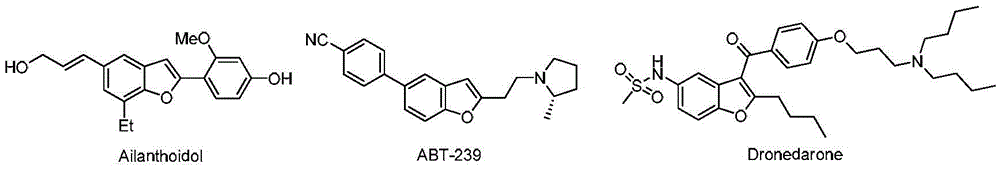
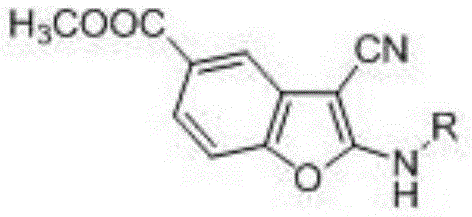
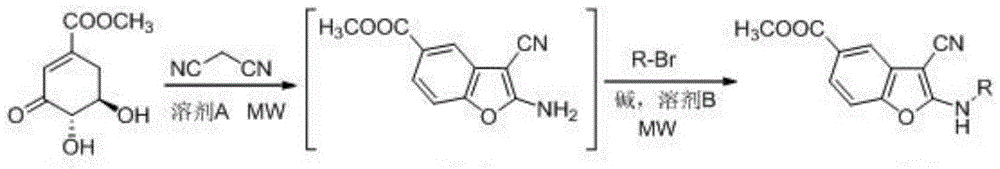
A kind of 2-alkylamino-3-cyanobenzofuran compound and its preparation method
A compound, the technology of cyanobenzene, which is applied in the field of 2-alkylamino-3-cyanobenzofuran compounds and its preparation, can solve the problems of difficult scale-up production, long reaction time, high price, etc., and achieve mild reaction conditions, The effect of short reaction time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of methyl 2-ethylamino-3-cyanobenzofuran-5-carboxylate
[0029] Add methyl 3-dehydroshikimate (0.19g, 1mmol), malononitrile (0.10g, 1.5mmol) and 10ml of water into the flask, and react in a microwave reactor at 85°C (240W) for 10min. After the completion, the solvent was removed by suction filtration, and the solid obtained above, sodium bicarbonate (0.17g, 2mmol), bromoethane (0.13g, 1.2mmol) and 8ml dimethyl sulfoxide were added to the reaction flask, and heated at 120°C (240W) Microwave reaction for 5 minutes, after the reaction was completed, add 40ml of water, extract with ethyl acetate (3×10ml), combine the extracts, dry over anhydrous magnesium sulfate, concentrate, and separate by column chromatography to obtain 0.20g of light yellow solid, yield 82%, mp 189 -190°C. 1 HNMR (400MHz, DMSO-d 6 )δ: 8.93(t, J=5.60Hz, 1H), 7.74(d, J=1.60Hz, 1H), 7.71(dd, J 1 =8.40Hz,J 2 =1.60Hz,1H),7.49(d,J=8.40Hz,1H),3.86(s,3H),3.47(m,2H),1.25(t,J=4.00Hz,3H); HRMS(ESI...
Embodiment 2
[0031] Preparation of methyl 2-ethylamino-3-cyanobenzofuran-5-carboxylate
[0032]Add methyl 3-dehydroshikimate (0.19g, 1mmol), malononitrile (0.07g, 1mmol) and 10ml of water into the flask, react in a microwave reactor at 85°C (240W) for 10min, and the reaction is complete Finally, the solvent was removed, and the solid obtained above, sodium bicarbonate (0.17g, 2mmol), bromoethane (0.13g, 1.2mmol) and 8ml dimethyl sulfoxide were added to the reaction flask, and microwaved at 120°C (240W) for 5min After completion of the reaction, add 40ml of water, extract with ethyl acetate (3 × 10ml), combine the extracts, dry over anhydrous magnesium sulfate, concentrate, and separate by column chromatography to obtain 0.16g of light yellow solid, yield 66%, mp 189-190 ℃. The structural analysis data are the same as in Example 1.
Embodiment 3
[0034] Preparation of methyl 2-ethylamino-3-cyanobenzofuran-5-carboxylate
[0035] Add methyl 3-dehydroshikimate (0.19g, 1mmol), malononitrile (0.13g, 2mmol) and 10ml of water into the flask, react in a microwave reactor at 85°C (240W) for 10min, and the reaction is complete Finally, the solvent was removed, and the solid obtained above, sodium bicarbonate (0.17g, 2mmol), bromoethane (0.13g, 1.2mmol) and 8ml dimethyl sulfoxide were added to the reaction flask, and microwaved at 120°C (240W) for 5min After completion of the reaction, add 40ml of water, extract with ethyl acetate (3 × 10ml), combine the extracts, dry over anhydrous magnesium sulfate, concentrate, and separate through column chromatography to obtain 0.20g of light yellow solid, yield 82%, mp 189-190 ℃. The structural analysis data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com