichia coliInorganic salts
The invention provides an Escherichia coli recombinant bacterial strain WJ004 for producing 3-dehydroshikimic acid. The enzyme activity of 3-dehydroshikimic acid dehydrogenase is reduced by lowering the expression of the 3-dehydroshikimic acid dehydrogenase or by not expressing the 3-dehydroshikimic acid dehydrogenase. The bacterial strain can grow normally in a glucose inorganic salt culture medium and can produce 3-dehydroshikimic acid without adding aromatic amino acid and derivatives thereof and other growth factors, so that the cost of the culture medium is reduced, and the production cost of the 3-dehydroshikimic acid is reduced. The recombinant bacterial strain does not contain plasmid and is stable in heredity.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Method for preparing aryl alkyl amine compounds with 3-dehydrogenation methyl shikimate
InactiveCN102382002AIncrease productionStable sourceOrganic compound preparationAmino-carboxyl compound preparationDehydrogenationMethyl benzoate
The invention relates to a method for preparing aryl alkyl amine compounds with 3-dehydrogenation methyl shikimate, in particular to a method for preparing 3,4-dyhydroxy-5-alkyl amino methyl benzoate compounds. The method includes steps of enabling 3-dehydrogenation methyl shikimate and aryl primary amine compounds to give a condensation-dehydrogenation reaction by means of catalysis of acid catalyst under the normal pressure and mixing conditions so that a six-membered ring framework gives an aromatization reaction, and then obtaining the 3,4-dyhydroxy-5-alkyl amino methyl benzoate compounds after the reacted mixture is concentrated, extracted, dried, filtered and recrystallized. The reaction is carried out for two to six hours at the temperature ranging from 20 DEG C to 50 DEG C. The method utilizes 3-dehydrogenation shikimic acid compounds which are renewable resources as raw materials, thereby having the advantages of fine atom economy, simplicity in operation, mild condition, high yield, low cost, less pollution and the like and being capable of realizing sustainable development.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Methods and materials for the production of shikimic acid
InactiveUS20070087424A1Sugar derivativesBacteria3-deoxy-D-arabino-heptulosonate-7-phosphate3-dehydroquinate synthase
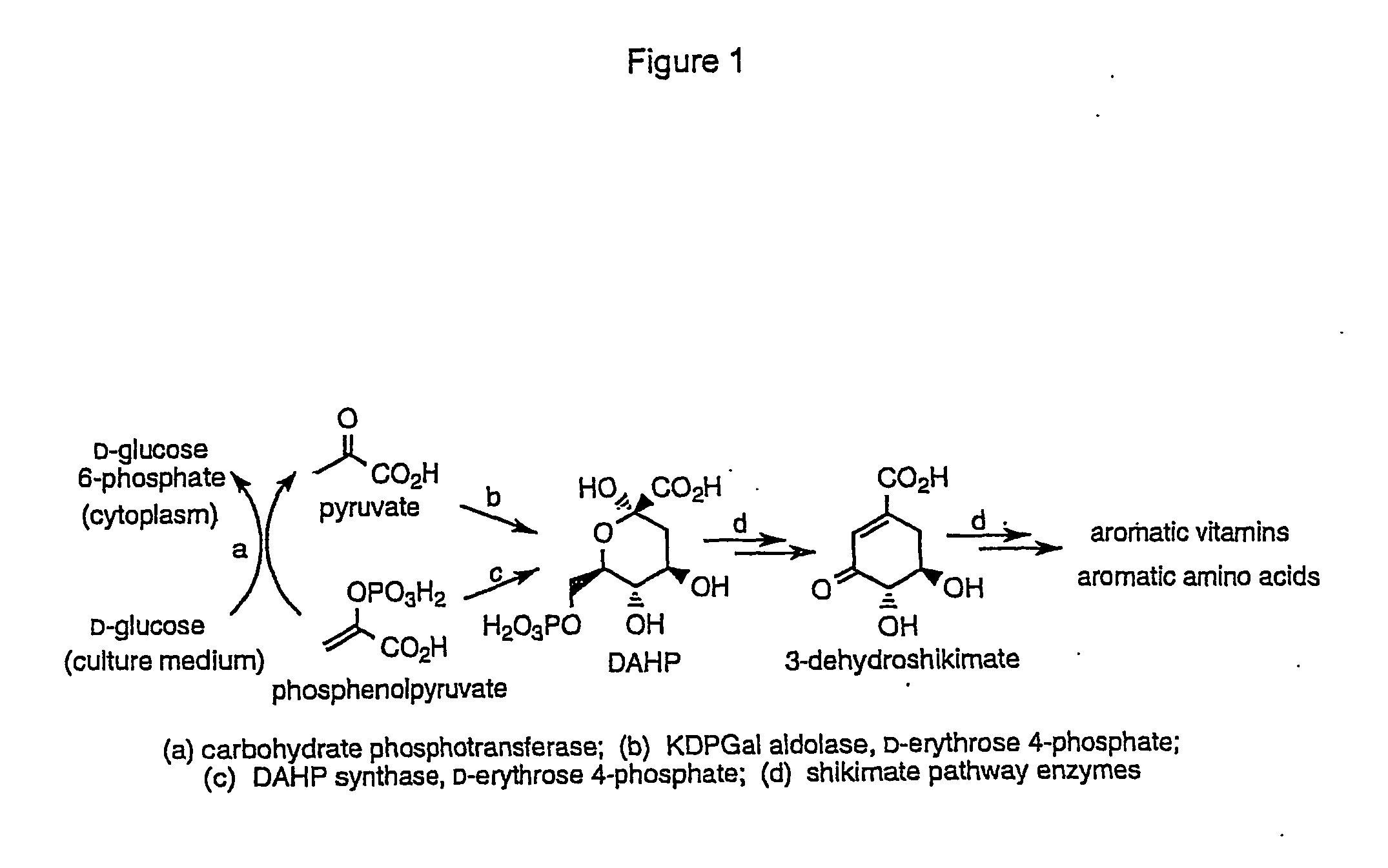
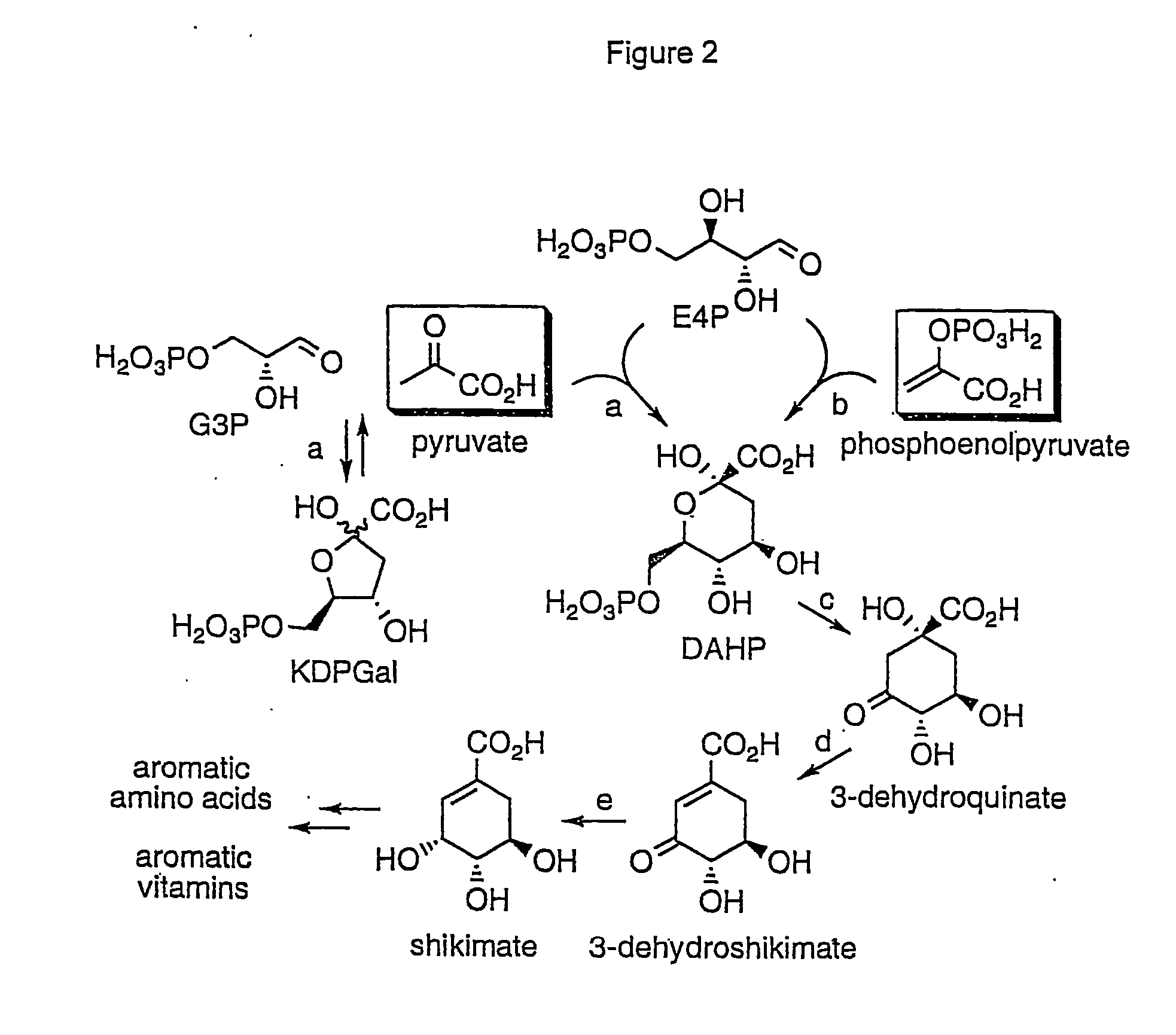
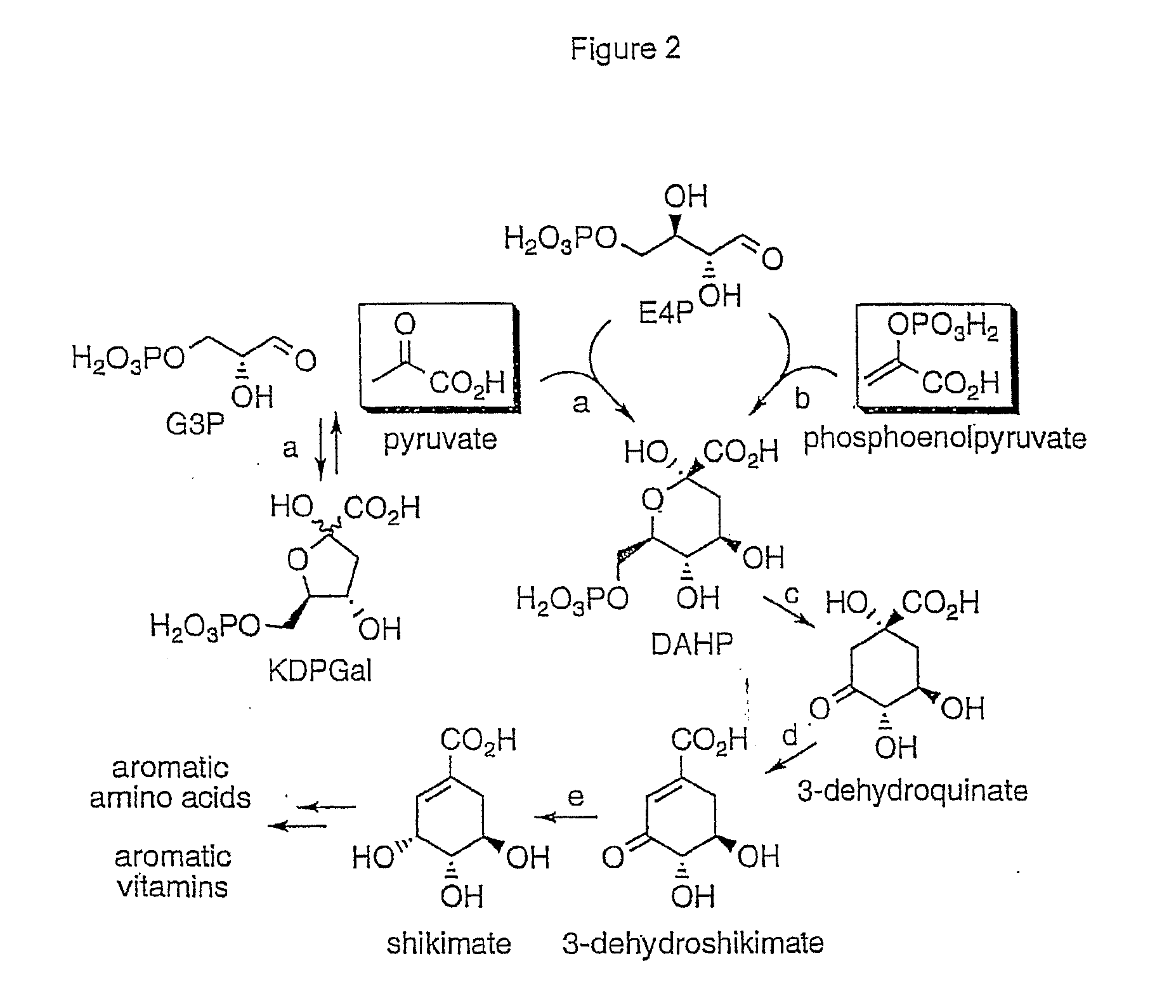
Novel enzymes and novel enzymatic pathways for the pyruvate-based synthesis of shikimate or at least one intermediate thereto or derivative thereof, nucleic acids encoding the enzymes, cells transformed therewith, and kits containing said enzymes, cells, or nucleic acid. A KDPGal aldolase is used to perform condensation of pyruvate with D-erythrose 4-phosphate to form 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP); a 3-dehydroquinate synthase is used to convert the DAHP to 3-dehydroquinate (DHQ); DHQ dehydratase can then convert DHQ to the key shikimate intermediate, 3-dehydroshikimate.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
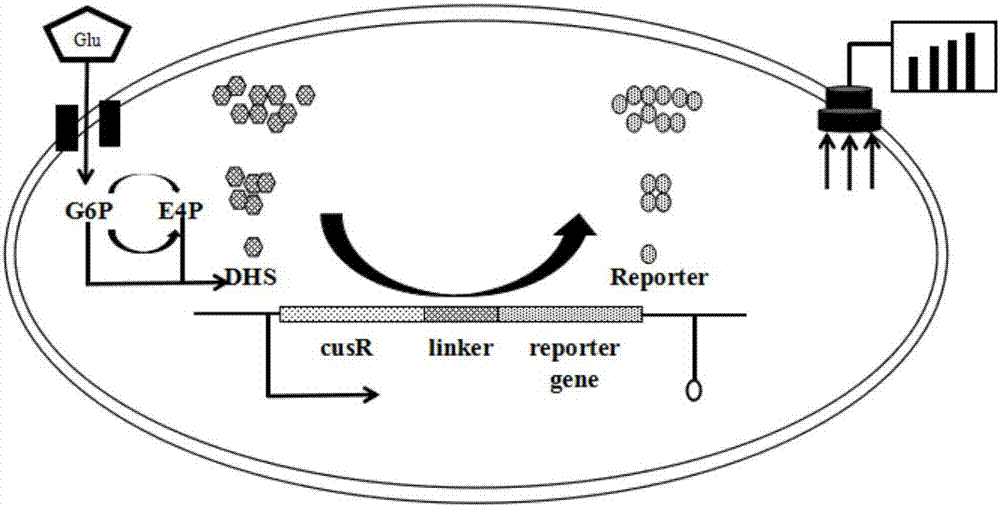
Biosensor capable of responding to 3-dehydroshikimic acid and application of biosensor
