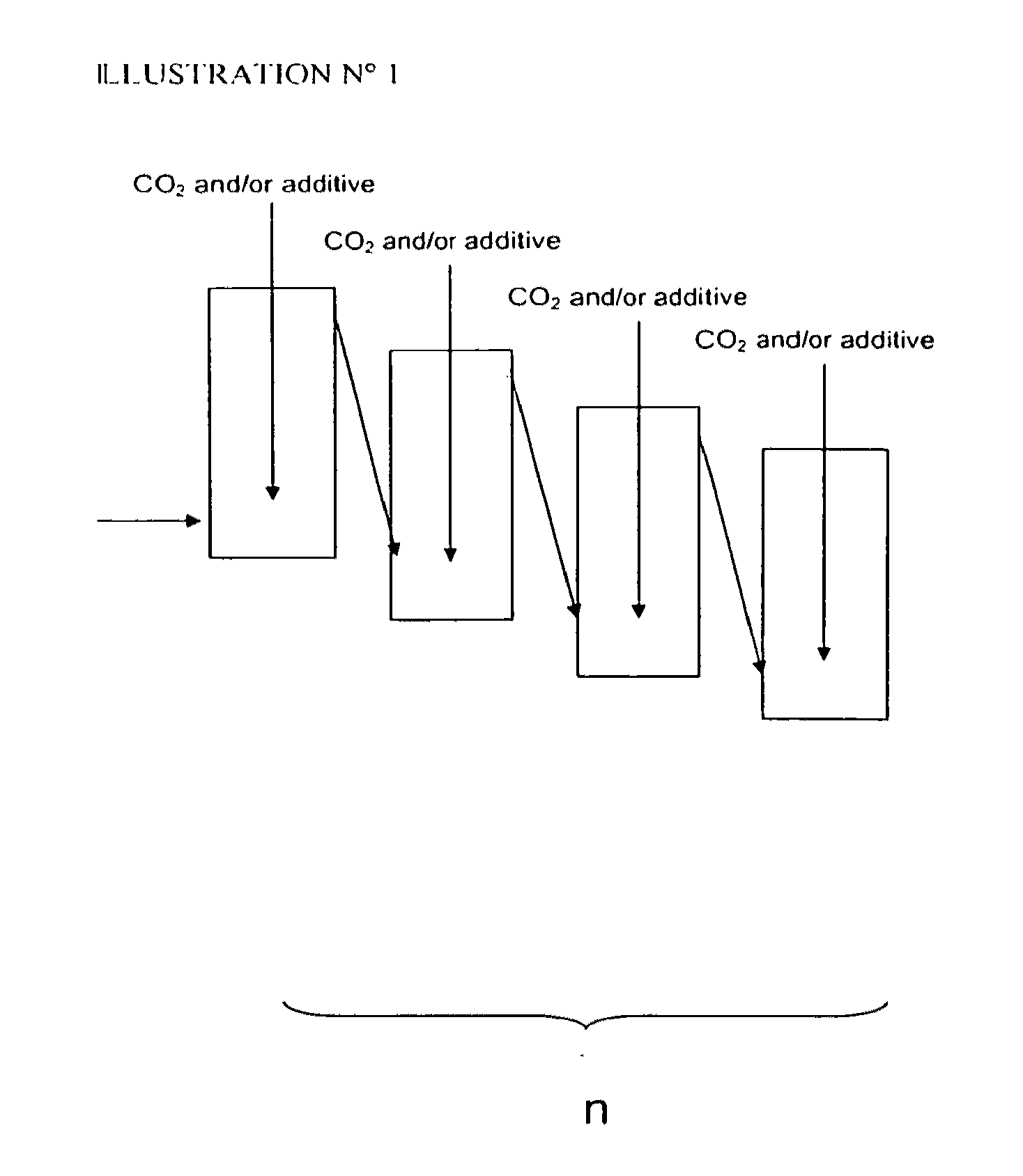
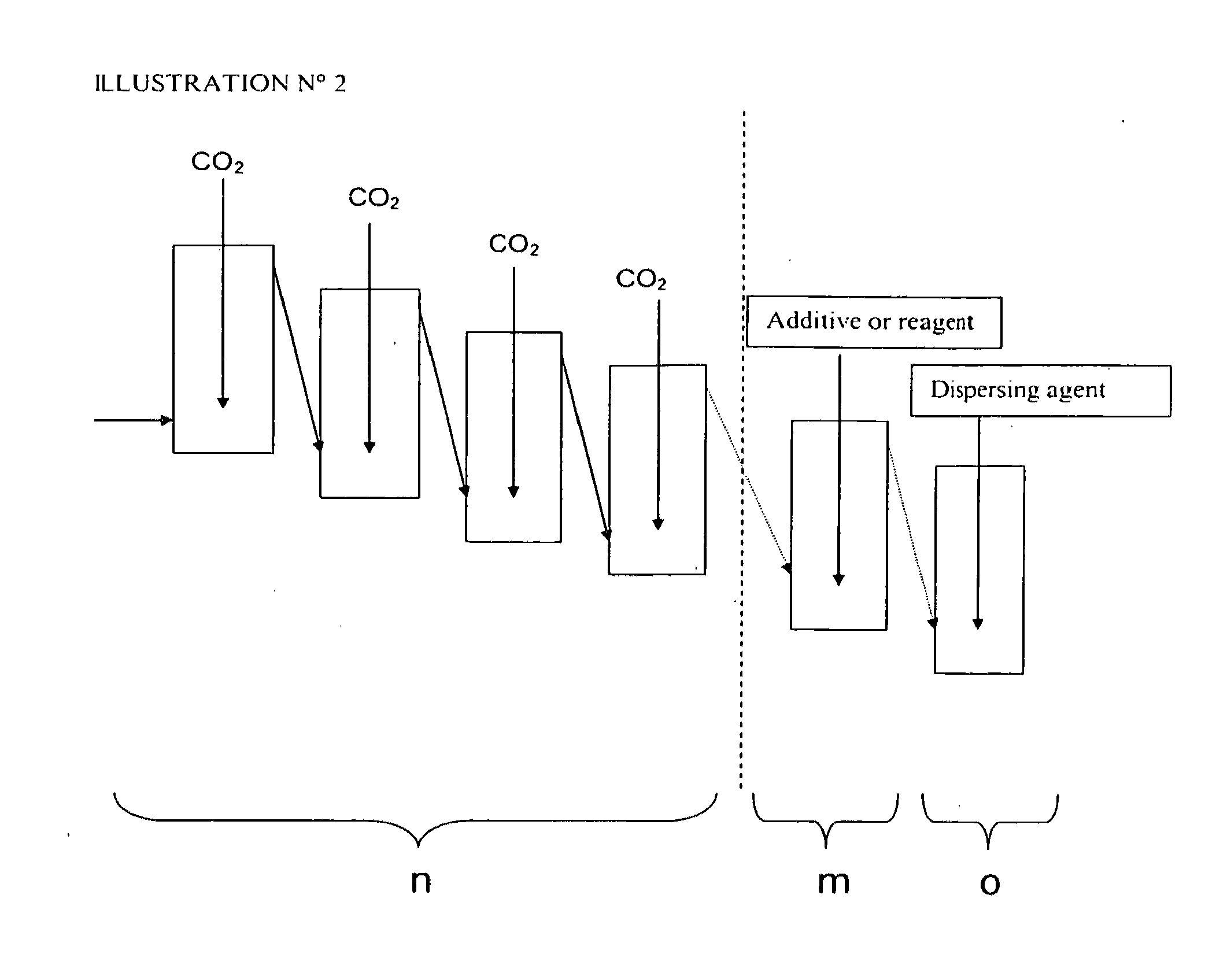
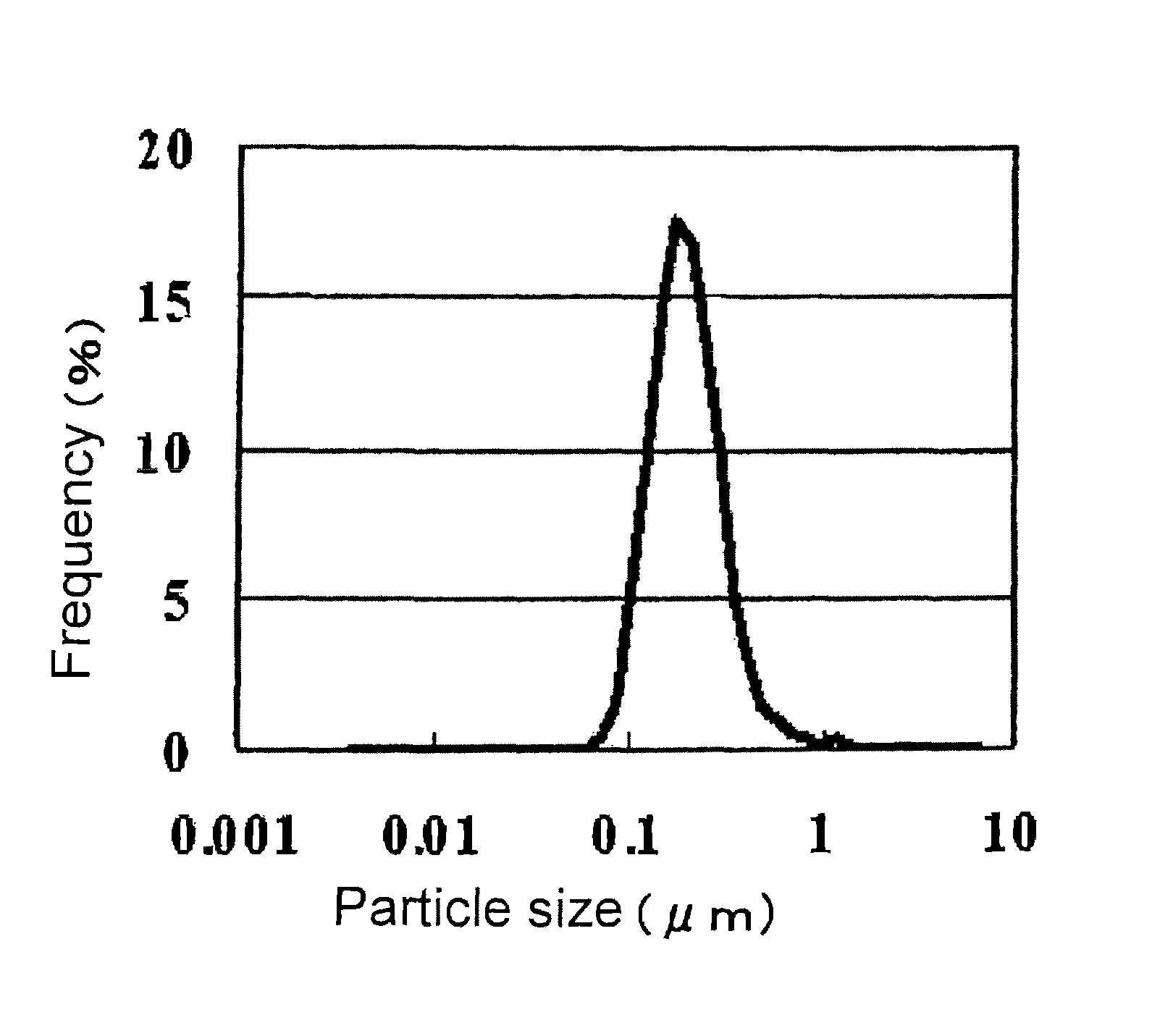
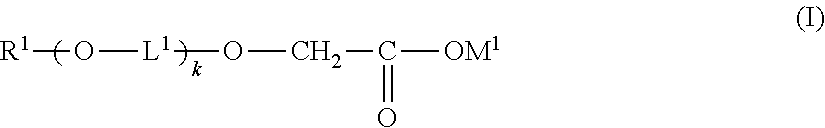
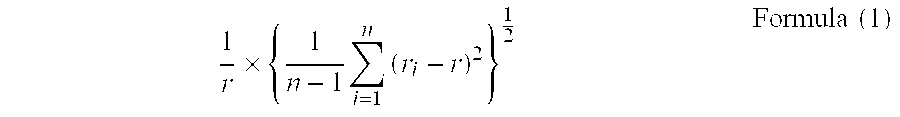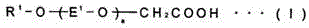Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
79results about "Strontium carbonates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for preparing micron/nano size inorganic particles
InactiveUS20050218540A1Easy to controlHigh purityMaterial nanotechnologyBarium carbonatesSulfideChemistry
The present invention discloses methods for making micron / nano sized (2 nm to 5 μm) particles of various inorganic materials such as mineral / oxides / sulphides / metals / ceramics using aqueous foam, Aqueous foams of various anionic, cationic, non-ionic surfactant, casein proteins and their mixtures has been used for the preparation of suitable inorganic materials growth. Large scale synthesis of advanced inorganic materials such as various ceramics, minerals, oxides, sulphides and metal micron / nanoparticles of controlled shape and size can be obtained by mixing appropriate metal ions with the suitable cationic / anionic / non-ionic / casein protein / their mixtures, which is bubbled by air to form aqueous foams and thereafter their reduction / reaction to form the final product.
Owner:COUNCIL OF SCI & IND RES
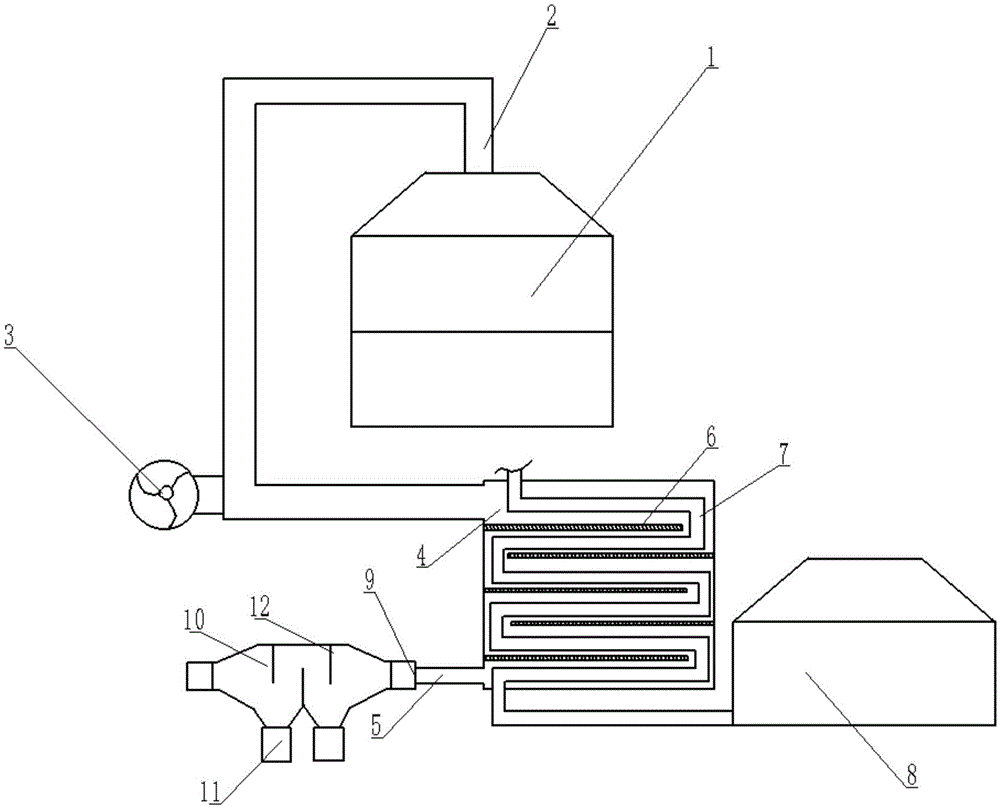
Supergravity field micro-reactor, and method for preparing nanometer material through liquid phase precipitation method
ActiveCN107224949AIncrease flow rateShort reaction residence timeMaterial nanotechnologyStrontium carbonatesMicroreactorSupporting system
