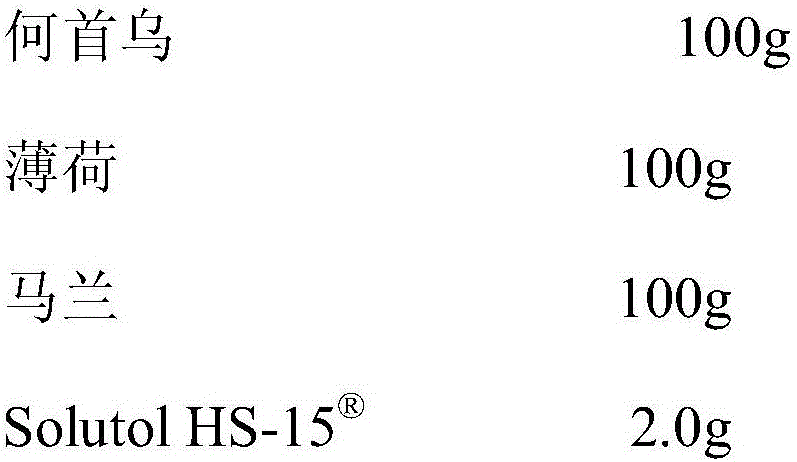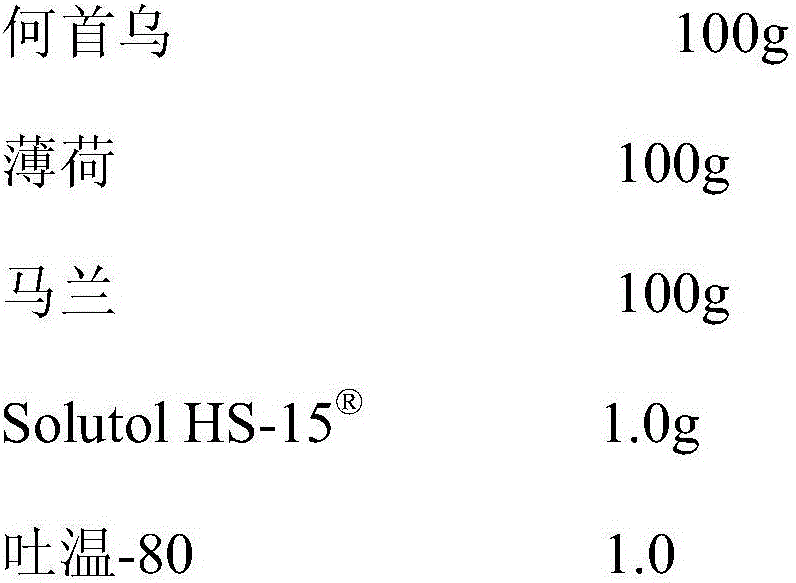Pharmaceutical composition capable of improving safety of compound radix polygoni multiflori injection
A technology of Polygonum multiflorum and its composition, which is applied in the field of medicine, can solve the problems of accelerating the pH value of the liquid medicine, the easy rancidity of polysorbate 80, and the decrease of the pH value of the solution, and achieve the effects of improving safety, low dosage, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
[0017] Preparation method: boil Polygonum Polygoni Multiflori and Peppermint with water twice, each time for 2 hours, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.27-1.30 (80°C), let it cool, add ethanol so that the ethanol content is 65%, stand overnight, filter, the filtrate is decompressed to recover ethanol and concentrate to a relative density of 1.30 (80°C), add 3 times the amount of water, stir well, refrigerate for 48 hours, filter, and the filtrate is concentrated to a relative density under reduced pressure About 1.15 (80°C), the liquid medicine is for later use; decoct malang twice with water, 1 hour each time, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.25-1.30 (80°C), let it cool, and add ethanol Make the ethanol content 65%, let it stand overnight, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.20 (80°C), a...
Embodiment 2
[0019]
[0020] Preparation method: boil Polygonum Polygoni Multiflori and Peppermint with water twice, each time for 2 hours, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.27-1.30 (80°C), let it cool, add ethanol so that the ethanol content is 65%, stand overnight, filter, the filtrate is decompressed to recover ethanol and concentrate to a relative density of 1.30 (80°C), add 3 times the amount of water, stir well, refrigerate for 48 hours, filter, and the filtrate is concentrated to a relative density under reduced pressure About 1.15 (80°C), the liquid medicine is for later use; decoct malang twice with water, 1 hour each time, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.25-1.30 (80°C), let it cool, and add ethanol Make the ethanol content 65%, let it stand overnight, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.20 (80°C), a...
Embodiment 3
[0022]
[0023]
[0024]Preparation method: boil Polygonum Polygoni Multiflori and Peppermint with water twice, each time for 2 hours, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.27-1.30 (80°C), let it cool, add ethanol so that the ethanol content is 65%, stand overnight, filter, the filtrate is decompressed to recover ethanol and concentrate to a relative density of 1.30 (80°C), add 3 times the amount of water, stir well, refrigerate for 48 hours, filter, and the filtrate is concentrated to a relative density under reduced pressure About 1.15 (80°C), the liquid medicine is for later use; decoct malang twice with water, 1 hour each time, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.25-1.30 (80°C), let it cool, and add ethanol Make the ethanol content 65%, let it stand overnight, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com