ActiveCN107974451AOvercoming technical biasBacteriaDNA/RNA fragmentationHigh-Throughput Screening MethodsScreening method
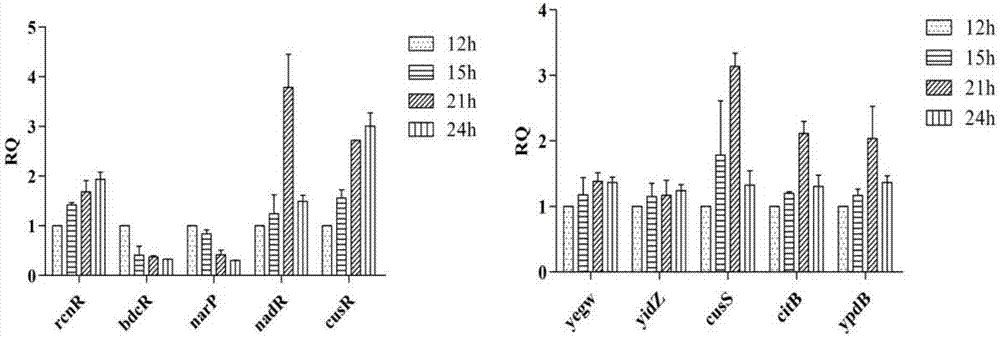
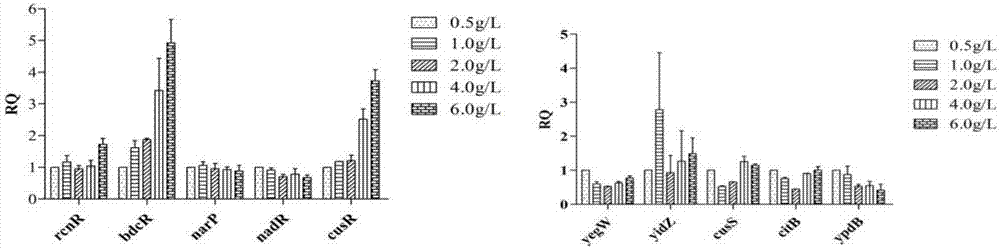
The invention achieves success in constructing a strain for producing 3-dehydroshikimic acid (DHS) via such means as genetic engineering or metabolic engineering, but the large-scale production of the3-dehydroshikimic acid via biological means faces the biggest bottleneck in the rapid acquisition of an efficient and stable-yield strain. The biosensor provided by the invention consists of a regulatory gene cusR and / or a promoter of the regulatory gene cusR and a reporter gene; positively correlated response to concentration of the 3-dehydroshikimic acid is achieved; and a technical bottleneckthat a 3-dehydroshikimic acid compound, which is colorless and is in lack of an effective chromogenic reaction, is difficult in achieving high-throughput screening; therefore, a strain mutagenesis library screening method for producing the 3-dehydroshikimic acid is further developed out.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
N-aryl oxazepine ketone compound and preparation method thereof
The invention discloses a 5-aryl-7-methoxycarbonyl dibenzo [b, e][1, 4] oxazepine-11(5H)-ketone compound and a preparation method thereof. The preparation method comprises the steps of carrying out condensation, isomerization and dehydration reaction on 3-dehydrogenated methyl shikimate and an aryl amine compound in the presence of an organic solvent and a catalyst in the microwave condition to obtain an aryl-substituted o-aminophenol intermediate; further adding o-halogen benzoic acid and an alkali neutralization catalyst in the microwave condition, cooling, pumping filtering, and carrying out recrystallization to obtain an N, N-diaryl-substituted o-aminophenol intermediate; and in the organic solvent, carrying out molecular lactonization cyclization reaction on the N, N-diaryl-substituted o-aminophenol intermediate in the presence of the catalyst and alkali to obtain the 5-aryl-7-methoxycarbonyl dibenzo [b, e][1, 4] oxazepine-11(5H)-ketone compound. The preparation method is simple, is short in the reaction time, is convenient for postprocessing and is high in the yield.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
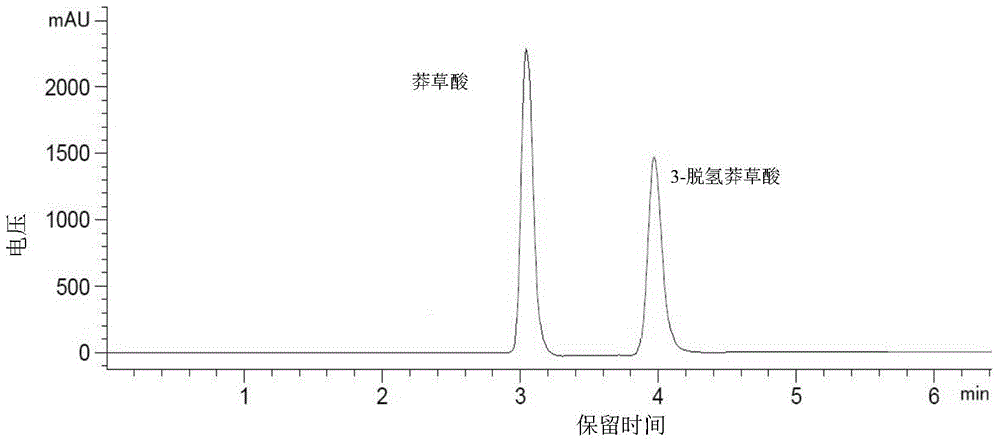
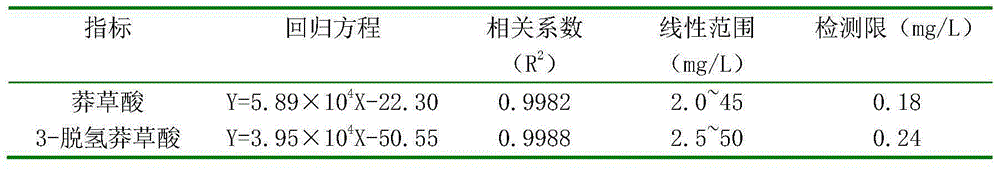
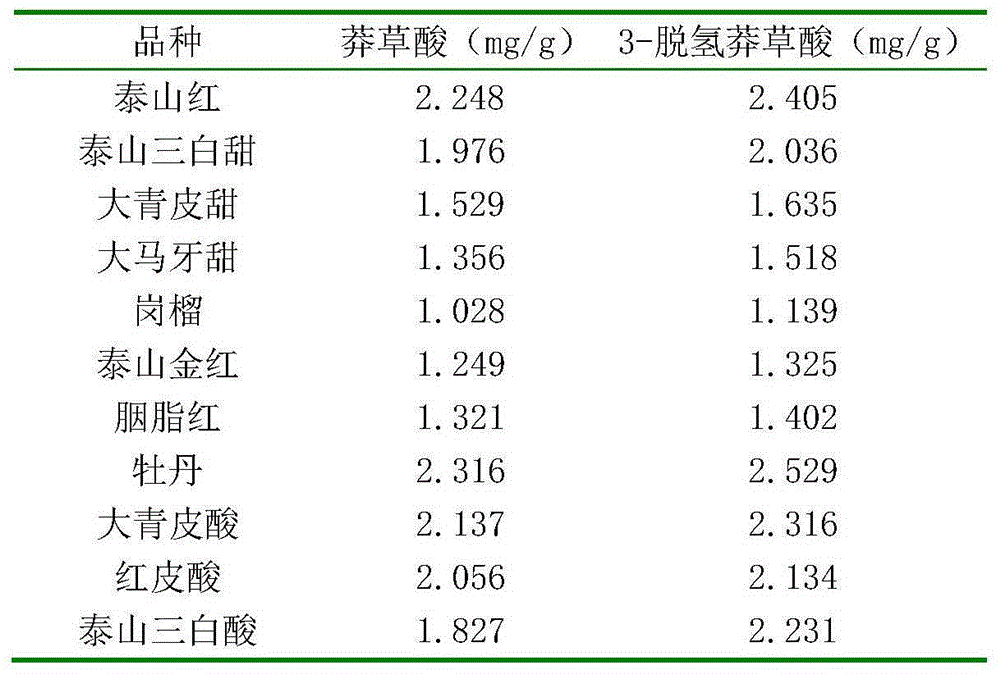
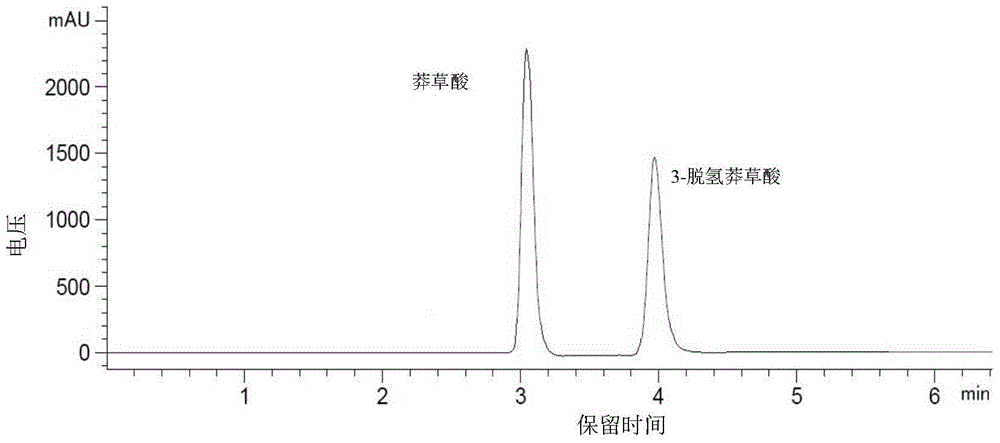
HPLC determination method for shikimic acid and 3-dehydrogenated shikimic acid in pomegranate peels
ActiveCN105116092AEasy to separateGood peak shapeComponent separationGallic acid esterIsocratic elution
The invention discloses an HPLC determination method for shikimic acid and 3-dehydrogenated shikimic acid in pomegranate peels. The method disclosed by the invention comprises the following steps: taking methanol-phosphoric acid water solution with the volume percent of 1% as a mobile phase and carrying out isocratic elution to simultaneously determine the content of shikimic acid and 3-dehydrogenated shikimic acid in the pomegranate peels, so as to pointedly detect shikimic acid and 3-dehydrogenated shikimic acid components in pomegranate fruits. The determination method disclosed by the invention is simple to operate, has short elution time, can detect comprehensively and has high detection efficiency, so that a theoretical foundation is laid for disclosing a metabolism mechanism of gallic acid in the pomegranate fruits.
