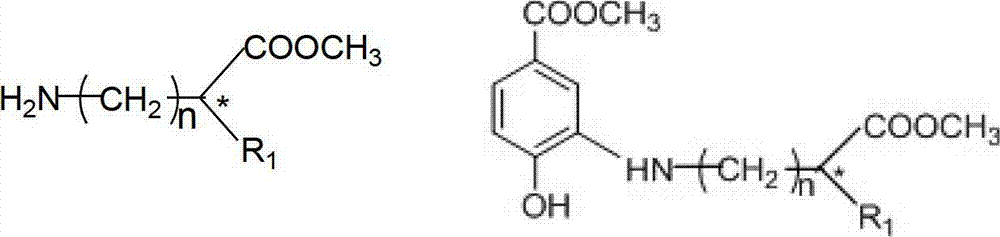
Preparation method of N-(5-methoxycarbonyl-2-hydroxyphenyl) amino acid ester compounds
A technology of ester compounds and hydroxyphenyl, applied in the field of preparation of N-amino acid ester compounds, can solve the problems of harsh reaction conditions, high reaction temperature, complex catalytic system, etc., and achieves no need for noble metal catalysis, mild reaction conditions, sustainable The effect of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of N-(5-methoxycarbonyl-2-hydroxyphenyl)-L-alanine methyl ester includes the following steps:
[0032] Take 3-dehydroshikimate methyl ester (0.93g, 5.0mmol), anhydrous aluminum trichloride (0.03g, 0.25mmol), 10g 3A molecular sieve in the reaction flask, and put 20ml of L-alanine methyl ester in ethanol solution ( 0.77g, 5.5mmol L-alanine methyl ester hydrochloride and 0.30g, 5.5mmol sodium methoxide in ethanol) were added to the above reaction flask. The above reaction system was reacted at 70°C for 6 hours, followed by TLC. After the reaction, it was cooled, filtered, the solvent was removed by rotary evaporation, column chromatography, eluted with ethyl acetate-petroleum ether, concentrated to remove the solvent to obtain a pale yellow oily liquid (([α] 25 / D = -63.79, c=0.59 (anhydrous ethanol) 1.03g, yield: 82%.
[0033] The structural characterization data of the product are as follows: 1 HNMR(400MHz,DMSO-d 6 )δppm: 10.40(s,1H),7.19(dd,J 1 =8.15,J 2 =1.7...
Embodiment 2
[0035] The preparation of N-(5-methoxycarbonyl-2-hydroxyphenyl)glycine methyl ester includes the following steps:
[0036] Take methyl 3-dehydroshikimate (0.93g, 5.0mmol), p-toluenesulfonic acid (0.05g, 0.25mmol), 10g3A molecular sieve in a reaction flask, and mix 20ml of methyl glycine in methanol (0.69g, 5.5mmol) Glycine methyl ester hydrochloride and 0.30 g, 5.5 mmol sodium methoxide in methanol) were added to the above reaction flask. The above reaction system was reacted at 65°C for 12 hours, followed by TLC. After the reaction was completed, it was cooled, filtered, and the solvent was removed by rotary evaporation, column chromatography, eluted with ethyl acetate-petroleum ether, and recrystallized to obtain a pale yellow solid 0.99 g, yield: 83%. m.p.110~112℃.
[0037] The structural characterization data of the product are as follows: 1 HNMR(400MHz,DMSO-d 6 )δppm: 10.36(s,1H),7.18(dd,J 1 =8.00,J 2 =1.74Hz,1H),6.89(d,J=1.74Hz,1H),6.75(d,J=8.00Hz,1H),5.30(s,1H),3.96(s,2H),...
Embodiment 3
[0039] The preparation of N-(5-methoxycarbonyl-2-hydroxyphenyl)-L-isoleucine methyl ester includes the following steps:
[0040] Take 3-dehydroshikimate methyl ester (0.93g, 5.0mmol), acetic acid (14.30μl, 0.25mmol), 10g3A molecular sieve in the reaction flask, add 20ml of L-isoleucine methyl ester in ethanol solution (1.00g, 5.5mmol L-isoleucine methyl ester hydrochloride and 0.30g, 5.5mmol sodium methoxide in ethanol) were added to the above reaction flask. The above reaction system was reacted at 70°C for 6 hours, followed by TLC. After the reaction, it was cooled, filtered, the solvent was removed by rotary evaporation, column chromatography, eluted with ethyl acetate-petroleum ether, and recrystallized to obtain a pale yellow solid (([α] 25 / D = -83.80, c=0.28 (anhydrous ethanol) 1.18g, yield: 80%. m.p.109~111℃.
[0041] The structural characterization data of the product are as follows: 1 HNMR(400MHz,DMSO-d 6 )δppm: 10.47(s,1H),7.19(dd,J 1 =8.18,J 2 =1.86Hz,1H), 7.03(d,J=1.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com