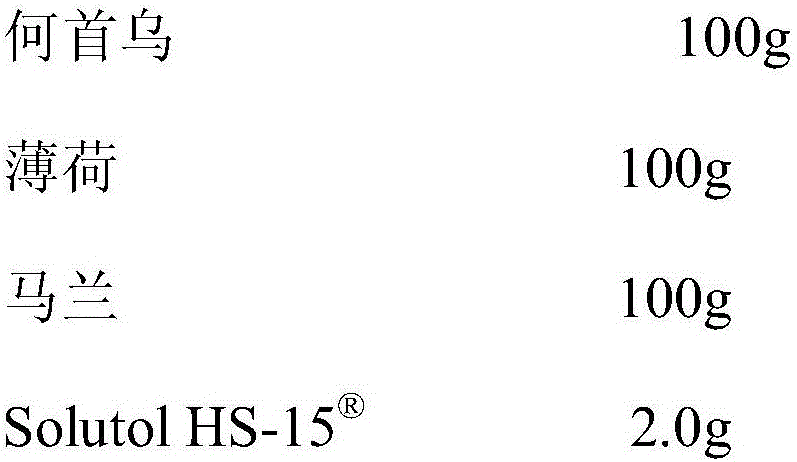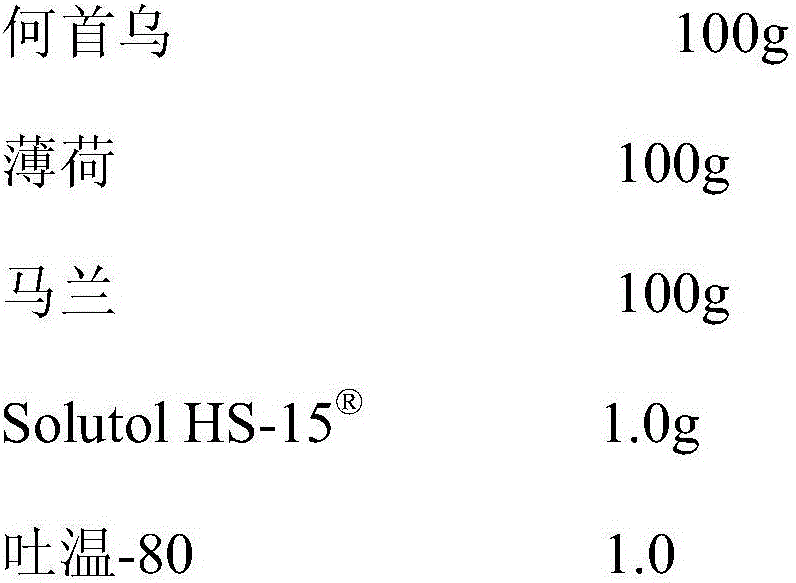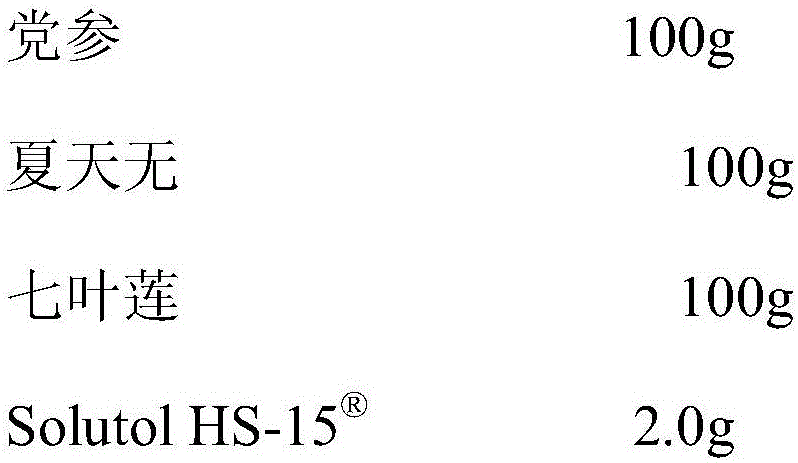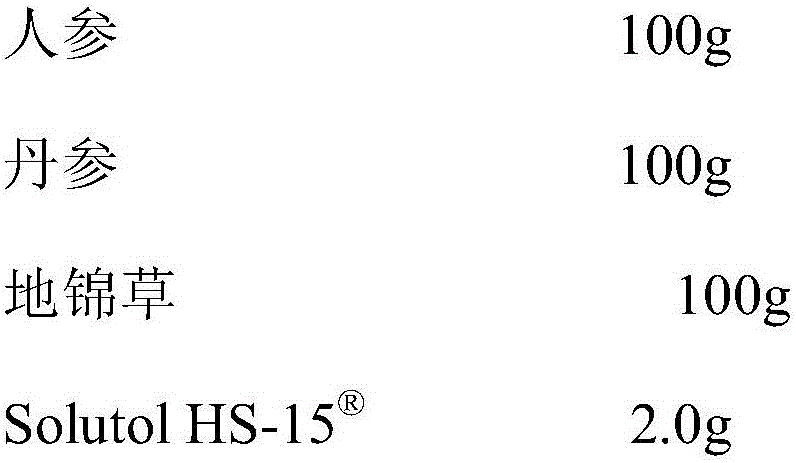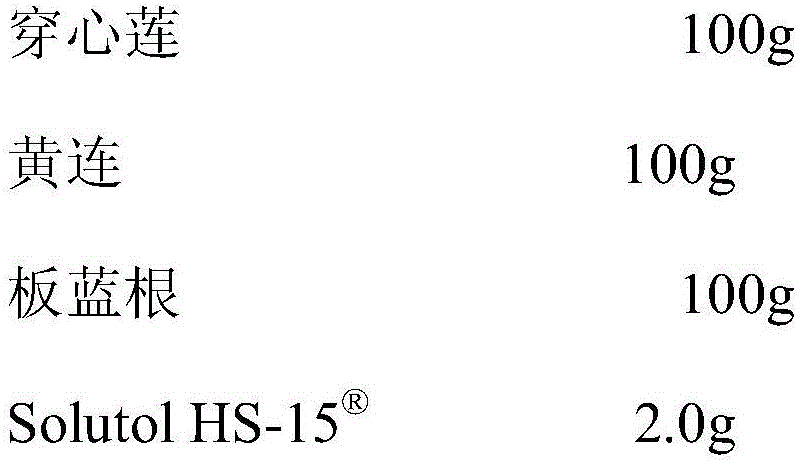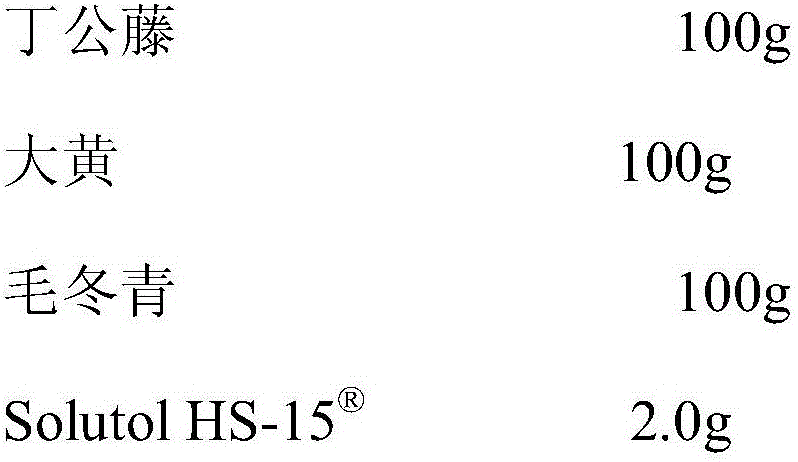Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37results about How to "The effect of solubilization is obvious" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alcohol base gasoline solubilizer and preparation method thereof
InactiveCN101544919ANo pollution in the processNo special smellLiquid carbonaceous fuelsFuranAntioxidant
The invention discloses alcohol base gasoline solubilizer and a preparation method thereof. The solubilizer comprises isopropyl ether, acetamide, alkylphenol polyether, methyl tert-butyl ether, fatty acid methyl ester, furan, tertiary butanol, antioxidant and anti-swelling agent. The preparation method comprises the following steps: weighing isopropyl ether, tertiary butanol, acetamide, alkylphenol polyether, methyl tert-butyl ether, fatty acid methyl ester, furan, antioxidant and anti-swelling agent according to a certain weight ratio, adding isopropyl ether and tertiary butanol in a reaction kettle for vacuumization, warming the reaction kettle, adding acetamide, stirring, adding alkylphenol polyether, stirring and cooling through a cooler, adding methyl tert-butyl ether, fatty acid methyl ester, furan, antioxidant and anti-swelling agent, and uniformly stirring to