Owner:SHANDONG INST OF POMOLOGY
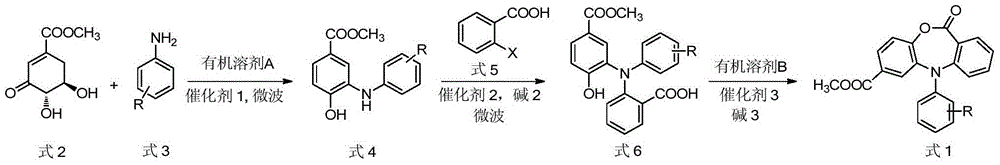
Preparation method of N-(2-hydroxy-5-methoxycarbonyl phenyl) acridone compound
The invention discloses a preparation method of a N-(2-hydroxy-5-methoxycarbonyl phenyl) acridone compound, and the preparation method is as follows: condensation, isomerization and dehydration reaction of methyl-3-dehydroshikimate and an aryl amine compound under organic solvent, catalyst 1 and microwave condition to obtain a diarylamine intermediate; continuing addition of o-halogenated benzoic acid, an acid and catalyst 2 for reaction under microwave condition, then cooling, filtration and recrystallization to obtain a triarylamine intermediate; Friedel-crafts acylation reaction of the triarylamine intermediate for cyclization under the function of catalyst 3 to obtain the N-(2-hydroxy-5-methoxycarbonyl phenyl) acridone compound. The method has the advantages of simple operation, short reaction time, and easy postprocessing.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Method for preparing epicatechol gallate and protocatechuic acid ester compounds from natural shikimic acid
InactiveCN102010338BIncrease productionStable sourceOrganic compound preparationCarboxylic acid esters preparationBenzoic acidAromatization
The invention discloses a method for preparing epicatechol gallate and protocatechuic acid ester compounds from a natural shikimic acid. The method comprises the following steps of: performing an esterification reaction of the shikimic acid serving as a raw material to obtain shikimic acid ester; obtaining 3-dehydro-shikimic acid ester under the action of 2-iodyl benzoic acid; and realizing aromatization under the action of a catalyst to obtain epicatechol gallate or protocatechuic acid ester compound. A renewable raw material serves as a starting material and a polyhydroxy substituted benzoic acid is formed by a brand new method, so the method has the advantages of simpleness in operation, mild reaction condition, high yield, capacity of realizing sustainable development and the like.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
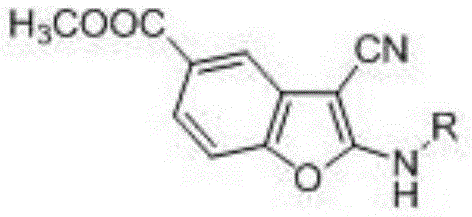
Preparation method of compound containing benzofuran structure
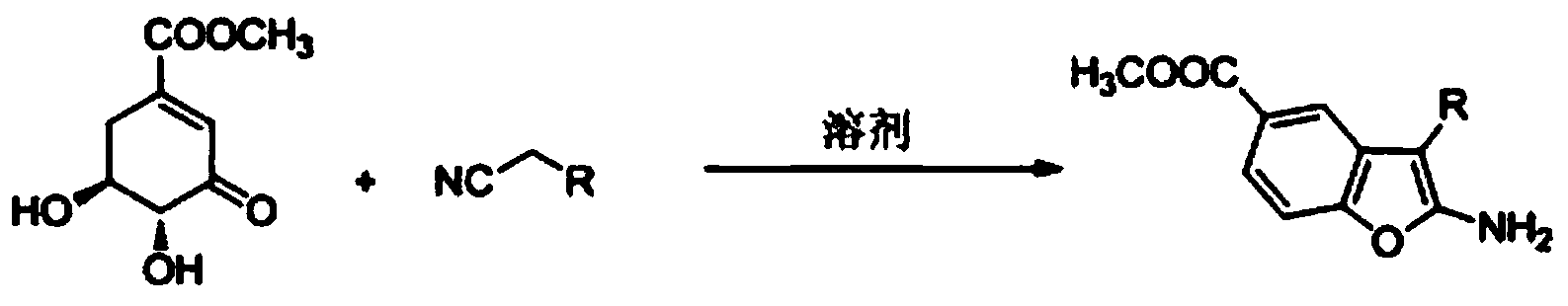
ActiveCN104387350ARealize sustainable developmentMild conditionsOrganic chemistryChemical synthesisFuran
The invention belongs to the technical field of chemical synthesis and discloses a preparation method of a compound containing a benzofuran structure. According to the preparation method, 3-methyl dehydroshikimate and a cyano-containing methylene compound are adopted as raw materials and reacted in the presence of a solvent and a catalyst to obtain the compound containing the benzofuran structure. The raw material 3-methyl dehydroshikimate, which is used in the invention, is prepared from renewable and non-food biomass resource shikimic acid as a raw material having Chinese characteristics by virtue of a simple and convenient method, therefore, the synthesis method is completely in line with the basic concept of modern green chemistry and the sustainable development of benzofuran compounds can be achieved.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