The invention relates to a supergravity field micro-reactor for preparing a nanometer material. The supergravity field micro-reactor comprises a micro-reaction assembly, a material receiving groove provided with a material outlet, a first motor, a transmission system, a material inlet system and a support system, wherein the micro-reaction assembly comprises an upper disc and a lower disc, micro-grooves are respectively distributed on the bottom surface of the upper disc and the top surface of the lower disc, the center position of the lower disc is provided with a material inlet micro-pore channel, a 0.03-0.1 mm gap is arranged between the bottom surface of the upper disc and the top surface of the lower disc after the combination, the first motor drives the transmission system to operate so as to make the upper disc and the lower disc in the micro-reaction assembly rotate in opposite rotation directions, and an upper disc lifting system can be additionally arranged so as to conveniently adjust the gap between the bottom surface of the upper disc and the top surface of the lower disc in the micro-reaction assembly and wash the upper disc and the lower disc. With the supergravity field micro-reactor of the present invention, the nanometer material is prepared by using the liquid phase precipitation method, such that the high dispersion property and the uniform particle distribution of the nanometer material can be maintained while the treatment capacity can be increased and the clogging of the micro-reaction assembly can be avoided.
Owner:SICHUAN UNIV
Grinding method and product
ActiveUS20060027688A1Easy to prepareHigh crystallinityCalcium/strontium/barium carbonatesGermanium dioxideHigh activityYttrium
A particulate material is ground more efficiently using a mixture of at least two different sizes of yttrium-stabilized zirconia balls. The method facilitates preparation of photocatalysts with high activity.