obtain the alcohol base gasoline solubilizer. The invention has no toxicity, no pollution and no special odor, less adding amount, good solubilizing effect, and no heavy metal component, no generation of new pollution and no mechanical impurity, and can increase the octane of 90# gasoline to 95-99#.
Owner:XIAN JIAHONG PETROCHEM TECH
Pharmaceutical composition for improving safety of compound gastrodin injection
InactiveCN105079067AImprove securityReduced responseOrganic active ingredientsAntipyreticPolyethylene glycolHydroxystearic Acid
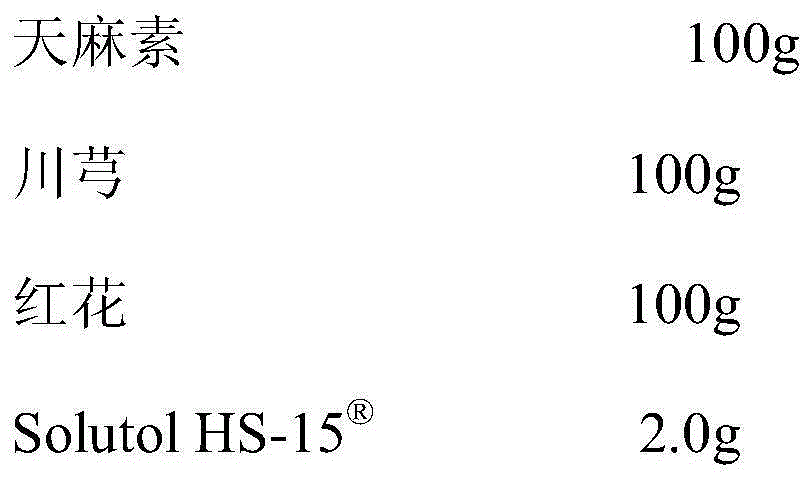
The invention discloses a pharmaceutical composition for improving the safety of a compound gastrodin injection. The pharmaceutical composition is mainly prepared from gastrodin extract, extract of chuanxiong rhizome, safflower extract and Solutol HS-15 for injections. According to the pharmaceutical composition for improving the safety of the compound gastrodin injection, cosolvent of polysorbate 80 which can cause potential safety hazards and affect product quality is replaced with cosolvent with better safety and a more obvious hydrotropy effect in the compound gastrodin injection, the safety of the Solutol HS-15 is higher than that of the polysorbate 80, the dosage of the Solutol HS-15 is lower than that of the polysorbate 80, the occurrence probability of untoward effects and risks of pharmaceuticals are reduced, and the safety of clinical medication is improved.