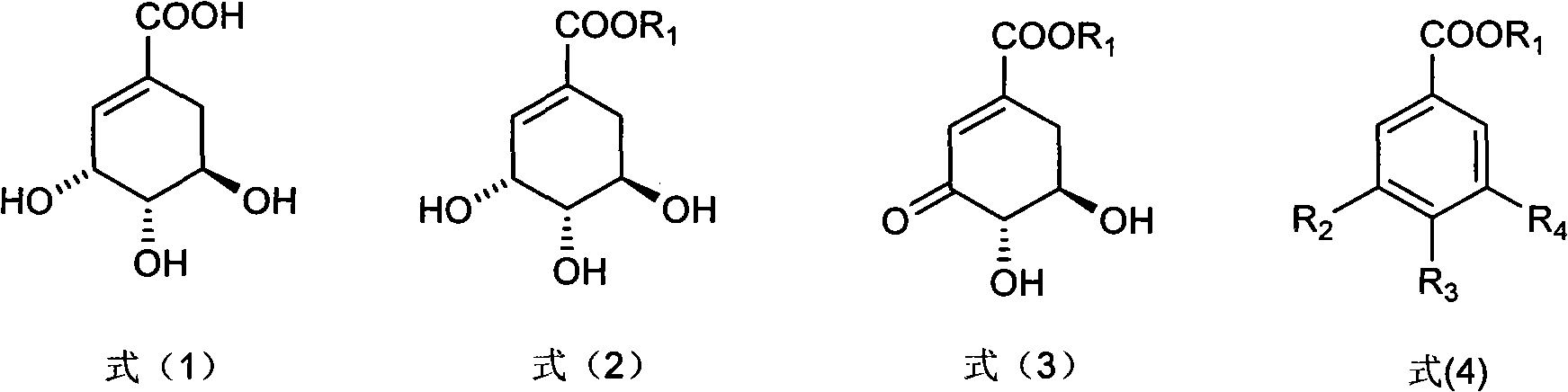
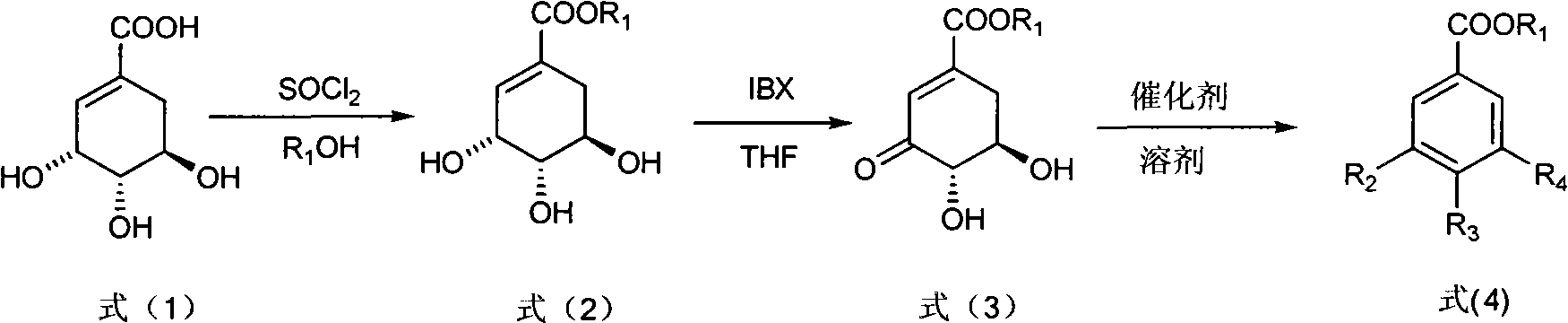
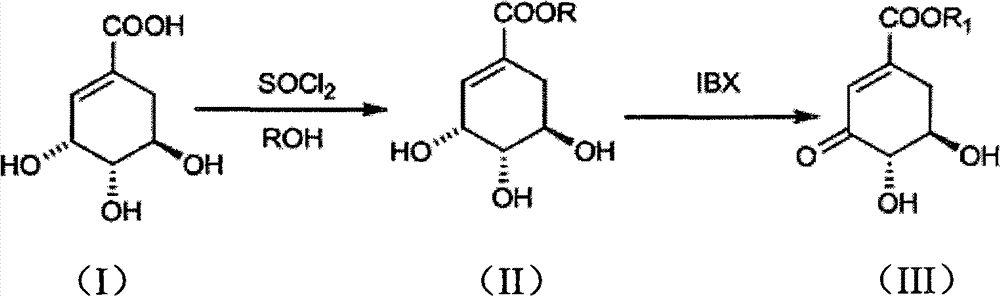
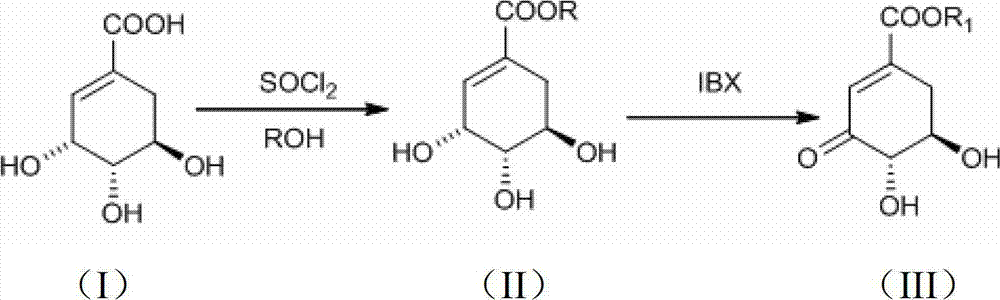
Preparation method of 3-dehydroshikimic ester compound
InactiveCN101973874BIncrease productionStable sourceOrganic compound preparationCarboxylic acid esters preparationAlcoholShikimic acid
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
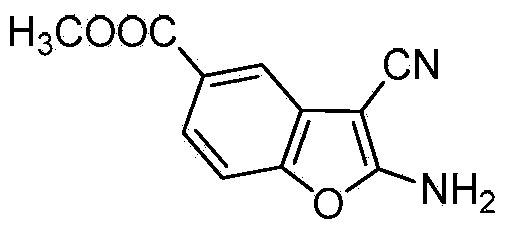
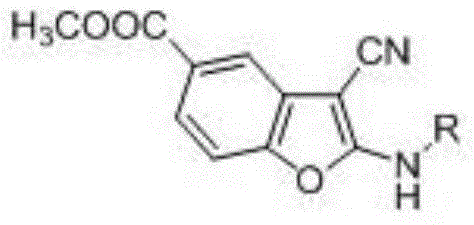
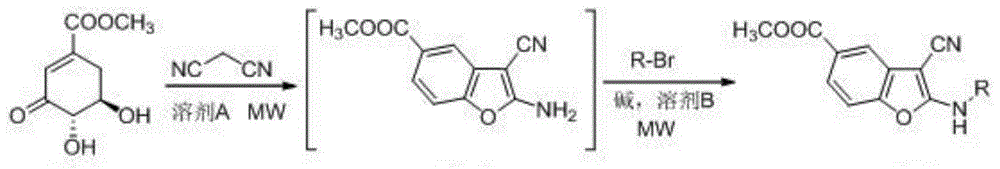
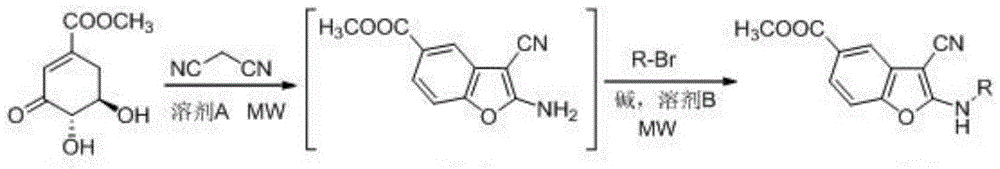
2-alkylamino-3-cyanobenzofuran type compound and preparation method thereof
ActiveCN104529962ARealize sustainable developmentMild reaction conditionsOrganic chemistryChemical synthesisMicrowave
The invention belongs to the technical field of chemical synthesis, and discloses a 2-alkylamino-3-cyanobenzofuran type compound and a preparation method thereof. The preparation method of the compound comprises the following steps: (1) dissolving 3-dehydroshikimic acid methyl ester and malononitrile into a solvent A, reacting under a microwave condition, and filtering after the reaction to obtain an intermediate; and (2) performing microwave or oil-bath heating reaction on the intermediate obtained in the step (1) and a bromo-substance in a solvent B under the catalysis of alkali, and separating and purifying a reaction product to obtain the 2-alkylamino-3-cyanobenzofuran type compound. The method for preparing the 2-alkylamino-3-cyanobenzofuran type compound by taking a non-aromatic compound as an initiator is a brand-new method provided by the invention, and has the advantages of simple and easily-available raw material, mild reaction condition, simple operation, short reaction time, high yield and low cost.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
HPLC method for determination of shikimic acid and 3-dehydroshikimic acid in pomegranate peel
ActiveCN105116092BPrevent browningEasy to separateComponent separationGallic acid esterIsocratic elution
The invention discloses an HPLC determination method for shikimic acid and 3-dehydrogenated shikimic acid in pomegranate peels. The method disclosed by the invention comprises the following steps: taking methanol-phosphoric acid water solution with the volume percent of 1% as a mobile phase and carrying out isocratic elution to simultaneously determine the content of shikimic acid and 3-dehydrogenated shikimic acid in the pomegranate peels, so as to pointedly detect shikimic acid and 3-dehydrogenated shikimic acid components in pomegranate fruits. The determination method disclosed by the invention is simple to operate, has short elution time, can detect comprehensively and has high detection efficiency, so that a theoretical foundation is laid for disclosing a metabolism mechanism of gallic acid in the pomegranate fruits.
Owner:SHANDONG INST OF POMOLOGY
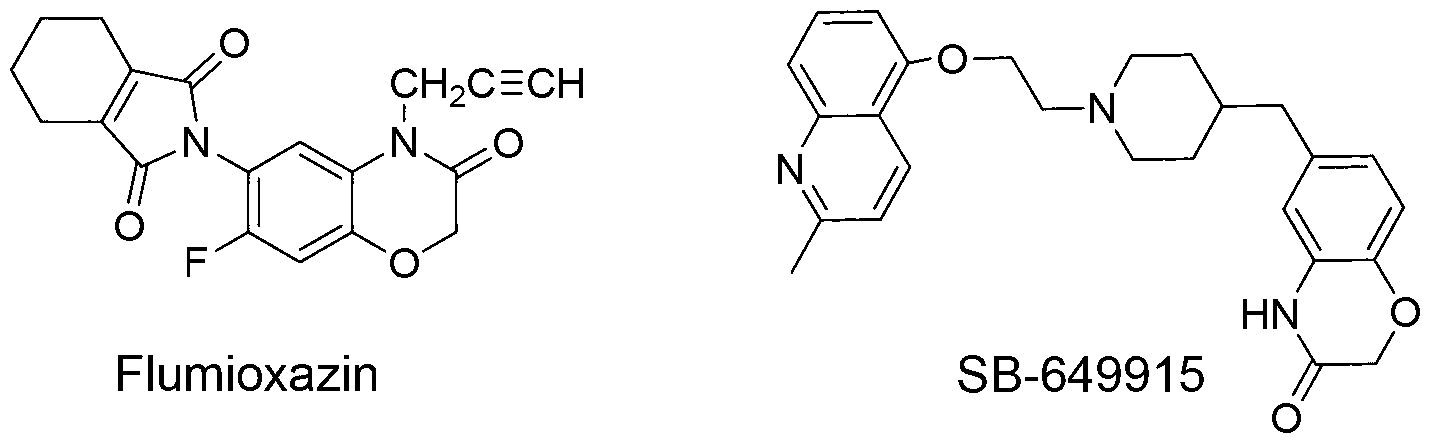
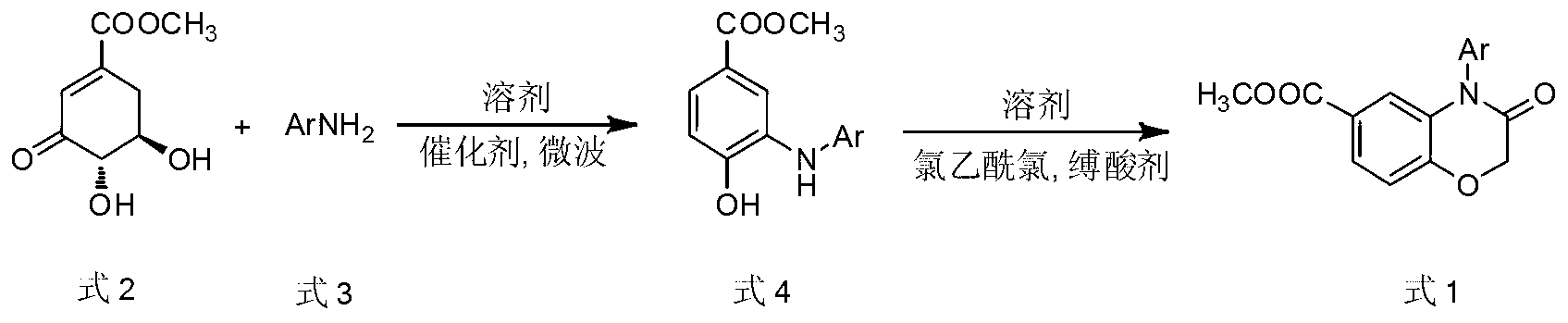
Method for preparing 4-aryl-6-methoxy carbonyl benzoxazinone compound
InactiveCN103288769BRealize sustainable development and utilizationShort reaction timeOrganic chemistryIsomerizationChloroacetyl chloride
