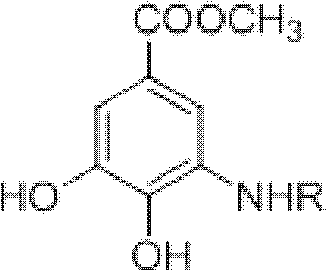
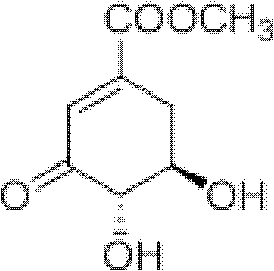
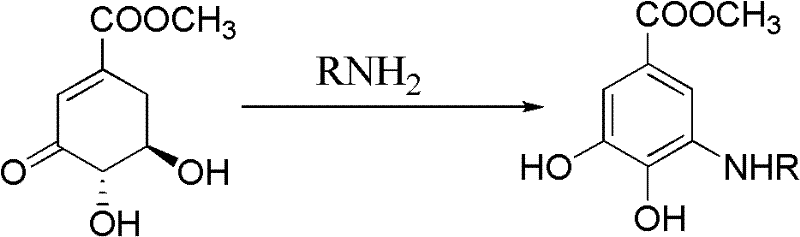
Method for preparing aryl alkyl amine compounds with 3-dehydrogenation methyl shikimate
A technology of arylalkylamine and acid methyl ester, which is applied in the field of preparation of arylalkylamine compounds, can solve the problems of low reactivity of alcohols and harsh reaction conditions, and achieve stable sources, mild reaction conditions, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 3, Preparation of methyl 4-dihydroxy-5-methylaminobenzoate:
[0029] 3-Dehydroshikimate methyl ester (0.93g, 5.0mmol), methylamine (0.75ml, 5.0mmol), formic acid (9.36μl, 0.25mmol) in the reaction flask, add 20ml CH 3 OH, stirred and reacted at 20°C for 3h, and the reaction was complete as detected by TLC. After completion of the reaction, concentrate (remove CH 3 OH) to dryness, extracted with ethyl acetate, combined the organic layers, and added anhydrous MgSO 4 After drying and filtering, the filtrate was concentrated, and then recrystallized from ethyl acetate-petroleum ether to obtain 0.55 g of methyl 3,4-dihydroxy-5-methylaminobenzoate as a yellow solid, yield: 56%. m.p.163-164°C. 1 H NMR (DMSO-d 6 , 400MHz) δ: 6.84 (d, J = 2.0Hz, 1H, 2-ArH), 6.60 (d, J = 2.0Hz, 1H, 6-ArH), 3.74 (s, 3H, OCH 3 ), 2.69 (s, 3H, CH 3 ); MS(EI): m / z=197[M] + , 182[M-CH 3 ] + , 166, 154.
Embodiment 2
[0031] 3, Preparation of methyl 4-dihydroxy-5-ethylaminobenzoate:
[0032] Methyl 3-dehydroshikimate (0.93g, 5.0mmol), ethylamine (0.38ml, 6.0mmol), acetic acid (14.30μl, 0.25mmol) were added to a reaction flask, 20ml DMSO was added, and the reaction was stirred at 50°C for 2h. TLC detects that the reaction is complete. After the reaction is complete, cool to 25°C, concentrate (remove DMSO) to dryness, extract with ethyl acetate, combine the organic layers, add anhydrous MgSO to the organic layer 4 After drying and filtering, the filtrate was concentrated, and then recrystallized from ethyl acetate-petroleum ether to obtain 0.77 g of methyl 3,4-dihydroxy-5-ethylaminobenzoate as a white solid, yield: 73%. m.p.180~182℃. 1 H NMR (DMSO-d 6 , 400MHz) δ: 6.84 (d, J=1.6Hz, 1H, 2-ArH), 6.66 (d, J=1.6Hz, 1H, 6-ArH), 3.73 (s, 3H, OCH 3 ), 3.07 (q, 2H, CH 2 ), 1.15(t,J 1 =7.2Hz,J 2 =6.8Hz, 3H, CH 3 ); MS(EI): m / z=211[M] + , 196[M-CH 3 ] + , 180, 164.
Embodiment 3
[0034] 3, the preparation of 4-dihydroxy-5-n-propylaminobenzoic acid methyl ester:
[0035] 3-Dehydroshikimate methyl ester (0.93g, 5.0mmol), n-propylamine (0.45ml, 5.5mmol), p-toluenesulfonic acid (0.01g, 0.05mmol) in a reaction flask, add 20ml t-BuOH, 30 ℃ The reaction was stirred for 4 h, and the reaction was complete as detected by TLC. After the reaction is complete, cool to 25°C, concentrate (remove t-BuOH) to dryness, extract with ethyl acetate, combine the organic layers, add anhydrous MgSO to the organic layer 4 After drying and filtering, the filtrate was concentrated, and then recrystallized from ethyl acetate-petroleum ether to obtain 0.71 g of gray-green solid methyl 3,4-dihydroxy-5-n-propylaminobenzoate, yield: 63%. m.p.159~160℃. 1 H NMR (DMSO-d 6 , 400MHz) δ: 9.34(s, 1H, OH), 6.83(d, J=1.6Hz, 1H, 2-ArH), 6.65(d, J=1.6Hz, 1H, 6-ArH), 3.73(s, 3H, OCH 3 ), 2.99(t,J 1 =6.8Hz,J 2 =7.2Hz, 2H, CH 2 ), 1.56 (m, 2H, CH 2 ), 0.91(t,J 1 =7.6Hz,J 2 =7.2Hz, 3H, CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com