Owner:CHENGDU AIBIKE BIOTECH
Medicine composition comprising butylphthalide and cosolvent
InactiveCN105999279AReduce dosageImprove solubilityOrganic active ingredientsNervous disorderSolubilityAcupuncture
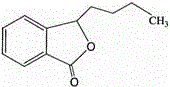
The invention relates to the field of medicine, particularly to a medicine composition comprising butylphthalide and cosolvent, and aims to adopt a novel cosolvent to improve the water solubility of butylphthalide, develop clinically required solid, semisolid or liquid preparations of butylphthalide, and better exert the therapeutical effect of butylphthalide. The composition can be used for preparing a plurality of dosage forms such as tablets, capsules, ointment, cream, gel, transfusion, aqueous acupuncture, powder for injection, oral liquid and the like. Compared with the prior art, the medicine composition realizes better safety and improvement on the water solubility of butylphthalide.
Owner:SICHUAN MANSAISI MEDICINE TECH CO LTD
Liquid deodorant and preparation method thereof
ActiveCN109481713APrevent regenerationEasy to deodorizeBiocideDisinfectantsSodium bicarbonateDeodorant
The invention belongs to the technical field of deodorant preparation, and particularly relates to a liquid deodorant and a preparation method thereof. The liquid deodorant comprises, in weight percent, 0.5-2.5% of beta-cyclodextrin, 1-4% of malic acid, 1-4% of citric acid, 0.1-2% of salicylic acid, 0.5-4% of sodium bicarbonate, 0.5-2% of polyoxyethylene nonionic surfactants, 0.5-2% of essence and80-95% of water. The liquid deodorant eliminates odor molecules in original existing environments by a physical absorption and chemical neutralization mode, sterilization and disinfection componentsare added, microbial reproduction is effectively inhibited, regeneration of the odor molecules is fundamentally prevented, the essence is added, and experience and enjoyment of the deodorant are greatly improved.
Owner:壹田(广州)生活健康用品有限公司
Indissolvable drug inclusion compound, inclusion method and chlortetracycline hydrochloride soluble powder
PendingCN112957481AImprove rapid dissolving abilityIncreased fast dissolving powerPowder deliveryTetracycline active ingredientsOrganic acidDrugs preparations
The invention provides an indissolvable drug inclusion method, and belongs to the technical field of biological medicines. The method comprises the following steps of stirring and dissolving beta-cyclodextrin in water, and preparing a beta-cyclodextrin solution; adding an indissolvable drug into the beta-cyclodextrin solution in batches, fully stirring, adding an organic acid, and stirring and mixing again to prepare an inclusion solution; and adding an organic solvent into the inclusion solution, stirring to separate out an inclusion product, standing at low temperature, and filtering out the product to obtain the inclusion product. The preparation method comprises the following steps of conducting inclusion of the water indissolvable drug raw material and beta-cyclodextrin with entrapment capacity by using an inclusion-hydrotropy-recrystallization process flow, assisting the hydrotropy effect of organic acid, and carrying out recrystallization precipitation to prepare the inclusion drug composition. The method is especially suitable for pharmaceutical preparations which are difficult in water solubility, not resistant to high temperature and unstable in aqueous solution.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
Preparing method for pharmaceutical composition capable of improving safety of compound ginkgo biloba injection
InactiveCN105902580AImprove securityReduced responseOrganic active ingredientsAntipyreticDipyridamoleHydroxystearic Acid
The invention discloses a preparing method for pharmaceutical composition capable of improving safety of compound ginkgo biloba injection. The pharmaceutical composition is mainly prepared from ginkgo biloba extract, dipyridamole extract, polysorbate extract and polyethylene glycol-12-hydroxyl stearate and is applied to injection. A cosolvent of polysorbate 80 which has safety hazard and can affect product quality in the compound ginkgo biloba injection is replaced by a cosolvent which is better in safety and more obvious in hydrotropy effect. The safety of the polyethylene glycol-12-hydroxyl stearate is higher than that of the polysorbate 80, the using amount of the polyethylene glycol-12-hydroxyl stearate is lower, the probability and the risk of untoward effects of medicine are reduced, and the safety of clinical medication is improved.
Owner:CHENGDU YICHUANGSI BIOLOGICAL SCI & TECH
Aquaculture pond water treatment agent
InactiveCN107162129AIncrease contact areaGood sustained release effectWater/sewage treatment using germicide/oligodynamic-processChemistryUrea
The present invention relates to an aquaculture pond water treatment agent, which comprises the following raw materials by weight: 3-5 parts of degradable porous microspheres, 1-2 parts of dibromohydantoin, 1-2 parts of an auxiliary material, 0.2-0.4 part of diniconazole, 1-2 parts of a rheum palmatum l extract, and 0.4-0.8 part of urea. The aquaculture pond water treatment agent of the present invention can effectively improve the solubility of dibromohydantoin in water while can avoid the pollution of the water treatment agent on fish, shrimp and other aquatic products, and can further provide the healthy and effective water treatment agent.