The invention discloses a method for preparing a 4-aryl-6-methoxy carbonyl benzoxazinone compound. The method comprises the steps of carrying out condensation, isomerization and dehydration reaction on 3-dehydrogenized methyl shikimate and aromatic primary amines in an organic solvent A under the existence of a catalyst and a micro-wave condition; adding an acid-binding agent to a reaction system in ice bath, and gradually dropwise adding an organic solvent B in which chloroacetyl chloride is dissolved; and then heating and carrying out condensation reaction to obtain the 4-aryl-6-methoxy carbonyl benzoxazinone compound. The adopted material 3-dehydrogenized methyl shikimate is a non-aromatic compound, can be prepared from a renewable biomass resource shikimic acid by a simple method, and is obtained independently on a fossil resource; sustainable development and utilization can be achieved; and furthermore, a one-pot method and a microwave assisting method are adopted, so that the method is simple and convenient to operate, short in reaction time, high in yield, clean and environment-friendly in reaction process and small in energy consumption.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
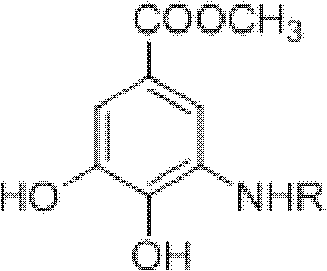
Preparation method of N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds
ActiveCN102816078AEfficient preparationPreparation sustainableCarbamic acid derivatives preparationOrganic compound preparationCarboxylic acidPhenol
The invention discloses a preparation method of N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds. The method comprises the steps of allowing reaction of N-arylation reagent and amino acid methyl ester in presence of organic solvent, catalyst and dehydrating agent; cooling product after reaction, filtering, concentrating, separating and / or recrystallizing to obtain N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds. The N-arylation reagent is methyl 3-dehydroshikimate, ethyl 3-dehydroshikimate or propyl 3-dehydroshikimate; has abundant resources and low cost; and belongs to renewable non-grain biomass resource. The method has the advantages of mild reaction condition, no need of noble metal catalysis, simple operation, high yield and large-scale preparation. Benzene ring of the product N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds contains a phenol hydroxyl group (2-site) and a carboxylic acid methyl ester (5-site), which provides a wide space for further derivatization.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Method for preparing alkylaryl amine compound through microwave synthesis
InactiveCN104370762ARealize sustainable development and utilizationShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationOrganic solvent4-hydroxybenzoic acid methyl ester
The invention discloses a method for preparing an alkylaryl amine compound through microwave synthesis. The method comprises the following steps of: reacting 3-dehydrogenated methyl shikimate and an alkylamine compound (formula 3) under the conditions of an organic solvent 1, a catalyst and a microwave, so that the hexatomic ring skeleton of 3-dehydrogenated methyl shikimate is subjected to aromatization; after reaction liquid is cooled, pouring the reaction liquid into a large quantity of saturated salt solutions, fast stirring to precipitate a solid or