Owner:GM GLOBAL TECH OPERATIONS LLC
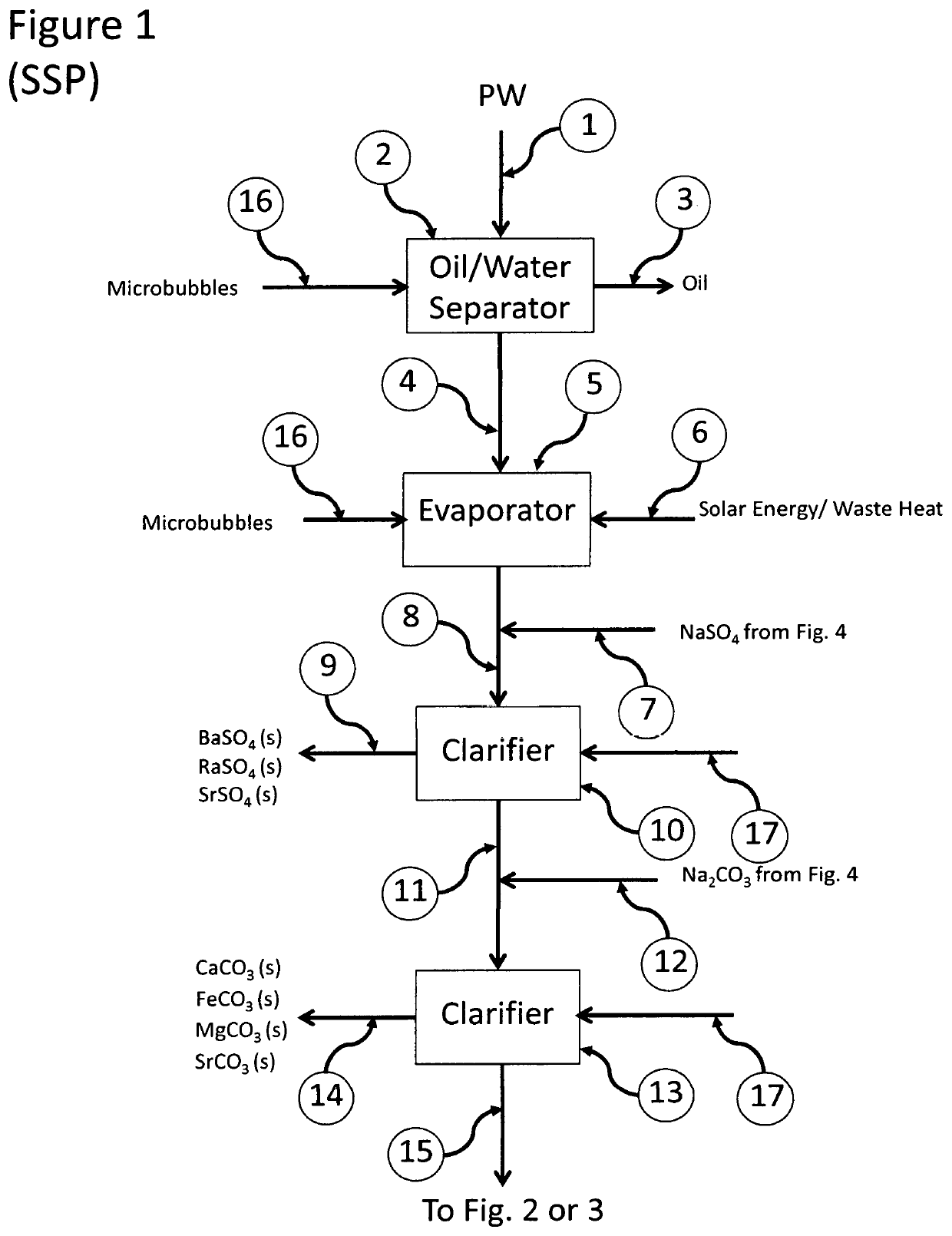
Recovery of Valuable Resources from Produced Water and Coal Combustion Products
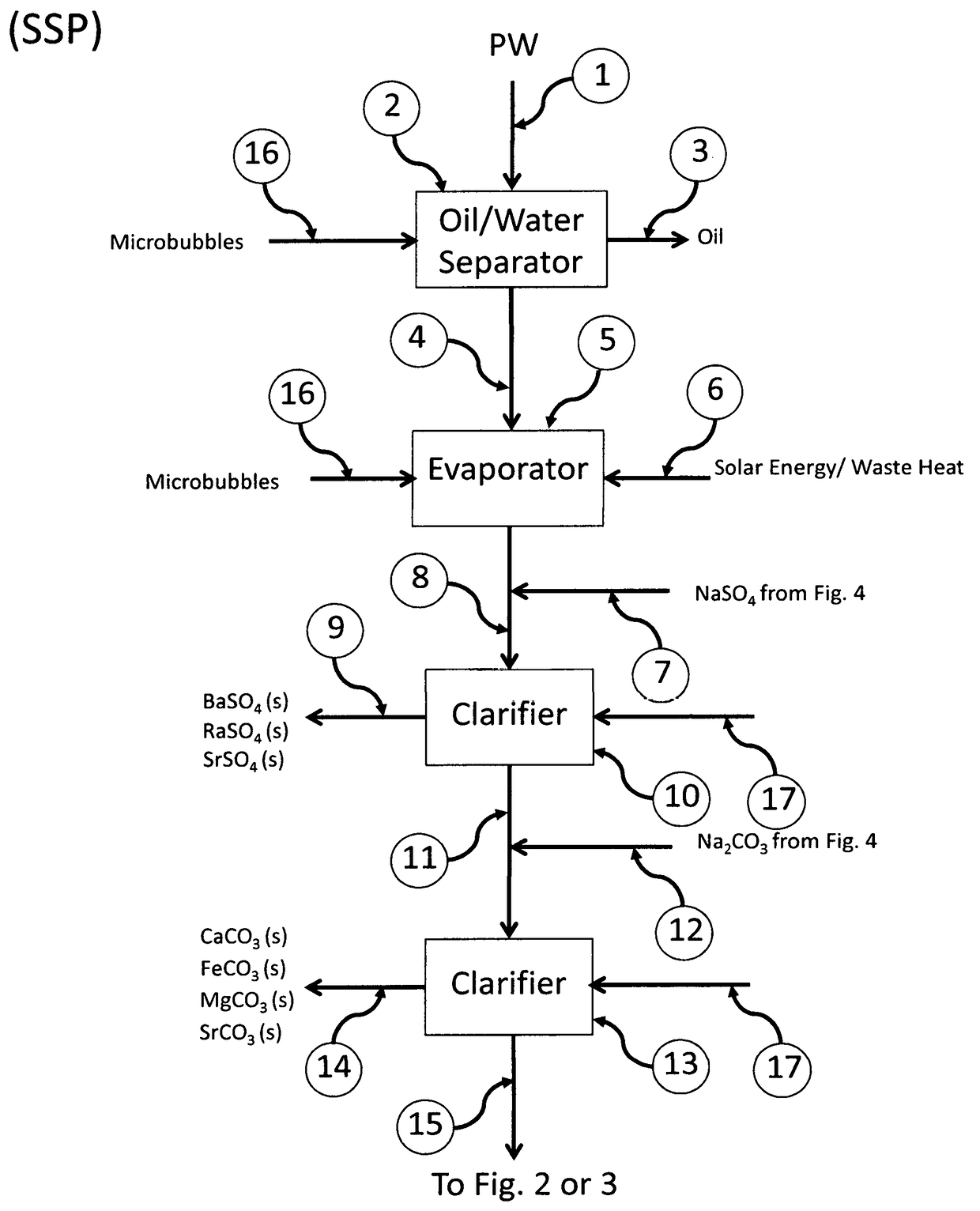
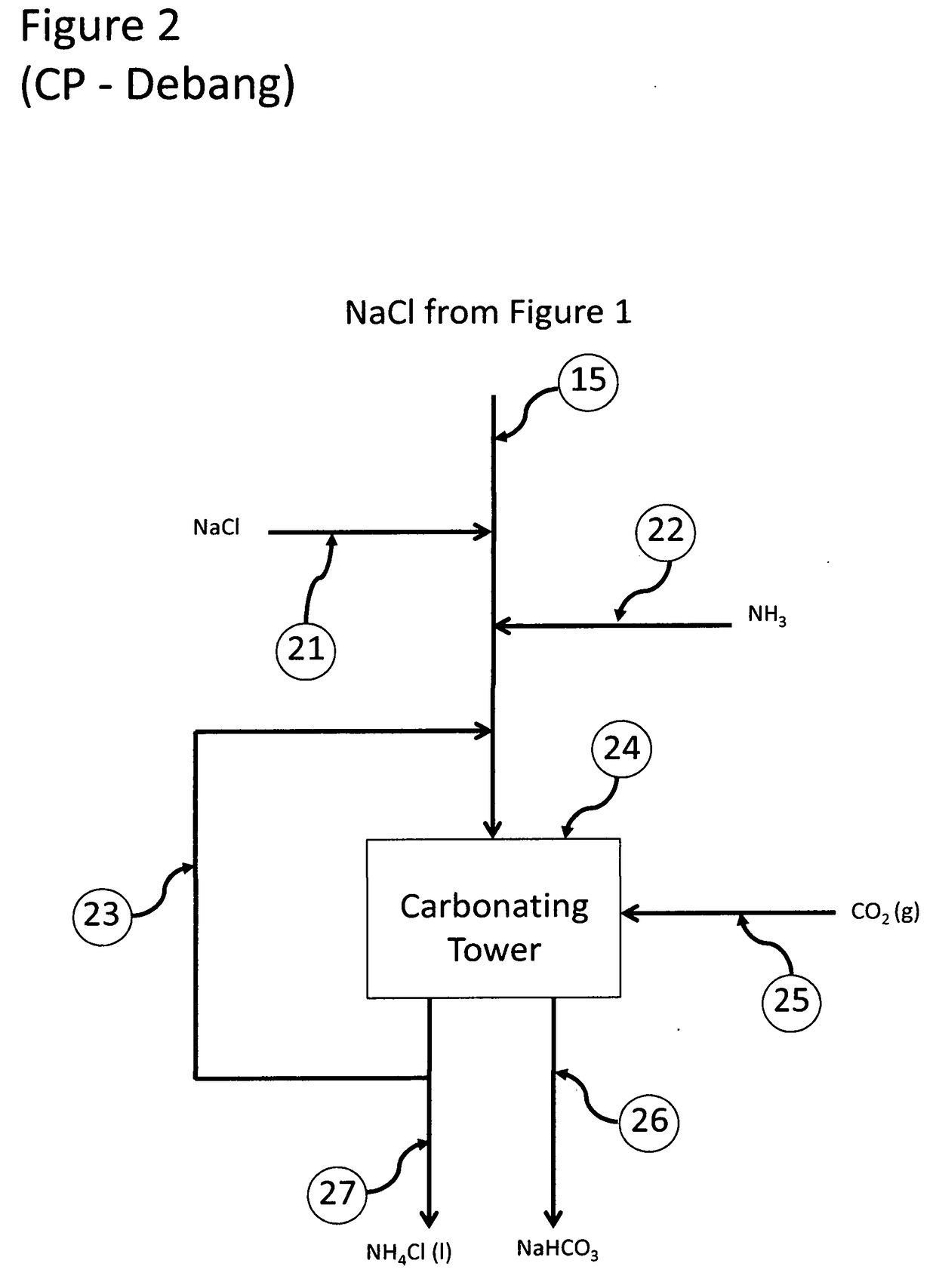
ActiveUS20180022617A1Increase valueIncrease wasteWaste water treatment from quariesStrontium carbonatesFlue gasWaste stream
The present invention relates to processes employing water produced from wells that, after suitable purification steps, is processed to recover resources that can be used to treat other waste streams, such as flue gases and ashes from combustion of fossil fuels.