Owner:XINGHUA HENGWEI BIOTECH
Pharmaceutical composition capable of improving safety of compound radix polygoni multiflori injection
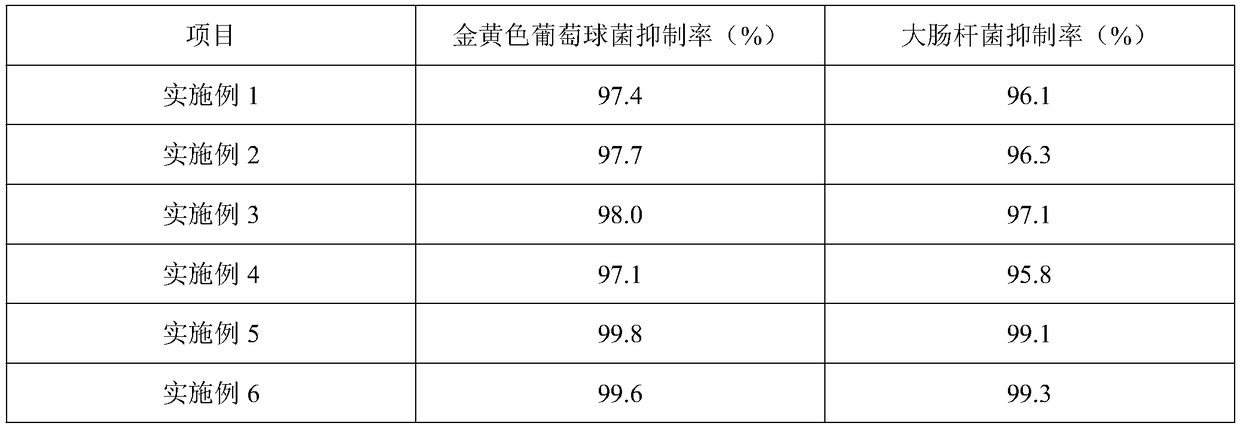
InactiveCN105920117AGood securityThe effect of solubilization is obviousPowder deliveryAntipyreticStearateKalimeris
The invention discloses a pharmaceutical composition capable of improving safety of a compound radix polygoni multiflori injection. The pharmaceutical composition is characterized in that the pharmaceutical composition for injection is mainly prepared from a radix polygoni multiflori extract, a herba menthae extract, an Indian kalimeris herb extract and polyglycerol dodecahydroxyl stearate, each 100 ml of solution contains the radix polygoni multiflori extract containing 10-35 g of radix polygoni multiflori, the herba menthae extract containing 20-75 g of herba menthae and the Indian kalimeris herb extract containing 10-35 g of Indian kalimeris herb, and the dosage ratio of radix polygoni multiflori to herba menthae to Indian kalimeris herb is 1:2:1. According to the pharmaceutical composition capable of improving the safety of the compound radix polygoni multiflori injection, cosolvent which is good in safety and obvious in solubilization assisting effect is adopted to replace the cosolvent polysorbate 80 which has potential safety hazards and affects the product quality in the compound radix polygoni multiflori injection, the safety of polyglycerol dodecahydroxyl stearate is higher than that of polysorbate 80, the dosage of polyglycerol dodecahydroxyl stearate is lower than that of polysorbate 80, probability and risks of adverse reactions of drugs are reduced, and the safety of clinical medication is improved.
Owner:成都市斯贝佳科技有限公司
Method for improving safety of compound radix angelicae sinensis injection solution
InactiveCN107661369AImprove securityThe effect of solubilization is obviousAntipyreticAnalgesicsMedicinePanax notoginseng extract
The invention discloses a method for improving the safety of compound angelica injection. The method mainly prepares a pharmaceutical composition for injection from angelica extract, astragalus extract, notoginseng extract and methyl methacrylate. The method of the present invention adopts the co-solvent with better safety and more obvious solubilizing effect to replace the co-solvent phenoxyethanol which has potential safety hazards and affects product quality in the compound angelica injection, and the safety of methyl methacrylate is higher than that of phenoxyethanol Moreover, the dosage is lower, which reduces the probability and risk of adverse drug reactions and improves the safety of clinical medication.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
Levodopa nasal dry powder for treating Parkinson's dyskinesia and preparation method thereof
ActiveCN114533676ARapidly absorbed into the bloodThe effect of solubilization is obviousPowder deliveryOrganic active ingredientsCyclodextrinLactose
The invention discloses a levodopa nasal dry powder for treating Parkinson's dyskinesia and a preparation method thereof, the levodopa nasal dry powder is a mixture obtained by co-grinding or co-crushing levodopa and a carrier, or a mixture obtained by co-grinding or co-crushing levodopa, a carrier and other auxiliary components; and the carrier is hydroxypropyl beta-cyclodextrin (HP-beta-CD), alpha-cyclodextrin, methylated-beta-cyclodextrin, sodium alginate or lactose. According to the levodopa dry powder preparation disclosed by the invention, levodopa is rapidly absorbed into blood through a nose to achieve relatively high bioavailability and plasma concentration, so that dyskinesia caused by a switching effect is rapidly relieved, and the problems of instability of levodopa and the like caused by a solution preparation are solved. A subsequent animal pharmacokinetic test proves that the co-ground material can achieve the effect of being rapidly absorbed into blood, which means that the dyskinesia caused by the Parkinson's switch effect is possibly rapidly relieved.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Pharmaceutical composition for improving safety of compound Herba Artemisiae Scopariae injection
InactiveCN106389322AImprove securityReduced responsePowder deliveryAntipyreticPanax notoginseng extractPolyethylene glycol
