Preparation method of compound containing benzofuran structure
A technology for benzofuran and compound is applied in the field of preparation of compounds containing benzofuran structure, can solve the problems of low yield, high price, serious environmental pollution of reaction reagents and the like, and achieves the effects of high yield and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
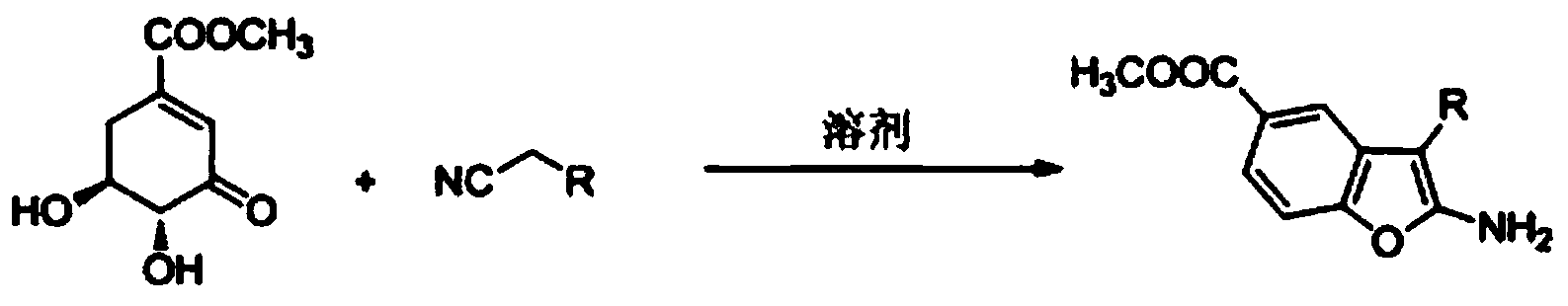
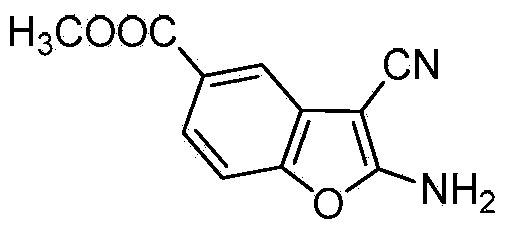
[0021] The preparation of 2-amino-3-cyanobenzofuran-5-carboxylic acid methyl ester comprises the following steps:
[0022] Add methyl 3-dehydroshikimate (0.37g, 2mmol), malononitrile (0.13g, 2mmol) and distilled water (10ml) into a 50ml two-necked flask equipped with a thermometer and a reflux condenser, and slowly heat up to 90°C , stirred for about 4 hours, a large amount of white solids were precipitated, and the reaction was detected by TLC. After the reaction was completed, it was cooled and filtered, and the solids were recrystallized with ethyl acetate to obtain white needle-like crystals of 2-amino-3-cyanobenzofuran-5- Methyl carboxylate 0.42g, yield 97%.
[0023] Compound characterization data are as follows: m.p.>200℃. 1 H NMR (400MHz, DMSO-d 6 ), δ: 8.50 (s, 2H, -NH 2 ), 7.74 (d, 1H, J=1.6Hz, 4-ArH), 7.70 (dd, 1H, J 1 =8.4Hz,J 2 = 1.6Hz, 6-ArH), 7.48(d, 1H, J=8.4Hz, 7-ArH), 3.86(s, 3H, -OCH 3 ); 13 C NMR (100MHz, DMSO-d 6 ), δ: 52.2, 60.1, 110.0, 114.7, 116....
Embodiment 2
[0026] The preparation of 2-amino-3-cyanobenzofuran-5-carboxylic acid methyl ester comprises the following steps:
[0027] Add methyl 3-dehydroshikimate (0.37g, 2mmol), malononitrile (0.20g, 3mmol) and distilled water (10ml) into a 50ml two-necked flask equipped with a thermometer and a reflux condenser, and slowly heat up to 100°C , stirred for about 3 hours, a large amount of white solids were precipitated, and the reaction was detected by TLC. After the reaction was completed, it was cooled and filtered, and the solid was recrystallized with ethyl acetate to obtain white needle-like crystals of 2-amino-3-cyanobenzofuran-5- Methyl carboxylate 0.41g, yield 95%.
[0028] Compound characterization data are as follows: m.p.>200℃. 1 H NMR (400MHz, DMSO-d 6 ), δ: 8.50 (s, 2H, -NH 2 ), 7.74 (d, 1H, J=1.6Hz, 4-ArH), 7.70 (dd, 1H, J 1 =8.4Hz,J 2 = 1.6Hz, 6-ArH), 7.48(d, 1H, J=8.4Hz, 7-ArH), 3.86(s, 3H, -OCH 3 ); 13 C NMR (100MHz, DMSO-d 6 ), δ: 52.2, 60.1, 110.0, 114.7, 116.6...
Embodiment 3
[0031] The preparation of 2-amino-3-cyanobenzofuran-5-carboxylic acid methyl ester comprises the following steps:
[0032] Add methyl 3-dehydroshikimate (0.37g, 2mmol), malononitrile (0.15g, 2.2mmol) and ethanol (10ml) into a 50ml two-necked flask equipped with a thermometer and a reflux condenser, and reflux for about 3h. The reaction was detected by TLC. After the reaction was completed, it was cooled, and the solvent was removed by rotary evaporation. The solid was recrystallized from ethyl acetate to obtain 0.38 g of white needle-like crystals, with a yield of 89%.
[0033] Compound characterization data are as follows: m.p.>200℃. 1 H NMR (400MHz, DMSO-d 6 ), δ: 8.50 (s, 2H, -NH 2 ), 7.74 (d, 1H, J=1.6Hz, 4-ArH), 7.70 (dd, 1H, J 1 =8.4Hz,J 2 = 1.6Hz, 6-ArH), 7.48(d, 1H, J=8.4Hz, 7-ArH), 3.86(s, 3H, -OCH 3 ); 13 C NMR (100MHz, DMSO-d 6 ), δ: 52.2, 60.1, 110.0, 114.7, 116.6, 123.2, 125.8, 128.7, 150.5, 166.0, 166.6; IR(KBr), v / cm -1 :3409,3320,2950,2212,1718,1646,158...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com