Owner:CORT STEVEN L
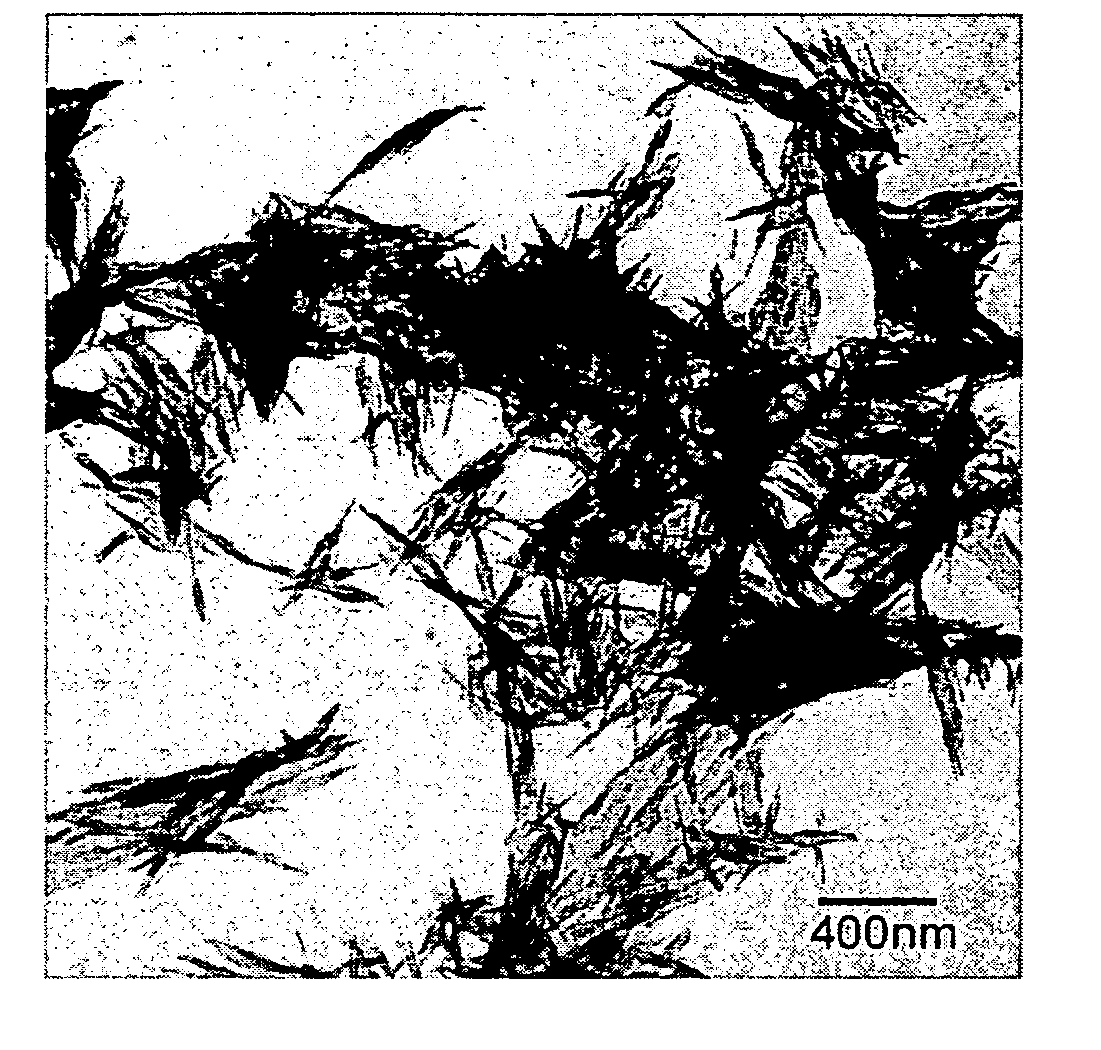
Biomimetic preparation method of strontium carbonate crystal
InactiveCN108675335AUse low concentrationCompact structureStrontium carbonatesNanotechnologyStrontium carbonateStrontium chloride hexahydrate
The invention discloses a biomimetic preparation method of a strontium carbonate crystal, wherein a coronary strontium carbonate nano crystal is prepared by a biomimetic method in a specific communication device by adopting strontium chloride hexahydrate and ammonium bicarbonate as reactants and sesbania gum as a biological regulation agent. According to the target product coronary strontium carbonate nano crystal, the corolla diameter is 3,000-6,000nm; the corolla is composed of nano rods with diameter being 50-80nm; the corolla structure is tight, with purity being greater than or equal to 99% and yield being 97-99%. The method disclosed by the invention has the characteristics of mild conditions, low energy consumption, large corolla diameter, tight corolla structure, high purity, highyield, low preparation cost, etc.
Owner:NANCHANG HANGKONG UNIVERSITY
Process for the preparation of micron/nano sized inorganic material
InactiveUS20080260615A1Economic and efficientMaterial nanotechnologyStrontium carbonatesSuper structureSulfide
The invention discloses methods for making micron / nano meter sized particles of various inorganic materials such as minerals / oxides / sulphides / metals / ceramics at a steadily expanding liquid-liquid interface populated by suitable surfactant molecules that spontaneously organize themselves into superstructures varying over large length-scales. This experiment is realized in a radial Hele-Shaw cell where the liquid-liquid interfacial growth rate and consequently time scales such as arrival of surfactant molecules to the interface, the hydrodynamic flow effect to modulate the material organization into super structures at the dynamic charged interface.
Owner:COUNCIL OF SCI & IND RES
Carbonate Crystal, Manufacturing Method Thereof, And Transparent Optical Resin Composition
InactiveUS20090124744A1Large aspect ratioFormed easily and efficientlyMaterial nanotechnologyPolycrystalline material growthTransmittanceCarbonate
The object of the present invention is to provide a carbonate crystal, which has oriented birefringence, is needle- or rod-like, and is able to negate the birefringence without sacrificing the light transmittance of a transparent polymeric resin when it exists in the transparent resin; a manufacturing method of the carbonate crystal; and a transparent optical resin composition comprising the carbonate resin. The carbonate crystal has an aspect ratio of two or greater, the average major axis length of 400 nm or shorter, and the variation coefficient expressed in Formula (1) below is 0.40 or less:1r×{1n-1∑i=1n(ri-r)2}12Formula(1)wherein r denotes an average major axis length, n denotes the number of particles used for the measurement of the major axis length, and n denotes the major axis length of the ith particle measured.
Owner:FUJIFILM CORP
Nano particle synthesizing process
InactiveCN1883786AIdeal sizeIdeal distributionMaterial nanotechnologyProductsNanoparticleSpray nozzle
Disclosed is a method for synthesizing nanometer particles, which characterized in the steps: providing at least one atomization nozzle and one reaction chamber, which communicate to each other; providing at least two reactants, at least one of which is liquid phase reactant; atomizing the liquid phase reactant by the atomization nozzle and jetting to the reaction chamber; mixing at least two reactants and depositing particles of nanometer structure.