The invention discloses a pharmaceutical composition for improving safety of a compound Herba Artemisiae Scopariae injection. The pharmaceutical composition is used for injection and is mainly prepared from Herba Artemisiae Scopariae extract, Bupleuri Herba extract, Radix Notoginseng extract and polyoxyl 15 hydroxystearate. A cosolvent which is better in safety and more obvious in dissolving assisting effect replaces polysorbate 80 which is used as a cosolvent, has potential safety hazards and affects quality of products in the compound Herba Artemisiae Scopariae injection, the safety of the polyoxyl 15 hydroxystearate is higher than that of the polysorbate 80, the use amount is lower, probability and risks of adverse reactions of drugs are reduced, and safety of clinical medication is improved.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Preparation method of pharmaceutical composition for improving safety of compound radix codonopsis injection solution
InactiveCN106361826AThe effect of solubilization is obviousImprove securityPowder deliveryAntipyreticAcetic acidTert butyl
The invention discloses a preparation method of a pharmaceutical composition for improving the safety of a compound radix codonopsis injection solution. The pharmaceutical composition is a pharmaceutical composition for injection, which is mainly prepared from radix codonopsis extract, rhizoma corydalis decumbentis extract, scandent schefflera root extract and tert-butyl peroxyacetate. According to the preparation method provided by the invention, a co-solvent, which has better safety and more obvious auxiliary dissolving effect, is used for replacing a co-solvent polysorbate 80 which has potential safety hazards on the compound radix codonopsis injection solution and influences product quality, the safety of the tert-butyl peroxyacetate is higher than that of the polysorbate 80 and the use amount of the tert-butyl peroxyacetate is lower; the probability and risks of untoward effects caused by medicines are reduced and the safety of clinical medication is improved.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Method for improving safety of compound rhizoma corydalis decumbentis injection solution
InactiveCN107661440AImprove securityThe effect of solubilization is obviousPowder deliveryAntipyreticAcrylic resinSodium benzoate
The invention discloses a method for improving the safety of compound summer injection. According to the method, the pharmaceutical composition for injection is mainly prepared from the extracts of summer grass, snow lotus extract, silver orchid extract and enteric-coated acrylic resin. The method of the present invention adopts the co-solvent sodium benzoate that security is better, the solubilizing effect is more obvious to replace the co-solvent sodium benzoate that has potential safety hazard and influence product quality in compound summer non-injection, and the safety of enteric-coated acrylic resin is higher than sodium benzoate and The dosage is lower, reducing the probability and risk of adverse drug reactions, and improving the safety of clinical medication.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
A kind of non-fried sweet potato processing technology
ActiveCN111869845BImprove flavor qualityImprove nutritional qualityFruits/vegetable preservation by coatingFood ingredient as coating agentFood gradeProcess engineering
Owner:BEIJING FUYINONG CHESTNUTS
A kind of Dexibuprofen injection and preparation method thereof
ActiveCN104146951BReduce dosageThe effect of solubilization is obviousOrganic active ingredientsAntipyreticIbuprofen InjectionSolvent
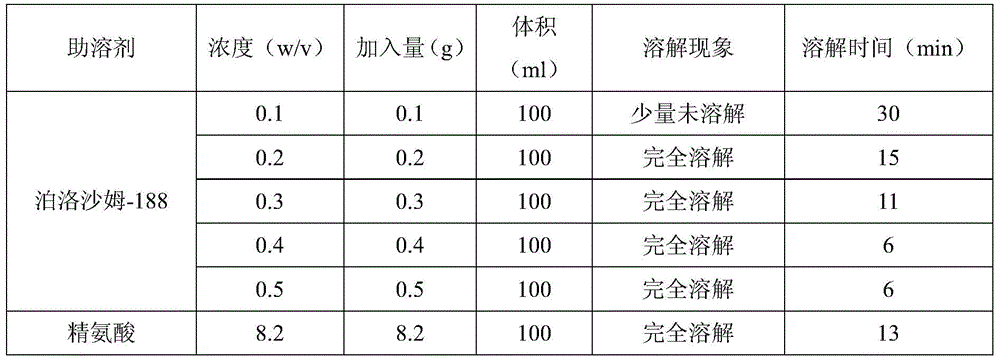
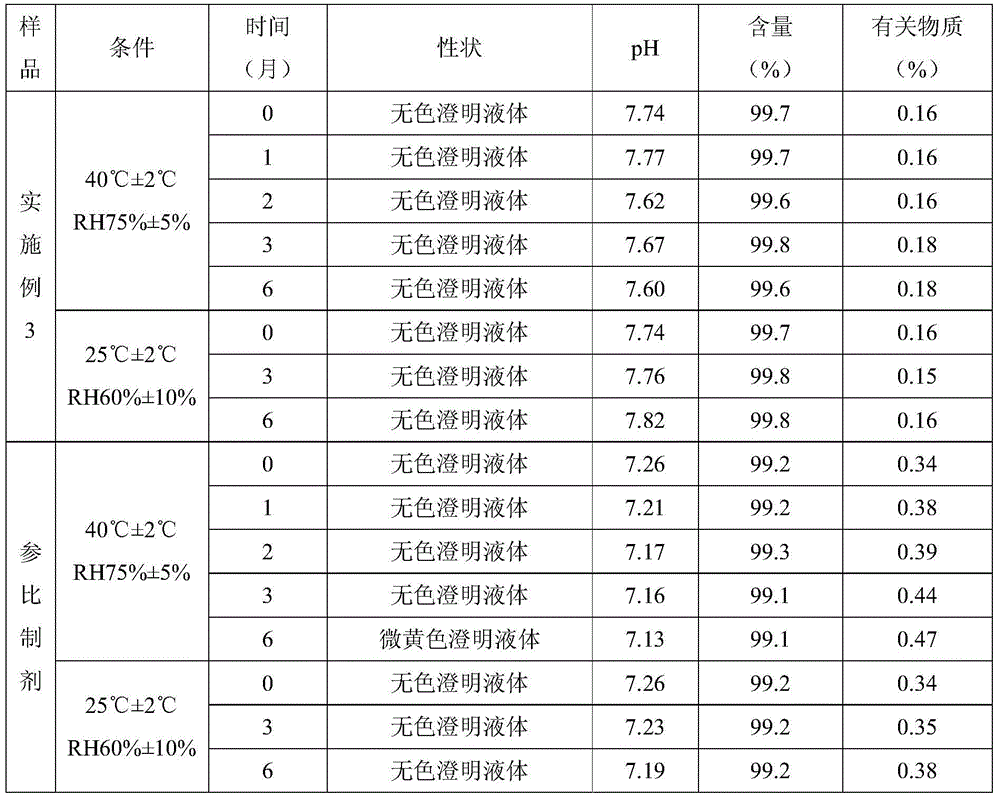
The invention relates to a dexibuprofen injection and a preparation method thereof, and particularly relates to a dexibuprofen injection prepared by using poloxamer-188 as a cosolvent and a preparation method thereof. In the injection, the weight ratio of dexibuprofen to poloxamer-188 is 100:(2-5). The invention provides the effective method which is capable of quickly dissolving dexibuprofen, the auxiliary usage is small, and the safety is high. Drug stability tests and animal safety tests indicate that the dexibuprofen product prepared by the method is safe and stable, and has a good drug effect.