extracting by using an organic solvent, drying, and carrying out column chromatography to obtain a 3-alkanamine-4-methyl hydroxybenzoate compound (formula I). The method disclosed by the invention can be used for obtaining the 3-alkanamine-4-methyl hydroxybenzoate compound by realizing the amine accelerating aromatization reaction of 3-dehydrogenated methyl shikimate by adopting microwave synthesis, and has the advantages of short reaction time, simplicity and convenience for operation, convenience for post-processing and cleanness in reaction.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
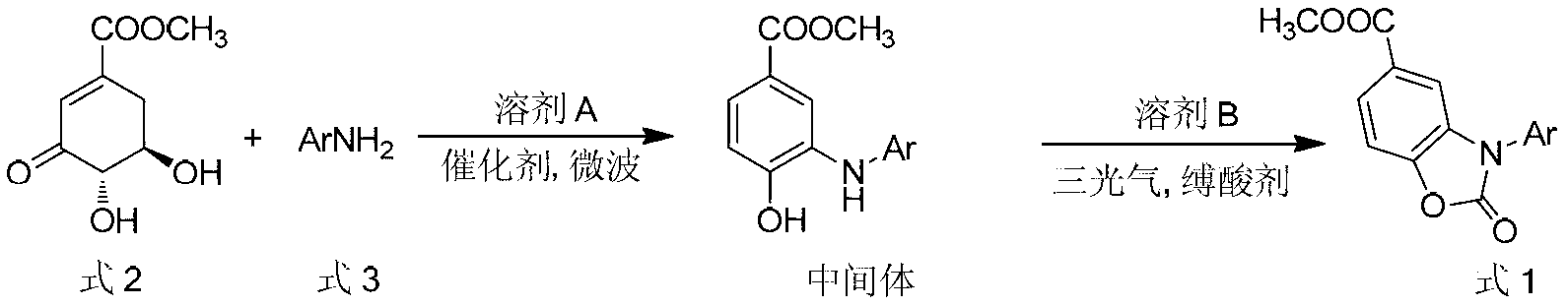
3-aryl-5-methoxycarbonyl benzoxazolinone compound preparation method
InactiveCN103224475ARealize sustainable development and utilizationShort reaction timeOrganic chemistrySolvent3-dehydroshikimate
The present invention discloses a 3-aryl-5-methoxycarbonyl benzoxazolinone compound preparation method, which comprises that: 3-methyl-3-dehydroshikimate is adopted as a raw material; the 3-methyl-3-dehydroshikimate and an aryl primary amine compound are subjected to a condensation-dewatering reaction under conditions of a solvent, a catalyst and microwave, such that a six membered ring skeleton is subjected to aromatization so as to obtain an intermediate 3-arylamino-4-methyl hydroxybenzoate compound; and the intermediate and triphosgene are subjected to a condensation reaction in the presence of a solvent and an acid-binding agent to obtain the3-aryl-5-methoxycarbonyl benzoxazolinone compound. According to the present invention, the used raw material 3-methyl-3-dehydroshikimate is a non-aromatic compound, and can be prepared from shikimic acid by using a simple method, the obtaining does not depend on fossil resources, sustainable development and utilization can be achieved, a reaction time is short, operation is simple, no pressure increase reaction device is required, post-treatment is convenient, and a yield is high.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Method for preparing aryl alkyl amine compounds with 3-dehydrogenation methyl shikimate
InactiveCN102382002BIncrease productionStable sourceOrganic compound preparationAmino-carboxyl compound preparationDehydrogenationMethyl benzoate
The invention relates to a method for preparing aryl alkyl amine compounds with 3-dehydrogenation methyl shikimate, in particular to a method for preparing 3,4-dyhydroxy-5-alkyl amino methyl benzoate compounds. The method includes steps of enabling 3-dehydrogenation methyl shikimate and aryl primary amine compounds to give a condensation-dehydrogenation reaction by means of catalysis of acid catalyst under the normal pressure and mixing conditions so that a six-membered ring framework gives an aromatization reaction, and then obtaining the 3,4-dyhydroxy-5-alkyl amino methyl benzoate compounds after the reacted mixture is concentrated, extracted, dried, filtered and recrystallized. The reaction is carried out for two to six hours at the temperature ranging from 20 DEG C to 50 DEG C. The method utilizes 3-dehydrogenation shikimic acid compounds which are renewable resources as raw materials, thereby having the advantages of fine atom economy, simplicity in operation, mild condition, high yield, low cost, less pollution and the like and being capable of realizing sustainable development.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Preparation method of N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds
ActiveCN102816078BEfficient preparationPreparation sustainableCarbamic acid derivatives preparationOrganic compound preparationCarboxylic acidPhenol
The invention discloses a preparation method of N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds. The method comprises the steps of allowing reaction of N-arylation reagent and amino acid methyl ester in presence of organic solvent, catalyst and dehydrating agent; cooling product after reaction, filtering, concentrating, separating and / or recrystallizing to obtain N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds. The N-arylation reagent is methyl 3-dehydroshikimate, ethyl 3-dehydroshikimate or propyl 3-dehydroshikimate; has abundant resources and low cost; and belongs to renewable non-grain biomass resource. The method has the advantages of mild reaction condition, no need of noble metal catalysis, simple operation, high yield and large-scale preparation. Benzene ring of the product N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds contains a phenol hydroxyl group (2-site) and a carboxylic acid methyl ester (5-site), which provides a wide space for further derivatization.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Methods and Materials for the Production of Shikimic Acid
ActiveUS20110045539A1Sugar derivativesAntibiotics3-dehydroquinate synthase3-deoxy-D-arabino-heptulosonate-7-phosphate
Novel enzymes and novel enzymatic pathways for the pyruvate-based synthesis of shikimate or at least one intermediate thereto or derivative thereof, nucleic acids encoding the enzymes, cells transformed therewith, and kits containing said enzymes, cells, or nucleic acid. A KDPGal aldolase is used to perform condensation of pyruvate with D-erythrose 4-phosphate to form 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP); a 3-dehydroquinate synthase is used to convert the DAHP to 3-dehydroquinate (DHQ); DHQ dehydratase can then convert DHQ to the key shikimate intermediate, 3-dehydroshikimate.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Microwave synthesis method for diarylamine compound
InactiveCN103214385BRealize sustainable development and utilizationShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationMicrowave methodIsomerization
The invention discloses a microwave synthesis method for a diarylamine compound. The method comprises the following steps of: subjecting 3-methyl dehydroshikimic acid and a primary arylamine compound to a condensation reaction, an isomerization reaction and a dehydration reaction under the action of an organic solvent, a catalyst and microwaves to enable a hexatomic ring framework to be aromatized; and then, cooling a reaction liquid, pouring the reaction liquid into saturated brine, rapidly stirring the solution, separating out a solid, and carrying out suction filtration, drying and recrystallization to obtain a 3-amido-4-hydroxybenzoic acid methyl ester compound. The raw material 3-methyl dehydroshikimic acid adopted in the method is a non-aromatic compound and can be prepared from shikimic acid by using a simple and convenient method without depending on fossil resources; sustainable exploitation and utilization can be realized; in addition, the microwave synthesis method adopts a microwave method which is short in reaction time, simple and convenient in operation, convenient in after-treatment and high in yield; and the microwave synthesis method is clean in reaction, friendly to the environment and low in energy consumption.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Method for preparing 4-aryl-6-methoxy carbonyl benzoxazinone compound