Owner:HONG FU JIN PRECISION IND (SHENZHEN) CO LTD +1
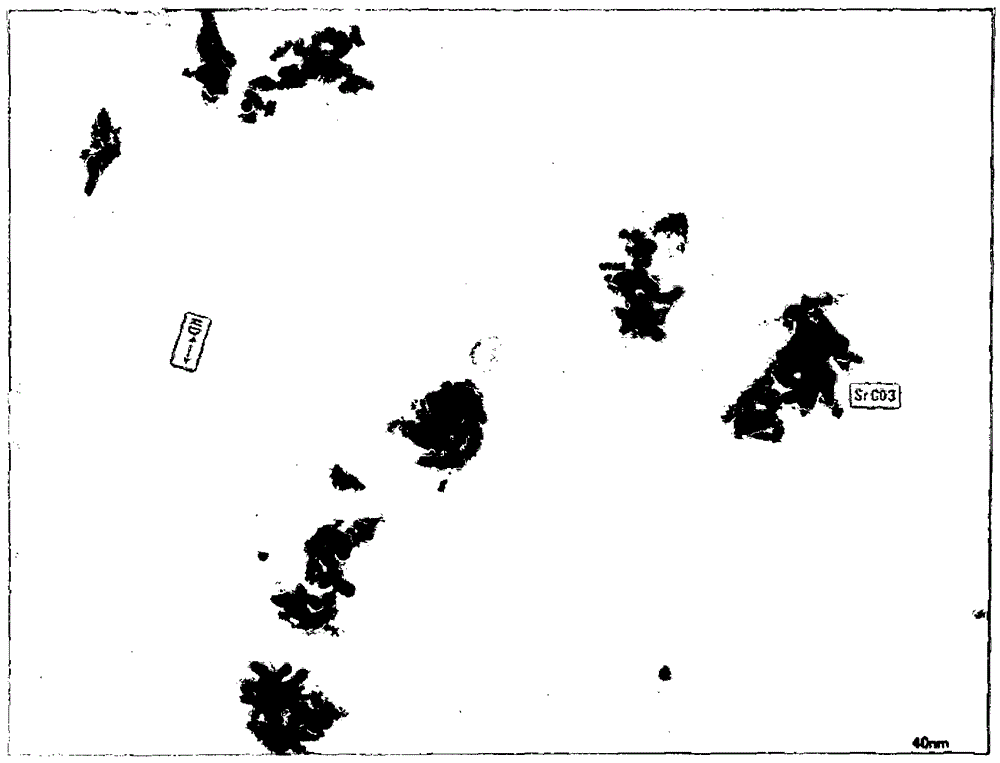
Method for preparing high-purity strontium salt
The invention relates to a preparation method of high-purity strontium salt, comprising the following steps: placing the high-purity strontium salt solution in an ultrasonic water bath, adding a dispersant, and stirring to dissolve the dispersant; adding an appropriate amount of NH4HCO3 solution dropwise into the SrCl2 solution, At the same time, adjust the pH value with ammonia water to ensure the presence of a large amount of CO32-, so that the Sr2+ conversion is complete; while it is hot, it is suction filtered, washed with distilled water and absolute ethanol several times in turn, and the filtrate is jointly detected with nitric acid and silver nitrate until there is no Sr2+ in the filtrate. Chloride ions; the product is placed in a drying oven to dry to obtain strontium carbonate powder. The method of the invention is simple, low in cost and easy to realize, and the prepared strontium carbonate powder has uniform particles and high purity.
Owner:吴礼秀
Method for producing strontium carbonate from carbon dioxide in exhaust gas
InactiveCN106044820AEfficient use ofEmission reductionStrontium carbonatesStrontium carbonateCarbon dioxide production
The invention provides a method for producing strontium carbonate by utilizing carbon dioxide in waste gas, comprising the following steps: S1, preparation of crude strontium; S2, leaching of crude strontium; S3, purification of CO in waste gas 2 ; S4, carbonization of leaching solution; S5, desulfurization of strontium carbonate slurry. The beneficial effect of the present invention is that the tail gas produced in the calcination process is used to purify CO2, and then used in the production of strontium carbonate. control features. Low cost and strong market competitiveness. The entire production process is closed-loop, with high yield, reducing my carbon dioxide emissions and turning waste into treasure.
Owner:SHIJIAZHUANG ZHENGDING JINSHI CHEM
Recovery of valuable resources from produced water and coal combustion products
ActiveUS10875785B2Increase valueIncrease wasteWaste water treatment from quariesStrontium carbonatesWaste streamFlue gas
The present invention relates to processes employing water produced from wells that, after suitable purification steps, is processed to recover resources that can be used to treat other waste streams, such as flue gases and ashes from combustion of fossil fuels.
Owner:CORT STEVEN L
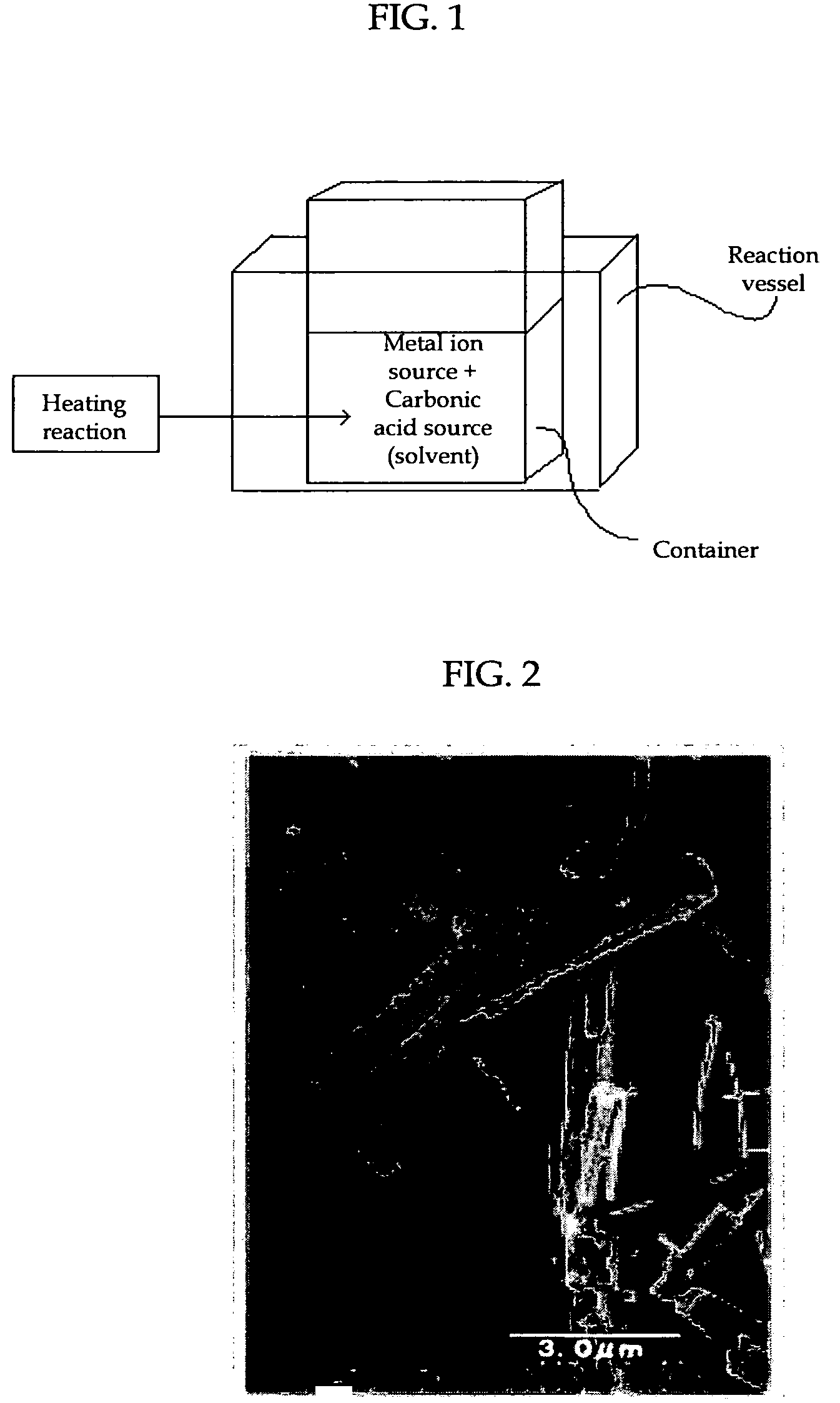
Process for Producing Carbonate Particles
InactiveUS20080267854A1High crystallinityLess-prone to agglomerationStrontium carbonatesMagnesium carbonatesFull width at half maximumX-ray
To provide a process for producing carbonate particles, capable of efficient, easy formation of carbonate particles which have high crystallinity, less prone to agglomeration and offer orientation birefringence, particularly carbonate particles that are needle- or rod-shaped, and of controlling the particle size. In the process a metal ion source and a carbonate ion source are heated together in a liquid of 55° C. or higher for reaction to produce carbonate particles with an aspect ratio of greater than 1, wherein the metal ion source contains at least one metal ion selected from the group consisting of Sr2+, Ca2+, Ba2+, Zn2+ and Pb2+. The carbonate particles are preferably needle- or rod-shaped, pH of the liquid after heating reaction is preferably 8.20 or more, and in its X-ray diffraction spectrum the full-width at half maximum of the diffraction peak corresponding to (111) plane is preferably less than 0.8°.
Owner:FUJIFILM CORP
Preparation method of strontium carbonate for producing glass substrate
The invention discloses a preparation method of strontium carbonate for producing a glass substrate. The preparation method comprises the steps of adding a purified strontium chloride solution and an ammonium bicarbonate solution into a multi-zone feeding and multi-zone reaction crystallization reaction kettle, adding a crystal form control agent for reaction, separating reaction materials, rinsing a solid, and adding the rinsed solid into a drum-type multiple temperature zone roasting furnace for roasting to form strontium carbonate for producing the glass substrate. According to the method, the crystal form control agent is added in a reaction; the aggregation problem of crystals can be solved; the change of a product from a club-shaped crystal form to a spherical crystal form can be achieved; and the particle size uniformity of the product is improved. The multi-zone feeding and multi-zone reaction crystallization reaction kettle is adopted; the multi-zone feeding is achieved; at the same time, the reaction kettle is internally provided with multiple reaction zones; a matrix supersaturation degree in a reaction process is sufficiently reduced; the reaction crystallization performance is improved; and the crystal form optimized product is obtained.
Owner:SOUTHWEAT UNIV OF SCI & TECH
Different types of mineral matter containing carbonate with reduced fossil fuel carbon dioxide emission on breakdown, together with their synthesis process and their uses
The invention concerns a new synthetic mineral matter containing carbonate, the decomposition of which reduces the rate of fossil fuel carbon dioxide emission. It also concerns its manufacture in batches, or in a batch-continuous manner, or in a continuous manner, together with its uses in the pharmaceutical field, the field of human or animal foodstuffs, or again the papermaking field with, notably, manufacture of paper, filler or coating, or again every other paper surface treatment, together with the fields of water-based or non-water-based paints, together with the field of plastics, such as that of breathable polyethylene films, or again the field of printing inks.