Owner:沈阳双鼎制药有限公司
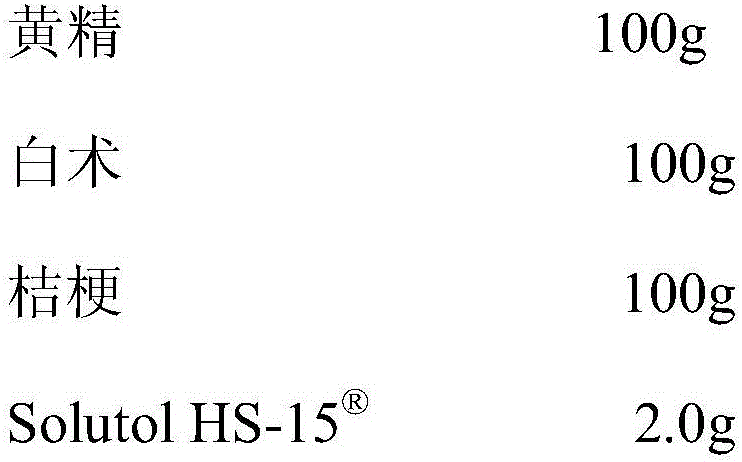
Pharmaceutical composition capable of improving safety of compound rhizoma polygonati injection
InactiveCN105878731AGood securityThe effect of solubilization is obviousPowder deliveryAntipyreticStearateRhizome
The invention discloses a pharmaceutical composition capable of improving the safety of a compound rhizoma polygonati injection. The pharmaceutical composition is characterized in that the pharmaceutical composition is an injection pharmaceutical composition prepared from a rhizoma polygonati extract, a bighead atractylodes rhizome extract, a platycodon root extract and glycerin polyoxyethylene ether hydroxystearate, wherein each 100ml solution contains 15 to 45g of rhizoma polygonati extract prepared from rhizoma polygonati, 10 to 10g of bighead atractylodes rhizome extract prepared from bighead atractylodes rhizome and 5 to 15g of platycodon root extract prepared from platycodon root, and the dosage ratio of the rhizoma polygonati to the bighead atractylodes rhizome to the platycodon root is 3 to 2 to 1. According to the pharmaceutical composition disclosed by the invention, a cosolvent which has better safety and more obvious solubilization effect is adopted to substitute a cosolvent polysorbate 80, which has potential safety hazards and affects the product quality, in the compound rhizoma polygonati injection, and the glycerin polyoxyethylene ether hydroxystearate has higher safety and lower dosage than those of the polysorbate 80, such that the probability and risk of adverse reactions of the pharmaceutical composition are reduced, and the safety of the clinical medication is improved.
Owner:CHENGDU YISHENGKE BIOTECH
Medicine compound for increasing safety of compound codonopsis pilosula injection
InactiveCN106309569AImprove securityReduced responsePowder deliveryAntipyreticAcetic acidCodonopsis pilosula
The invention discloses a medicine compound for increasing the safety of a compound codonopsis pilosula injection. The medicine compound is an injection medicine compound which is made from codonopsis pilosula extract, corydalis amabilis extract, schefflera arboricola extract and tert-butyl peroxyacetate. According to the invention, a cosolvent with higher safety and more obvious fluxing effect is used for replacing the cosolvent polysorbate 80 with potential safety hazards and influences on product quality in the compound codonopsis pilosula injection, the safety of tert-butyl peroxyacetate is higher than the safety of polysorbate 80 and the dosage is less, so that the probability and risk of untoward effects of the medicine can be reduced and the safety of clinical medication can be increased.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Alcohol base gasoline solubilizer and preparation method thereof
InactiveCN101544919BNo pollution in the processNo special smellLiquid carbonaceous fuelsFuranAntioxidant
The invention discloses alcohol base gasoline solubilizer and a preparation method thereof. The solubilizer comprises isopropyl ether, acetamide, alkylphenol polyether, methyl tert-butyl ether, fatty acid methyl ester, furan, tertiary butanol, antioxidant and anti-swelling agent. The preparation method comprises the following steps: weighing isopropyl ether, tertiary butanol, acetamide, alkylphenol polyether, methyl tert-butyl ether, fatty acid methyl ester, furan, antioxidant and anti-swelling agent according to a certain weight ratio, adding isopropyl ether and tertiary butanol in a reaction kettle for vacuumization, warming the reaction kettle, adding acetamide, stirring, adding alkylphenol polyether, stirring and cooling through a cooler, adding methyl tert-butyl ether, fatty acid methyl ester, furan, antioxidant and anti-swelling agent, and uniformly stirring to obtain the alcohol base gasoline solubilizer. The invention has no toxicity, no pollution and no special odor, less adding amount, good solubilizing effect, and no heavy metal component, no generation of new pollution and no mechanical impurity, and can increase the octane of 90# gasoline to 95-99#.
