Levodopa nasal dry powder for treating Parkinson's dyskinesia and preparation method thereof
A technology of levodopa nasal dry powder and levodopa, which is applied in directions such as medical preparations without active ingredients, medical preparations containing active ingredients, powder delivery, etc., can solve the problem of limited nasal volume, unstable levodopa and easy Degradation, can not meet and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of levodopa nasal dry powder:
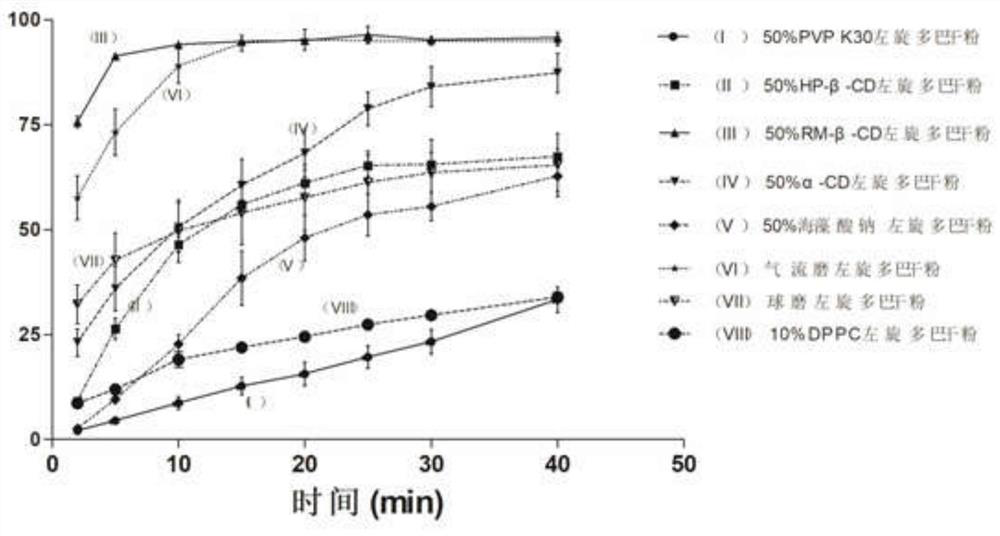
[0039] Ⅰ. Weigh 2.5g of L-dopa, put it in a sealed container with 2.5g of any methylated-β-cyclodextrin (RM-β-CD), mix well, dry the mixture in an oven at 80°C for 0.5h, take it out and set it to dry Cool down to room temperature in the machine; in an environment with relative humidity < 50%, put the mixture into the agate ball milling cavity of a German flying ball mill, add grinding beads, and perform planetary ball milling after sealing. After 10 minutes, it was stopped for 10 minutes, and the cycle was repeated 72 times. The total grinding time was 12 hours. After grinding, carry out subpackaging under the condition of <50% RH to obtain the levodopa nasal dry powder (I) for the treatment of Parkinson's dyskinesia;
[0040] Ⅱ. Weigh 2.5g of levodopa in the same way, respectively mix with 2.5g of polyvinylpyrrolidone 30 (PVP K30), 2.5g of hydroxypropyl-β-cyclodextrin (HP-β-CD), 2.5g of α-cyclodextrin (α-CD) and 2.5g sodium...
Embodiment 2
[0046] Weigh 2.5g of levodopa and place them in 2.5g of any methylated-β-cyclodextrin (RM-β-CD) and 2.5g of dimethyl-β-cyclodextrin (DM-β-CD) respectively. In a sealed container, mix well, vacuum dry at 80°C for 0.5h, take it out and put it in a desiccator to cool to room temperature; in an environment with relative humidity < 50%, put the mixture into the agate ball milling chamber of a German flying ball mill, add grinding beads, and seal it. Then carry out planetary ball milling, set the ball milling speed to 500 rpm, centrifugal force 4 kg, stop 10 min after grinding for 10 min, cycle 12 times, and the total grinding time is 2 h. After grinding, carry out sub-packaging under the condition of <50% RH, respectively obtain the levodopa nasal dry powder I (containing RM-β-CD) and II (containing DM-β) for the treatment of Parkinson's dyskinesia. -CD);
[0047] The dissolution experiment was carried out with reference to the method in Example 1, and the dissolution curve of lev...
Embodiment 3
[0049] Optimization of nasal dry powder formulation:
[0050] Weigh 4.5, 3.5, and 2.5 g of levodopa, respectively, and place them in a sealed container with 0.5, 1.5, and 2.5 g of any methylated-β-cyclodextrin, mix well, and dry the mixture in an oven at 80°C for 0.5 h. Cool down to room temperature in the machine; in an environment with relative humidity < 50%, put the mixture into the agate ball milling cavity of a German flying ball mill, add grinding beads, and carry out planetary ball milling after sealing. After 10 minutes, it was stopped for 10 minutes, and the cycle was repeated 12 times. The total grinding time was 2 hours.
[0051] The dissolution test of L-dopa dry powder containing different proportions of RM-β-CD was carried out according to the method of Example 1, and was compared with the dissolution of L-dopa by ball milling alone in Example 1.
[0052] Results The dissolution rate of L-dopa dry powder with 50% RM-β-CD content was significantly higher than th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com