Owner:BURI MATTHIAS +1
Alkaline earth metal carbonate micropowder
InactiveUS9102810B2Good dispersionMaterial nanotechnologyStrontium carbonatesPolymer scienceOrganic solvent
Dispersibility of an alkaline earth metal carbonate micropowder in a polymer resin or in an organic solvent is improved by treating the surface of the alkaline earth metal micropowder with a surfactant having hydrophilic groups and hydrophobic groups and groups that form anions in water.
Owner:UBE IND LTD
Process for obtaining precipitated calcium carbonate
ActiveUS20160167977A1Low costOvercome deficienciesPigmenting treatmentStrontium carbonatesPrecipitated calcium carbonateSlurry
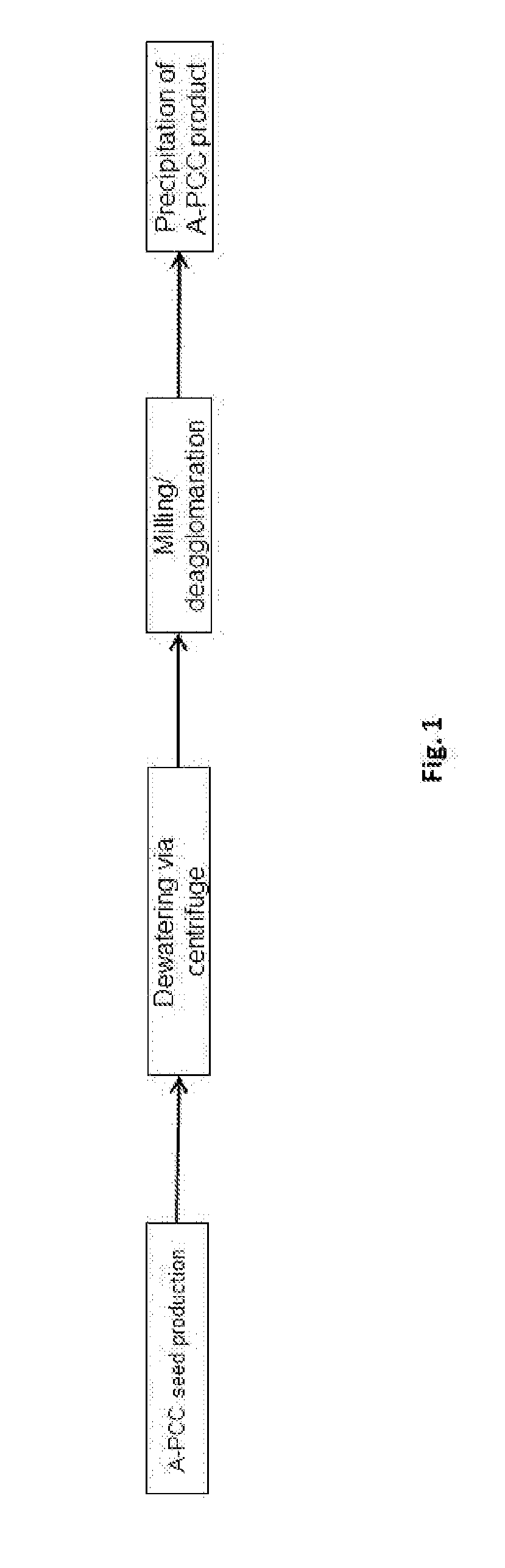
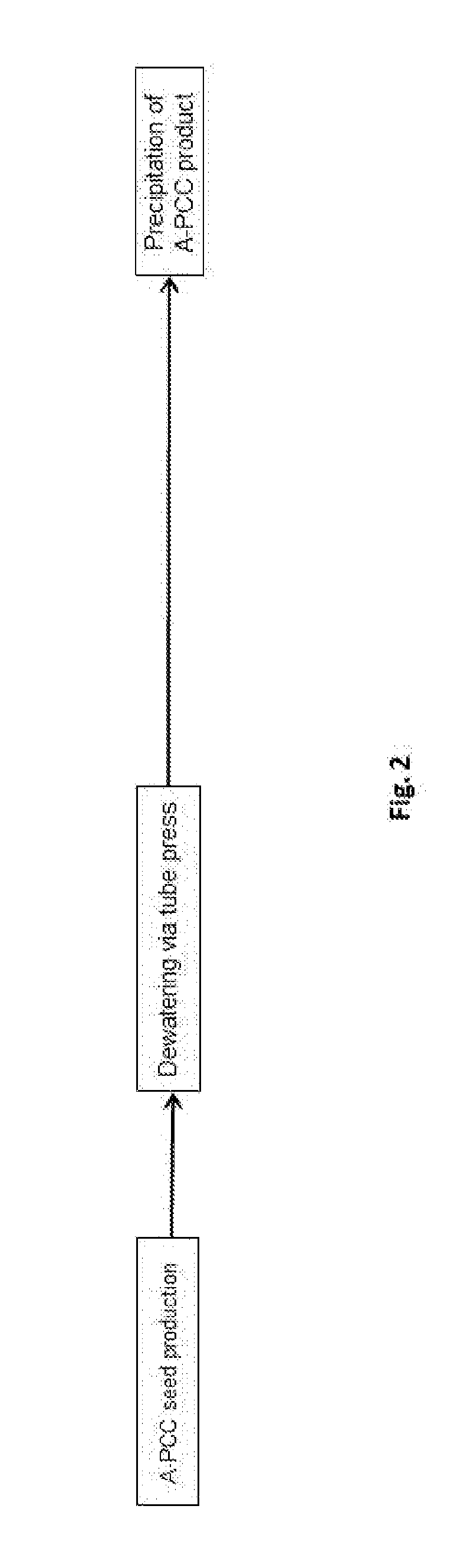
The present invention provides a process for preparing a precipitated calcium carbonate product comprising the steps of: (a) preparing an aqueous suspension of precipitated calcium carbonate seeds by carbonating a suspension of Ca(OH)2 in the presence of 0.005 to 0.030 moles of Sr, in the form of Sr(OH)2, per mole of Ca(OH)2, (b) dewatering and dispersing the precipitated calcium carbonate seeds prepared in step (a) to obtain an aqueous suspension of precipitated calcium carbonate seeds having a d50 of less than or equal to 0.1 to 0.3 um and a BET specific surface area of 10 to 30 m2 / g, and (c) forming an aqueous suspension of a precipitated calcium carbonate product by carbonating a slurry of Ca(OH)2 in the presence of 0.5 to 5% by dry weight of the precipitated calcium carbonate seeds obtained in step (b), wherein the precipitated calcium carbonate seeds have a d50 that is less than the d50 of the precipitated calcium carbonate product and the precipitated calcium carbonate seeds have an aragonitic polymorph content greater than or equal to the precipitated calcium carbonate product obtained in step (c) characterized in that the dewatering of step (b) is carried out by the use of a tube press.
Owner:OMYA INT AG
Method for preparing nano strontium carbonate
ActiveCN106115760ASimple preparation stepsReduce manufacturing costMaterial nanotechnologyStrontium carbonatesCarbanionImpurity
The invention discloses a method for preparing nano strontium carbonate and belongs to the technical field of strontium carbonate preparation. Celestite serves as the raw material and is smashed and then dissolved with an excessive amount of diluted sulphuric acid to move calcium carbonate therein, then strontium sulfate in the celestite is dissolved with diluted hydrochloric acid, filtering is conducted to remove insoluble substances like barium sulfate, then the obtained strontium chloride solution is mixed with automatically cultured microorganism colonies on the surface of the celestite, and organic matter, with negative charges, on the surface of microorganism cell surfaces is chelated with strontium ions in the solution; urea serves as the carbon source of microorganisms, the urea is continuously decomposed so that the concentration of carbanions in the solution around the microorganisms can be increased continuously, accordingly the concentration of part of carbanions and strontium ions in the solution is increased, the carbanions and the strontium ions are combined into strontium carbonate crystal precipitate, and then the precipitate is filtered and dried to obtain the nano strontium carbonate. The method has the advantages that preparation steps are simple, the impurity content of the obtained product is reduced by 70-80%, and activity is improved by 25-30%.
