Method for preparing 4-aryl-6-methoxy carbonyl benzoxazinone compound
A technology of methoxycarbonylbenzene and compounds, which is applied in the field of preparation of 4-aryl-6-methoxycarbonylbenzoxazinone compounds, can solve the problems of low total yield, poor selectivity, and limited application, and achieve Short reaction time, convenient post-processing, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
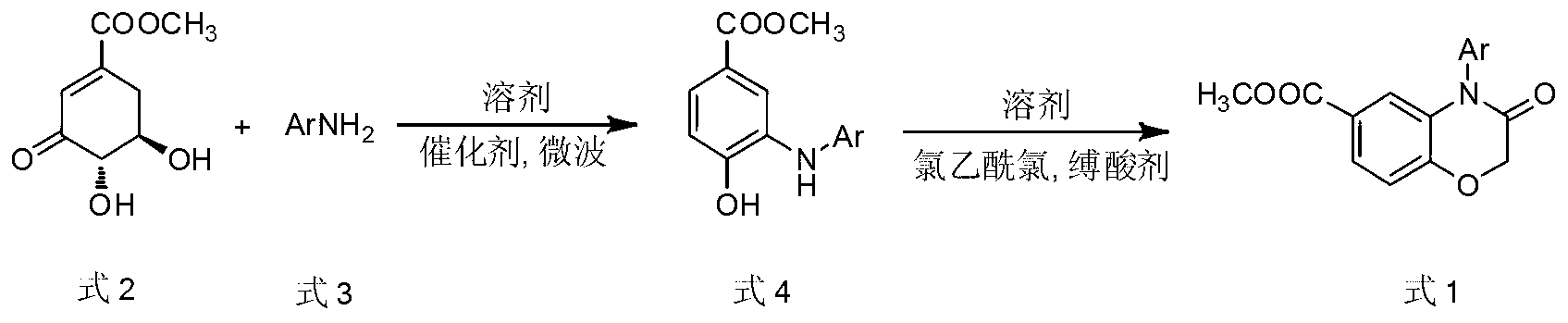
[0022] Example 1: Preparation of 4-phenyl-6-methoxycarbonyl-(2H)-1,4-benzoxazin-3(4H)-one
[0023] Methyl 3-dehydroshikimate (0.93g, 5.0mmol), aniline (0.47g, 5.0mmol), p-toluenesulfonic acid (0.05g, 0.25mmol), N,N-dimethylformamide (5ml) Add to microwave reaction vial. The above system was reacted in a microwave reactor at 130°C and a microwave power of 240W for 10 minutes. TLC detected that the aniline reaction was complete, and the intermediate 3-anilino-4-hydroxybenzoic acid methyl ester was obtained, and the intermediate was directly carried out in the next step without separation. . After the reaction system was cooled, potassium carbonate (4.15g, 30mmol) was added to the reaction solution under an ice bath and an acetonitrile solution (5ml) dissolved with chloroacetyl chloride (0.80ml, 12mmol) was added dropwise. After the addition was complete, the system was heated To 60 ° C, the reaction 2h. TLC detects that the reaction is complete, and the reaction solution is p...
Embodiment 2
[0024] Example 2: Preparation of 4-(4-methoxyphenyl)-6-methoxycarbonyl-(2H)-1,4-benzoxazin-3(4H)-one
[0025] Methyl 3-dehydroshikimate (1.02g, 5.5mmol), 4-methoxyaniline (0.62g, 5.0mmol), formic acid (9.36μl, 0.25mmol), N,N-dimethylformamide ( 5ml) into the reaction flask, and the above system was reacted in a microwave reactor at 150°C and a microwave power of 400W for 5min. TLC detected that the reaction of 4-methoxyaniline was complete, and the intermediate 3-(4`-methoxyanilino) was obtained. -Methyl 4-hydroxybenzoate, the intermediate is directly subjected to the next reaction without isolation. After cooling, potassium carbonate (4.15g, 30mmol) was added to the above reaction solution under an ice bath and acetonitrile solution (5ml) dissolved with chloroacetyl chloride (0.80ml, 12mmol) was added dropwise. 60°C, react for 2h. TLC detects that the reaction is complete, the reaction solution is poured into a beaker filled with 80ml of saturated saline, stirred rapidly, a...
Embodiment 3
[0026] Example 3: Preparation of 4-(4-methylphenyl)-6-methoxycarbonyl-(2H)-1,4-benzoxazin-3(4H)-one
[0027] Methyl 3-dehydroshikimate (1.12g, 6.0mmol), p-toluidine (0.54g, 5.0mmol), acetic acid (14.30μl, 0.25mmol), N,N-dimethylformamide (5ml) were added In the reaction flask, the above system was reacted in a microwave reactor at 130°C and a microwave power of 240W for 8 minutes, and the p-toluidine was completely reacted by TLC to obtain the intermediate 3-(4`-methylanilino)-4-hydroxybenzoic acid methyl ester, the intermediate was directly carried out to the next reaction without isolation. After cooling, potassium carbonate (4.15g, 30mmol) was added to the above reaction solution under an ice bath and acetonitrile solution (5ml) dissolved with chloroacetyl chloride (0.80ml, 12mmol) was added dropwise. 60°C, react for 2h. TLC detects that the reaction is complete, the reaction solution is poured into a beaker filled with 80ml of saturated saline, stirred rapidly, a large a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com