Owner:XIAN JIAHONG PETROCHEM TECH
Method for improving safety of compound herba sambuci chinensis injection solution
InactiveCN107661292AGood securityThe effect of solubilization is obviousPowder deliveryAntipyreticSolventMarasmius
The invention discloses a method for improving the safety of compound Luying injection. According to the method, the medicinal composition for injection is mainly prepared from the extract of Lu Ying, the extract of the hairy needles, the extract of the seeds of the iris and the hydrogenated castor oil. The method of the present invention adopts the co-solvent with better safety and more obvious solubilizing effect to replace the co-solvent carbomer that has potential safety hazards and affects product quality in the compound Luying injection, and the safety of hydrogenated castor oil is higher than that of carbomer. The dosage is lower, reducing the probability and risk of adverse drug reactions, and improving the safety of clinical medication.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
Medicine composition for improving safety of compound rhizoma corydalis decumbentis injection solution
InactiveCN107661289AImprove securityThe effect of solubilization is obviousPowder deliveryAntipyreticAcrylic resinSodium benzoate
The invention discloses a medicine composition for improving the safety of a compound rhizoma corydalis decumbentis injection solution. The medicine composition is mainly prepared from a rhizoma corydalis decumbentis extract, a flos saussureae involucratae extract, a herba cephalantherae erectae extract and an enteric coated acrylic resin. According to the medicine composition provided by the invention, a cosolvent with better safety and a more obvious dissolution-promotion effect is adopted to replace a cosolvent sodium benzoate with potential safety hazard and probability of affecting product quality in the compound rhizoma corydalis decumbentis injection solution, the safety of the enteric coated acrylic resin is higher than the safety of the sodium benzoate, the dosage of the enteric coated acrylic resin is lower than the dosage of the sodium benzoate, the probability and the risk of side effects happened by the medicine are reduced, and the safety of clinical medication is improved.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
Medicine composition for improving safety of compound humifuse euphorbia herb injection solution
InactiveCN107661290AImprove securityReduce dosageAntipyreticAnalgesicsDrug adverse reactionsMedicine
The invention discloses a pharmaceutical composition for improving the safety of compound dijincao injection. The pharmaceutical composition is a pharmaceutical composition for injection mainly made of Dijincao extract, Bailiangjin extract, Jigucao extract and hydroxypropyl acrylate. In the present invention, the co-solvent with better safety and more obvious solubilizing effect is used to replace the co-solvent glycerin in the compound Dijincao injection, which has potential safety hazards and affects product quality, and the safety of hydroxypropyl acrylate is higher than that of glycerol Moreover, the dosage is lower, which reduces the probability and risk of adverse drug reactions and improves the safety of clinical medication.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
Pharmaceutical composition capable of improving safety of compound smilax glabra injection
InactiveCN105902773AImprove securityReduced responsePowder deliveryAntipyreticMedicinePolyethylene glycol
The invention discloses a pharmaceutical composition capable of improving the safety of a compound smilax glabra injection. The pharmaceutical composition is characterized by being mainly prepared from a smilax glabra extract, a fructus shisandrae extract, a semen cuscutae extract and polyethylene glycol stearate, wherein each 100ml of solution contains 20-75g of smilax glabra extract of a smilax glabra material, 20-75g of the fructus shisandrae extract of a fructus shisandrae material and 10-35g of semen cuscutae extract of a semen cuscutae material, wherein the dosage ratio of smilax glabra to fructus shisandrae to semen cuscutae is 2 to 2 to 1. A cosolvent polysorbate 80 which has a potential safety hazard and affects the product quality in the compound smilax glabra injection is replaced with the cosolvent which is better in safety and more obvious in solubilizing effect; the polyethylene glycol stearate is higher than the polysorbate 80 in safety and lower than the polysorbate 80 in dosage; the probability and risk of an adverse effect of the medicine are reduced; and the clinical medication safety is improved.
Owner:成都市斯贝佳科技有限公司
Pharmaceutical composition for improving safety of compound puerarin injection
InactiveCN106389318AImprove securityReduced responseOrganic active ingredientsAntipyreticMedicineTraditional medicine
The invention discloses pharmaceutical composition for improving safety of compound puerarin injection. The pharmaceutical composition is mainly prepared from puerarin extract, Chinese thorowax root extract, corn cervi pantotrichum extract and dibutyl fumarate and is used for injection. A cosolvent with better safety and more significant solubilization effect is used for substituting for a cosolvent polysorbate 80 having potential safety hazards, affecting product quality and existing in the compound puerarin injection, dibutyl fumarate is higher than polysorbate 80 in safety and lower in consumption, the probability and the risk of adverse reactions of the composition are reduced, and the clinical medication safety is improved.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Preparation method of pharmaceutical composition for improving safety of compound ginseng injection
InactiveCN106361814AImprove securityReduced responsePowder deliveryAntipyreticPolyethylene glycolSalvia extract
The invention discloses a preparation method of a pharmaceutical composition for improving safety of a compound ginseng injection. The pharmaceutical composition is mainly prepared from ginseng extract, salvia extract, humifuse euphorbia herb extract and polyethylene glycol 15-hydroxystearic acid, and used for injection. The preparation method of the pharmaceutical composition uses cosolvent good in safety and obvious in dissolving aiding effect for replacing cosolvent polysorbate 80 in the compound ginseng injection, which has potential safety hazards and influences product quality, and the polyethylene glycol 15-hydroxystearic acid has safely higher than the cosolvent polysorbate 80, and furthermore the usage amount of the polyethylene glycol 15-hydroxystearic acid is lower than that of the cosolvent polysorbate 80, and therefore a probability and risk of adverse effects of drugs are reduced, and safety of clinical medication is improved.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Pharmaceutical composition capable of improving safety of compound manglietia injection
InactiveCN105943736AImprove securityReduced responsePowder deliveryAntipyreticMedicinal herbsSolanum nigrum extract
The invention discloses a pharmaceutical composition capable of improving safety of a compound manglietia injection. The pharmaceutical composition for injection is characterized by being mainly prepared from a manglietia extract, an extract of black nightshade herb, a banana extract and polyethylene glycol dodecahydroxyl stearate, wherein each 100ml of the solution (the pharmaceutical composition) consists of the manglietia extract of 10-20g of a manglietia medicinal material, the black nightshade herb extract of 10-20g of a black nightshade herb medicinal material and the banana extract of 1-2g of a banana medicinal material, and the dosage ratio of the manglietia medicinal material to the black nightshade herb medicinal material to the banana medicinal material is 10 to 10 to 1. According to the pharmaceutical composition disclosed by the invention, a cosolvent, namely polysorbate 80, which has safety hidden dangers and has influence on product quality in the compound manglietia injection is replaced by a cosolvent which is better in safety and is more remarkable in dissolve-assisting effect with a lower dosage, so that the probability and risks of adverse reactions of the medicine (the pharmaceutical composition) are reduced, and the safety of clinical administration is improved.