Owner:重庆庆龙新材料科技有限公司
Process for producing strontium carbonate and barium carbonate without continuous carbonization of hydrogen sulfide gas holder
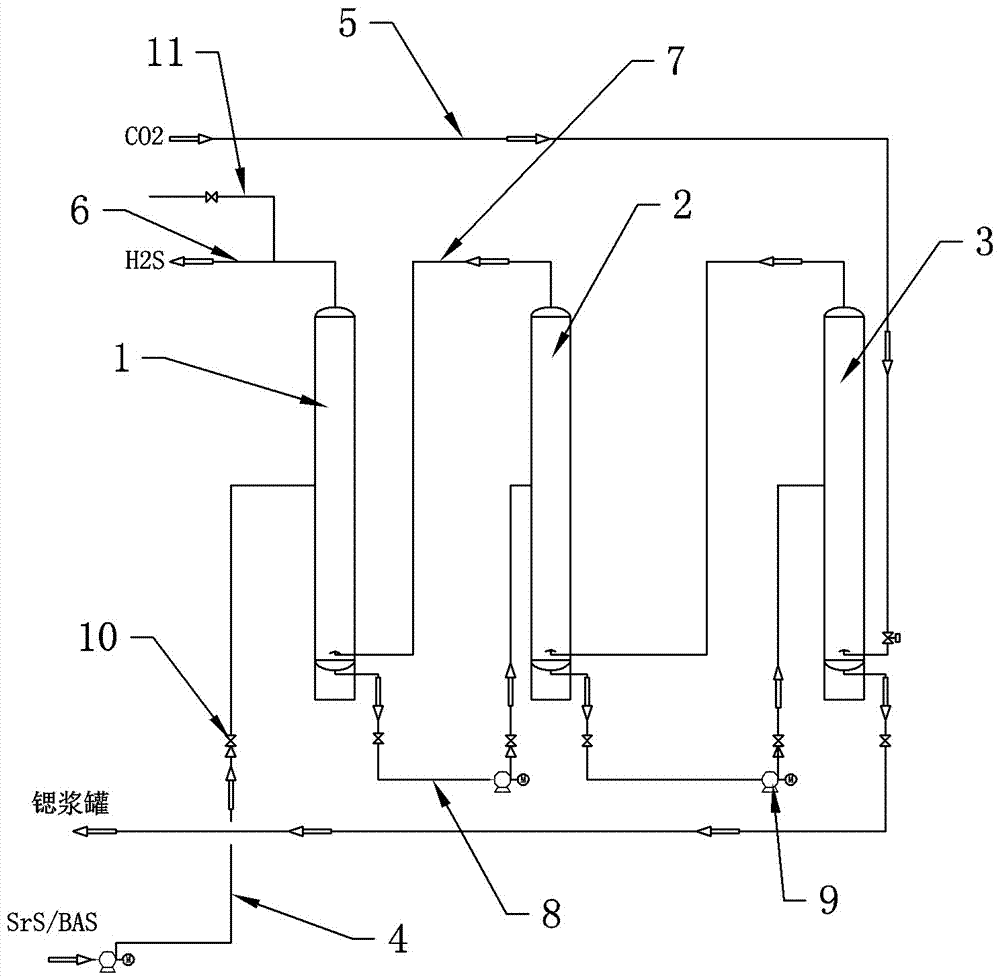
InactiveCN107140670ASubsequent production process is stableBarium carbonatesStrontium carbonatesStrontium carbonateStrontium sulfide
The application of the present invention discloses a process for continuously carbonizing strontium carbonate and barium carbonate without hydrogen sulfide gas tank, which includes the following process steps: the first step is to pass the strontium sulfide or barium sulfide liquid through the liquid feed pipe first from the first carbonization tower The upper part is sprayed down; the second step is to adjust the flow rate to maintain a stable liquid level in the first carbonization tower; the third step is to adjust the flow rate to maintain a stable liquid level in the second carbonization tower, and so on; The fourth step is to completely react carbon dioxide with strontium sulfide or barium sulfide solution, and the hydrogen sulfide gas produced is discharged from the exhaust pipe at the top of the first carbonization tower into the subsequent production process; the fifth step is to discharge strontium sulfide or barium strontium sulfide slurry , and then adjust the flow rate to maintain a stable liquid level in the last carbonization tower; the sixth step is to start the car and start continuous production according to the above process. By adopting the process of the invention, the hydrogen sulfide gas tank can be eliminated, and at the same time, the production stability of the follow-up process using hydrogen sulfide as a raw material can be guaranteed.
Owner:CHONGQING KINGLONG FINE STRONTIUM CHEM
Preparation method of spherical nano-strontium carbonate
InactiveCN110451544AGood spherical effectUniform particlesStrontium carbonatesNanotechnologyStrontium carbonateHypergravity
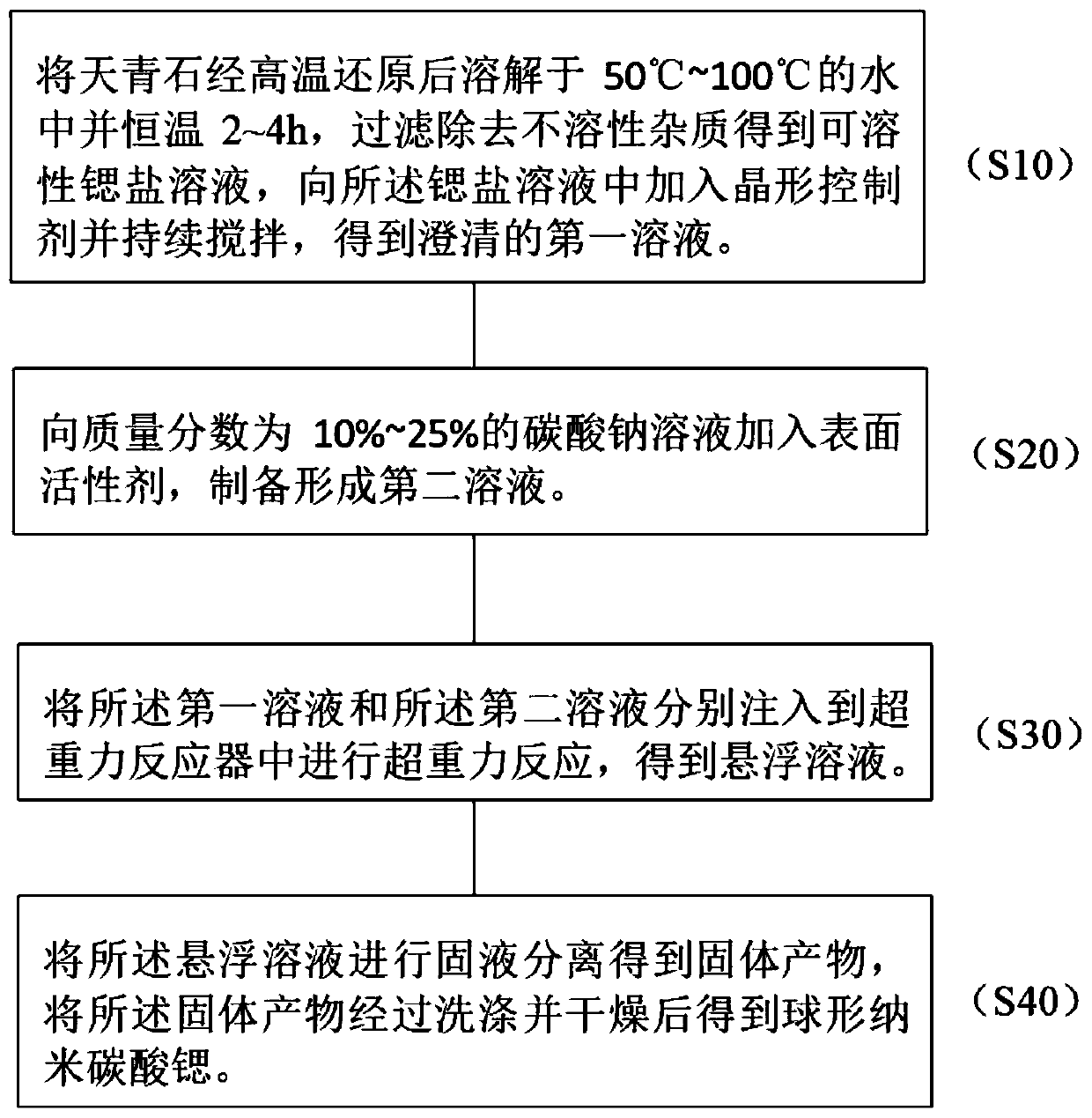
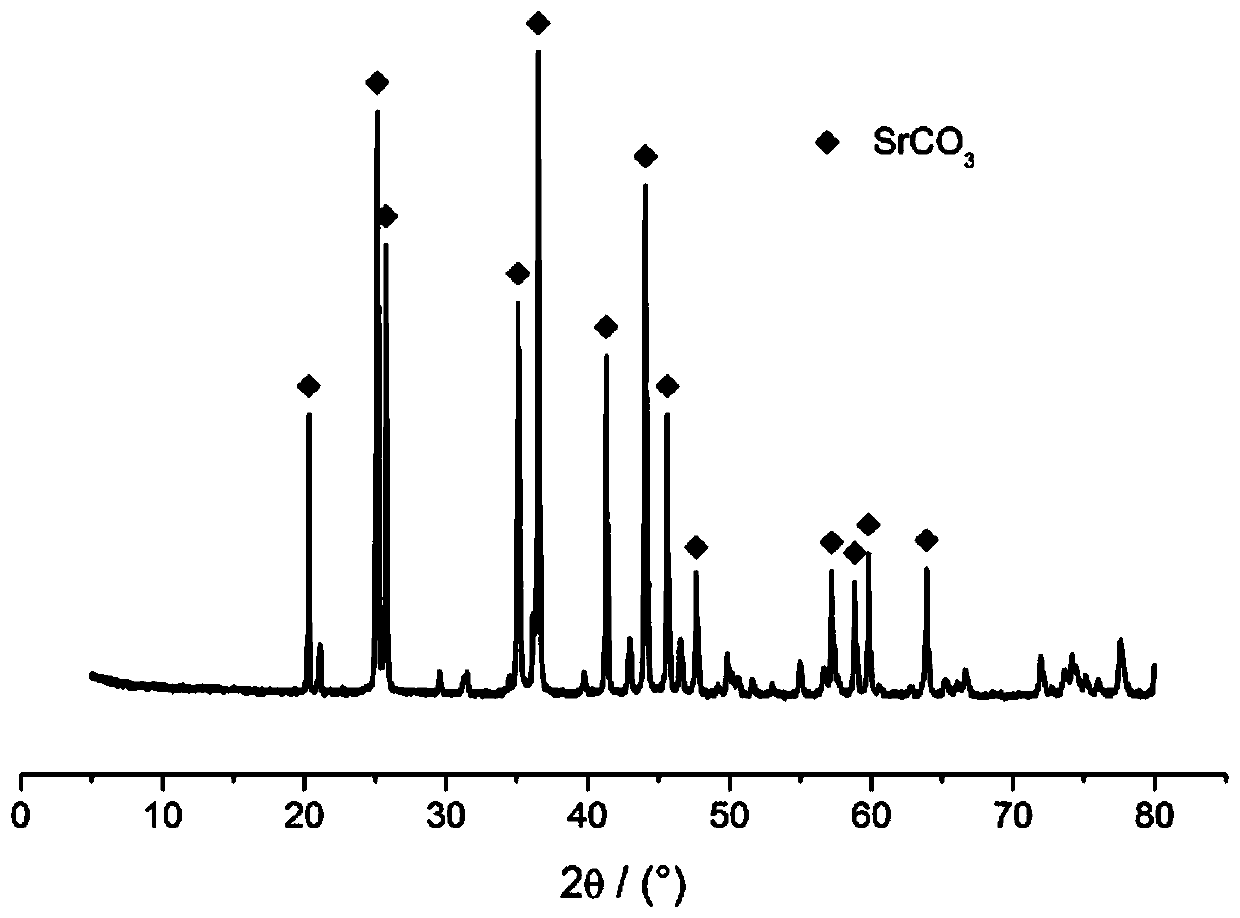
The invention discloses a preparation method of spherical nano-strontium carbonate. The method includes the steps of: S10. subjecting celestite to high temperature reduction, then dissolving the reduced celestite in water at 50DEG C-100DEG C and maintaining a constant temperature for 2-4h, filtering out insoluble impurities to obtain a soluble strontium salt solution, adding a crystal form controlagent into the strontium salt solution and performing stirring continuously to obtain a clarified first solution; S20. adding a surfactant into a sodium carbonate solution with a mass fraction of 10%-25% to prepare a second solution; S30. injecting a first solution and a second solution respectively into a hypergravity reactor for hypergravity reaction to obtain a slurry solution; and S40. subjecting the slurry solution to solid-liquid separation to obtain a solid product, and washing and drying the solid product to obtain spherical nano-strontium carbonate. The method provided by the invention can prepare spherical nano-strontium carbonate with good spherical effect, uniform particles and narrow particle size range.
