A kind of Dexibuprofen injection and preparation method thereof
A technology of dextroibuprofen and injection, applied in the field of medicine, can solve the problems such as no obvious innovation, insignificant effect of solubilization, increase of adverse reactions, etc., and achieve significant effect of solubilization, shorten production time, and speed up dissolution. effect of speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
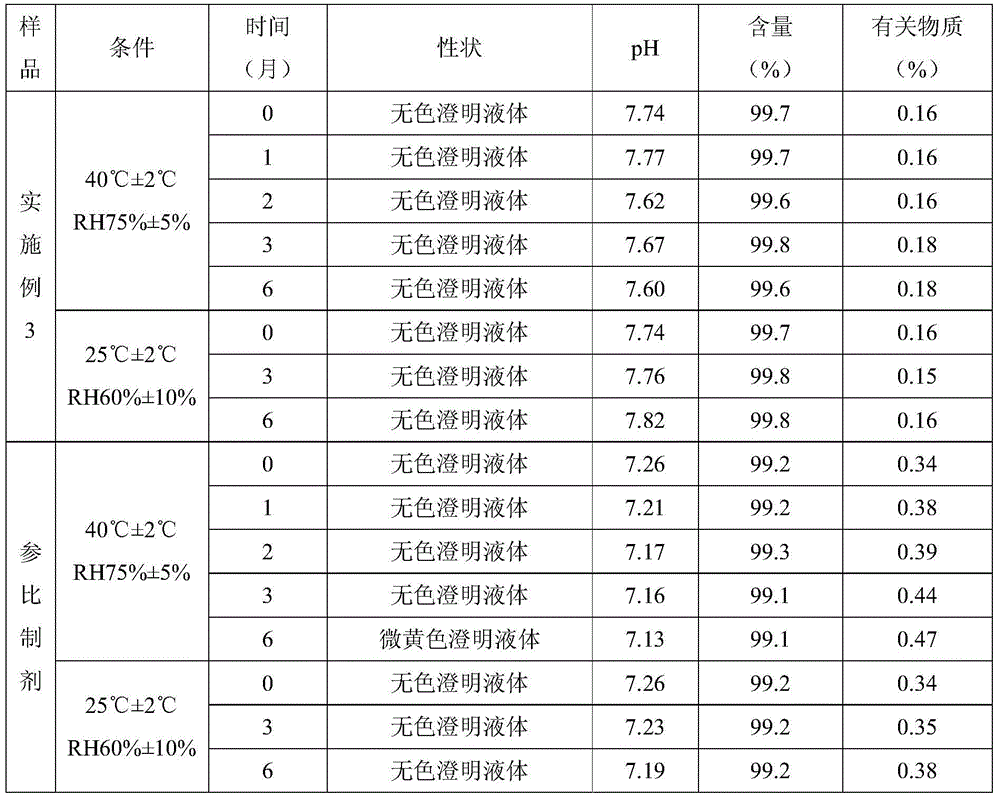
Examples
Embodiment 1
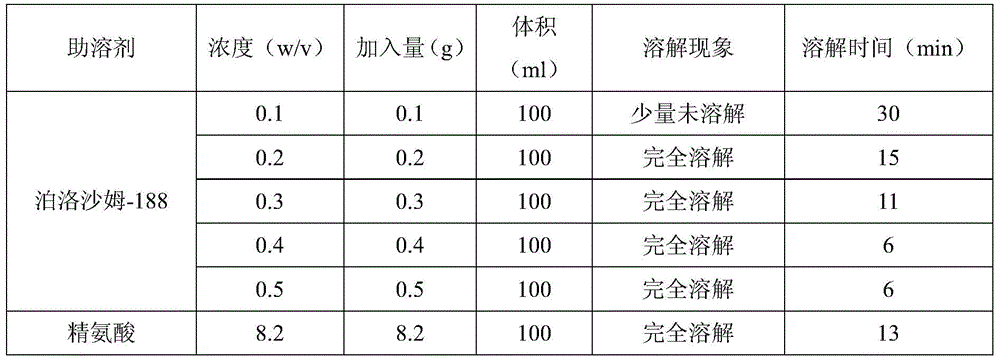
[0033] A preparation method of Dexibuprofen injection comprises adding 640ml of water for injection into a beaker, adding 1.6g of poloxamer-188 and stirring until completely dissolved, adjusting pH 7.5 to 8.5 with sodium hydroxide, adding 80g of Dextrobuprofen Stir the ibuprofen until it is completely dissolved, add 0.16g of activated carbon for needles, keep warm at 50-70°C for 15 minutes, filter to remove the carbon, add water for injection to 800ml, stir evenly, and filter the filtrate with a 0.22μm sterile microporous filter membrane , the filtrate was filled in ampoules, each 4ml, 200 in total, filled with nitrogen and sealed, sterilized at 121°C for 15 minutes.
Embodiment 2
[0035] A preparation method of Dexibuprofen injection comprises adding 640ml of water for injection into a beaker, adding 2.4g of poloxamer-188 and stirring until completely dissolved, adjusting the pH to 7.5~8.5 with sodium hydroxide, adding 80g of Dextrobuprofen Stir the ibuprofen until it is completely dissolved, add 0.16g of activated carbon for needles, keep warm at 50-70°C for 15 minutes, filter to remove the carbon, add water for injection to 800ml, stir evenly, and filter the filtrate with a 0.22μm sterile microporous filter membrane , the filtrate was filled in ampoules, each 4ml, 200 in total, filled with nitrogen and sealed, sterilized at 121°C for 15 minutes.
Embodiment 3
[0037] A preparation method of Dexibuprofen injection comprises adding 640ml of water for injection into a beaker, adding 3.2g of poloxamer-188 and stirring until completely dissolved, adjusting the pH to 7.5-8.5 with arginine, adding 80g of Dexabuprofen Stir the ibuprofen until it is completely dissolved, add 0.17g of activated carbon for needles, keep warm at 50-70°C for 15 minutes, filter to remove the carbon, add water for injection to 800ml, stir evenly, and filter the filtrate with a 0.22μm sterile microporous filter membrane , the filtrate was filled in ampoules, each 4ml, 200 in total, filled with nitrogen and sealed, sterilized at 121°C for 15 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com