Owner:CHENGDU YISHENGKE BIOTECH
Preparation method of drug composition for improving safety of compound crocus sativus injection
This invention discloses a preparation method of a drug composition for improving the safety of a compound crocus sativus injection. The drug composition for injection is mainly prepared from a crocus sativus extract, a pubescent Holly root extract, a radix stephaniae tetrandrae extract and butyl phthalyl butyl glycolate. A cosolvent good in safety and obvious in solubilizing-aiding effect is adopted to replace a cosolvent polysorbate 80 having potential safety hazard and affecting the product quality existing in the compound crocus sativus injection, the safety of the butyl phthalyl butyl glycolate is higher than that of the polysorbate 80, the usage amount of the butyl phthalyl butyl glycolate is lower, the probability and risk of adverse reaction of drugs are reduced, and the safety of clinical medication is improved.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
A polycarbonate additive mixing and adding system and using method thereof
ActiveCN109603618BMinimize impact on product qualityAvoid introducingTransportation and packagingRotary stirring mixersPolymer scienceStatic mixer
The invention belongs to the technical field of polycarbonate processing, and specifically relates to a polycarbonate additive mixing and adding system, comprising a mixing preparation tank, a filter, a feeding tank, a pumping pump and a static mixer sequentially connected through a delivery pipe; The terminator of polycarbonate is housed in the mixing and preparing tank, the inner wall of the mixing and preparing tank is wound with a heat conduction pipe, and the top of the mixing and preparing tank is provided with a feeding hopper for auxiliary agents to be put in, and the static mixer is provided with a There is an oligomeric polycarbonate feed inlet, and the pump is connected to the oligomeric polycarbonate feed inlet. The invention enables the auxiliary agent to be dissolved in the terminator required in the production process of polycarbonate, avoiding the introduction of a new auxiliary agent solvent, so that the auxiliary agent can better improve the quality and performance of polycarbonate products.
Owner:NINGBO ZHETIE DAPHOON CHEM
Pharmaceutical composition capable of improving safety of compound andrographis injection
The invention discloses a pharmaceutical composition capable of improving the safety of a compound andrographis injection. The pharmaceutical composition is a pharmaceutical composition for injection, prepared from andrographis extract, coptis chinensis extract, radix isatidis extract and hexafluorobutyl methacrylate. According to the invention, a cosolvent, polysorbate 80, which has potential safety hazards and influences the product quality in the compound andrographis injection is replaced with a cosolvent with better safety and more obvious solubilizing effect, the hexafluorobutyl methacrylate is higher in safety than the polysorbate 80 and is low in usage amount, the probability and risk of causing adverse reactions of the drug can be reduced, and the clinical medication safety is improved.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Pharmaceutical composition capable of improving safety of compound obtuseleaf erycibe stem injection
InactiveCN106344505AGood securityThe effect of solubilization is obviousAntipyreticAnalgesicsRheum officinaleMethacrylate
The invention discloses a pharmaceutical composition for improving the safety of compound Dinggongteng injection, the pharmaceutical composition is mainly composed of Dinggongteng extract, rhubarb extract, hair holly extract and hexafluorobutyl methacrylate for injection pharmaceutical composition. The present invention adopts the co-solvent with better safety and more obvious solubilizing effect to replace the co-solvent polysorbate 80, which has potential safety hazards and affects product quality in the compound Dinggongteng injection, and the safety of hexafluorobutyl methacrylate is higher than that of polysorbate The ester 80 is high and the dosage is lower, which reduces the probability and risk of adverse drug reactions and improves the safety of clinical medication.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Medicine composition for improving safety of compound radix angelicae sinensis injection solution
InactiveCN107661368AImprove securityThe effect of solubilization is obviousPowder deliveryAntipyreticDrug adverse reactionsPanax notoginseng extract
The invention discloses a pharmaceutical composition for improving the safety of compound angelica injection. The pharmaceutical composition is a pharmaceutical composition for injection mainly made of angelica extract, astragalus extract, notoginseng extract and methyl methacrylate. The present invention adopts the co-solvent with better safety and more obvious solubilizing effect to replace the co-solvent phenoxyethanol which has potential safety hazards and affects product quality in the compound angelica injection, and the safety of methyl methacrylate is higher than that of phenoxyethanol. The dosage is lower, reducing the probability and risk of adverse drug reactions, and improving the safety of clinical medication.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com