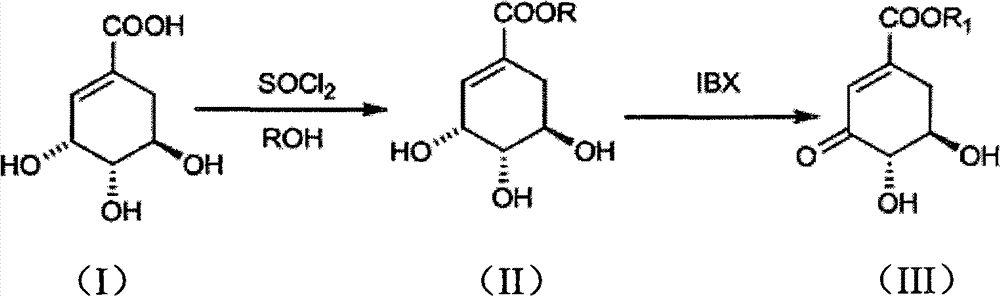
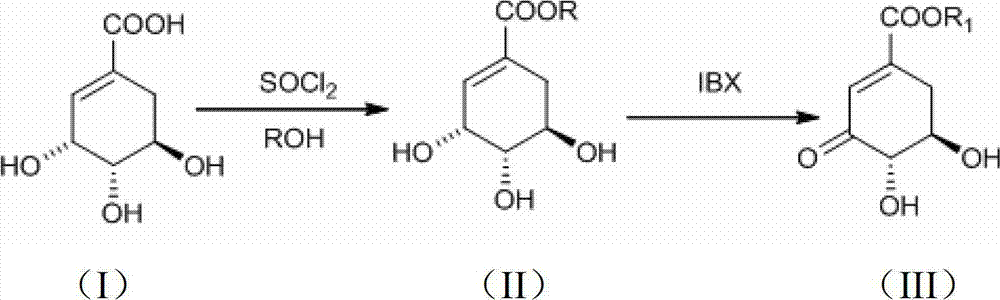
Preparation method of 3-dehydroshikimic ester compound
A technology of shikimate and compounds, which is applied in the field of preparation of 3-dehydroshikimate compounds, can solve problems such as not being suitable for large-scale production, high toxicity of diazomethane, and high requirements for equipment, so as to facilitate the expansion and The effect of protection, large output and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Add shikimic acid (17.4g, 0.1mol) and 150mL of anhydrous methanol into a three-necked round bottom flask, stir well, control the internal temperature of the reaction solution at 10°C, add thionyl chloride (15mL, 0.2mol) dropwise ). After the dropwise addition was completed, the internal temperature was controlled at 40° C., and the reaction was carried out for 3 hours. Concentrate to obtain a viscous liquid, which is recrystallized from ethyl acetate to obtain 14.76 g of white powdery solid methyl shikimate, yield: 78.5%.
[0025] The following is the product of step (1) 1 H NMR spectrum and MS spectrum data:
[0026] m.p.112~113℃. 1 H NMR (CD 3 COCD 3 , 400MHz) δ6.73(m, 1H, 2-H), 4.38(m, 1H, 3-H), 4.02(s, 1H, 4-OH D 2 O exchangeable), 4.00 (brs, 2H, 3, 5-OH D 2 Oexchangeable), 3.69(s, 3H, OCH 3 ), 3.85(m, 1H, 5-H), 3.68(m, 1H, 4-H), 2.64(dd, J 1 =17.6Hz,J 2 = 4.4Hz, 1H, 6α-H), 2.18(dd, J 1 =17.6Hz,J 2 =6.8Hz, 1H, 6β-H).MS(EI), m / z(%): 188(M+), 170(M + ...
Embodiment 2
[0031] (1) With embodiment 1 step (1).
[0032](2) Methyl shikimate (7.52 g, 40 mmol), IBX (13.44 g, 48 mmol) and 160 mL of tetrahydrofuran were put into a reaction flask, heated to 67° C., and stirred for 1 h. Filtration and concentration gave a light yellow solid. Recrystallized from ethyl acetate-petroleum ether to obtain 3.84 g of white fine needle crystals of methyl 3-dehydroshikimate, yield: 51.6%.
[0033] The characterization result of the product is the same as the step (2) of Example 1.
Embodiment 3
[0035] (1) With embodiment 1 step (1).
[0036] (2) Methyl shikimate (7.52g, 40mmol), IBX (13.44g, 48mmol) and 160mL of tetrahydrofuran were put into a reaction flask, heated to 50°C, and stirred for 6h. Filtration and concentration gave a light yellow solid. Recrystallized from ethyl acetate-petroleum ether to obtain 4.17 g of white fine needle crystals of methyl 3-dehydroshikimate, yield: 56.0%.
[0037] The characterization result of the product is the same as the step (2) of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com