InactiveCN103288769ARealize sustainable development and utilizationShort reaction timeOrganic chemistryIsomerizationChloroacetyl chloride
The invention discloses a method for preparing a 4-aryl-6-methoxy carbonyl benzoxazinone compound. The method comprises the steps of carrying out condensation, isomerization and dehydration reaction on 3-dehydrogenized methyl shikimate and aromatic primary amines in an organic solvent A under the existence of a catalyst and a micro-wave condition; adding an acid-binding agent to a reaction system in ice bath, and gradually dropwise adding an organic solvent B in which chloroacetyl chloride is dissolved; and then heating and carrying out condensation reaction to obtain the 4-aryl-6-methoxy carbonyl benzoxazinone compound. The adopted material 3-dehydrogenized methyl shikimate is a non-aromatic compound, can be prepared from a renewable biomass resource shikimic acid by a simple method, and is obtained independently on a fossil resource; sustainable development and utilization can be achieved; and furthermore, a one-pot method and a microwave assisting method are adopted, so that the method is simple and convenient to operate, short in reaction time, high in yield, clean and environment-friendly in reaction process and small in energy consumption.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
A kind of 2-alkylamino-3-cyanobenzofuran compound and its preparation method
ActiveCN104529962BBiologically activeGreat application potentialOrganic chemistryChemical synthesisMicrowave
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Method for preparing diaryl amine compound from 3-methyl dehydroshikimate
InactiveCN102391061BIncrease productionStable sourceOrganic compound preparationSulfonic acid amide preparationReaction temperatureLewis acid catalysis
The invention relates to a method for preparing diaryl amine compound, in particular 3-arylamine-4-hydroxyl methyl benzoate compound, from 3-methyl dehydroshikimate, which comprises the following steps: carrying out a condensation-dehydration reaction on the 3-methyl dehydroshikimate and aryl primary amine compound under the catalysis of a Lewis acid catalyst in normal pressure and agitation conditions, so that a hexatomic ring skeleton generates aromatization, wherein the reaction temperature is 20-80 DEG C, and the reaction time is 6-18 hours; and concentrating, extracting, drying, filtering and recrystallizing a reaction mixture to obtain the 3-arylamine-4-hydroxyl methyl benzoate compound. By using the method, renewable resource 3-dehydroshikimic acid compound is used as a raw material; the method has the advantages of good atom economy, simpleness in operation, moderate condition, high yield, low cost, less pollution and the like; and the sustainable development can be achieved.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com