Owner:QINGHAI UNIV FOR NATITIES
Mineral materials containing carbonate with reduced emission of combustible fossil carbonaceous gas on decomposition thereof and method for production and use thereof
The present invention relates to novel synthetic minerals containing carbonates, the decomposition of which reduces carbon dioxide emissions from fossil fuels. The invention further relates to its batchwise, batch-continuous or continuous production and its use in the field of pharmaceuticals, food for humans or animals, or papermaking, especially for the production, filling or coating of paper. Any other surface treatment of cloth or paper, as well as in the field of aqueous or non-aqueous paints, and in the field of plastic materials such as breathable polyethylene films, or in the field of printing inks.
Owner:OMYA DEV AG
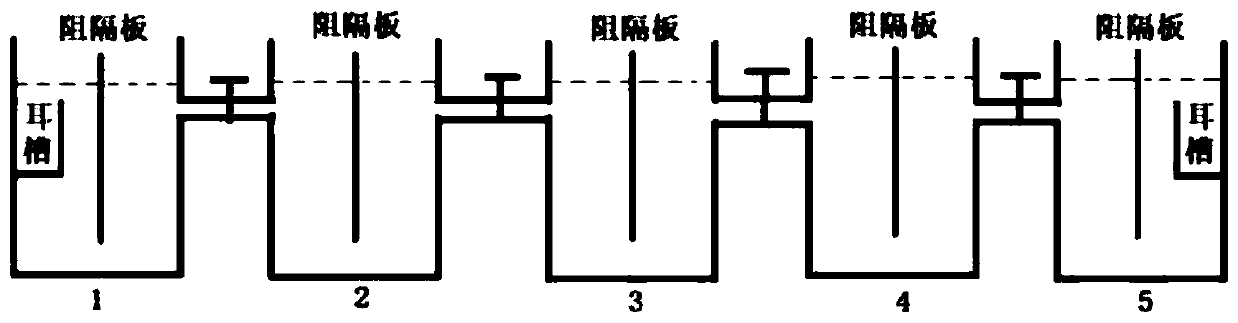
Method and device for preparing strontium carbonate crystals through self-diffusion at room temperature
InactiveCN109133136AReduce consumptionImprove production efficiencyStrontium carbonatesStrontium carbonateSelf-diffusion
The invention relates to a method and a device for preparing strontium carbonate crystals through self-diffusion at room temperature. According to the method, with strontium dichloride hexahydrate andammonium hydrogen carbonate as reactants and glycine as a biomimetic control agent, granular strontium carbonate crystals are prepared; the method comprises the following specific steps: in a reaction device, adding a first batch of the strontium dichloride hexahydrate and the ammonium hydrogen carbonate, then adding 0.020% of a glycine solution, reacting for 24h by standing at room temperature,supplementing a second batch of the reactants and a third batch of the reactants, respectively continuing reacting for 24h by standing, filtering a reaction product, washing for three times by using distilled water, and drying for 1h at 105 DEG C to obtain the granular strontium carbonate crystals with the grain diameter of 110-440nm, wherein the product purity is higher than or equal to 99% and the yield is 96-98%. The reaction device consists of a reactor 1 (5), a reactor 2 (9) and a reactor 3 (13). The method and the device have the advantages of high preparation efficiency, simple and convenient operation, high technological stability, low energy consumption, low production cost and the like.
Owner:NANCHANG HANGKONG UNIVERSITY
Carbonates and Method for Producing the Same
InactiveUS20080241048A1Efficient productionEfficient methodPigmenting treatmentStrontium carbonatesAlcoholAqueous solution
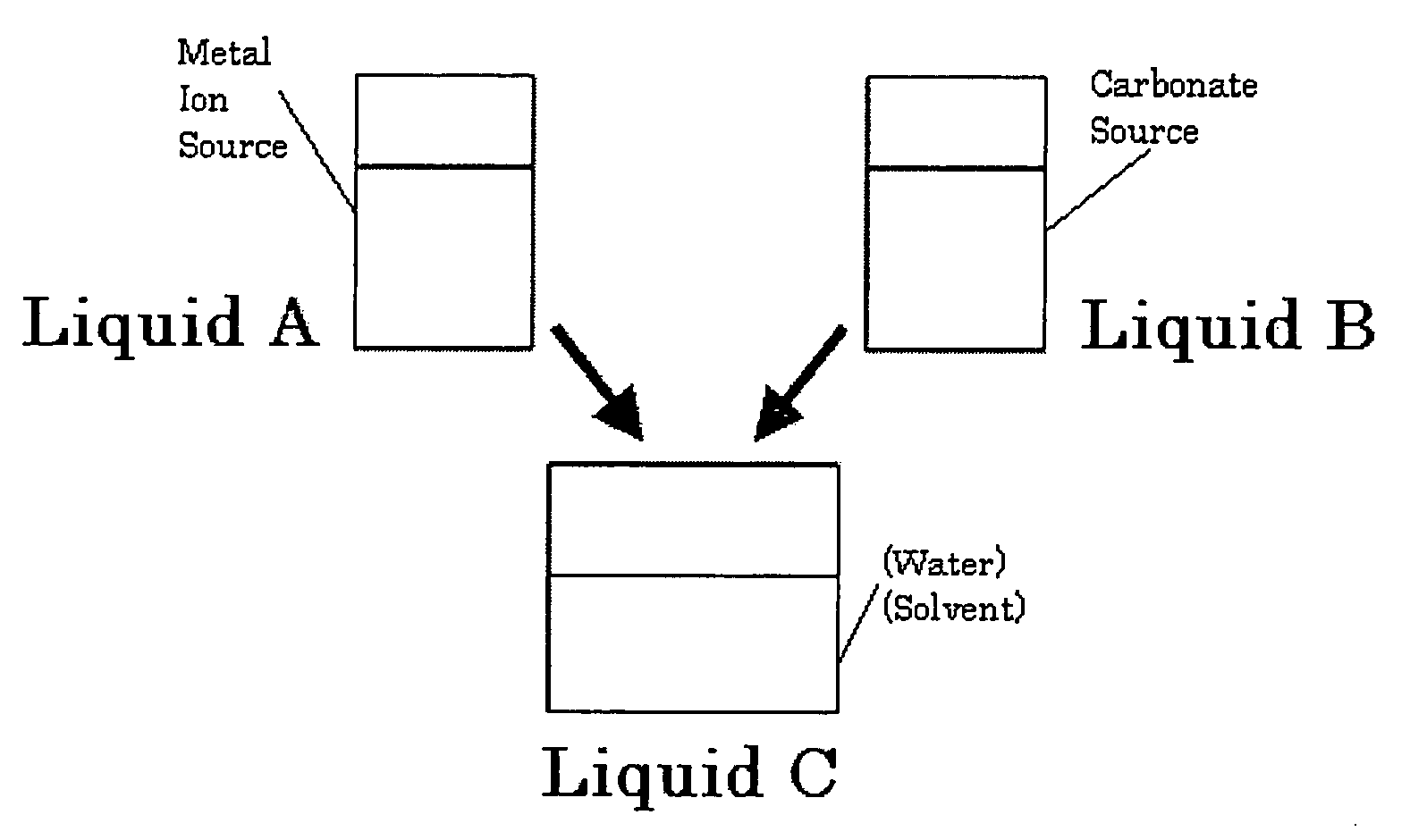
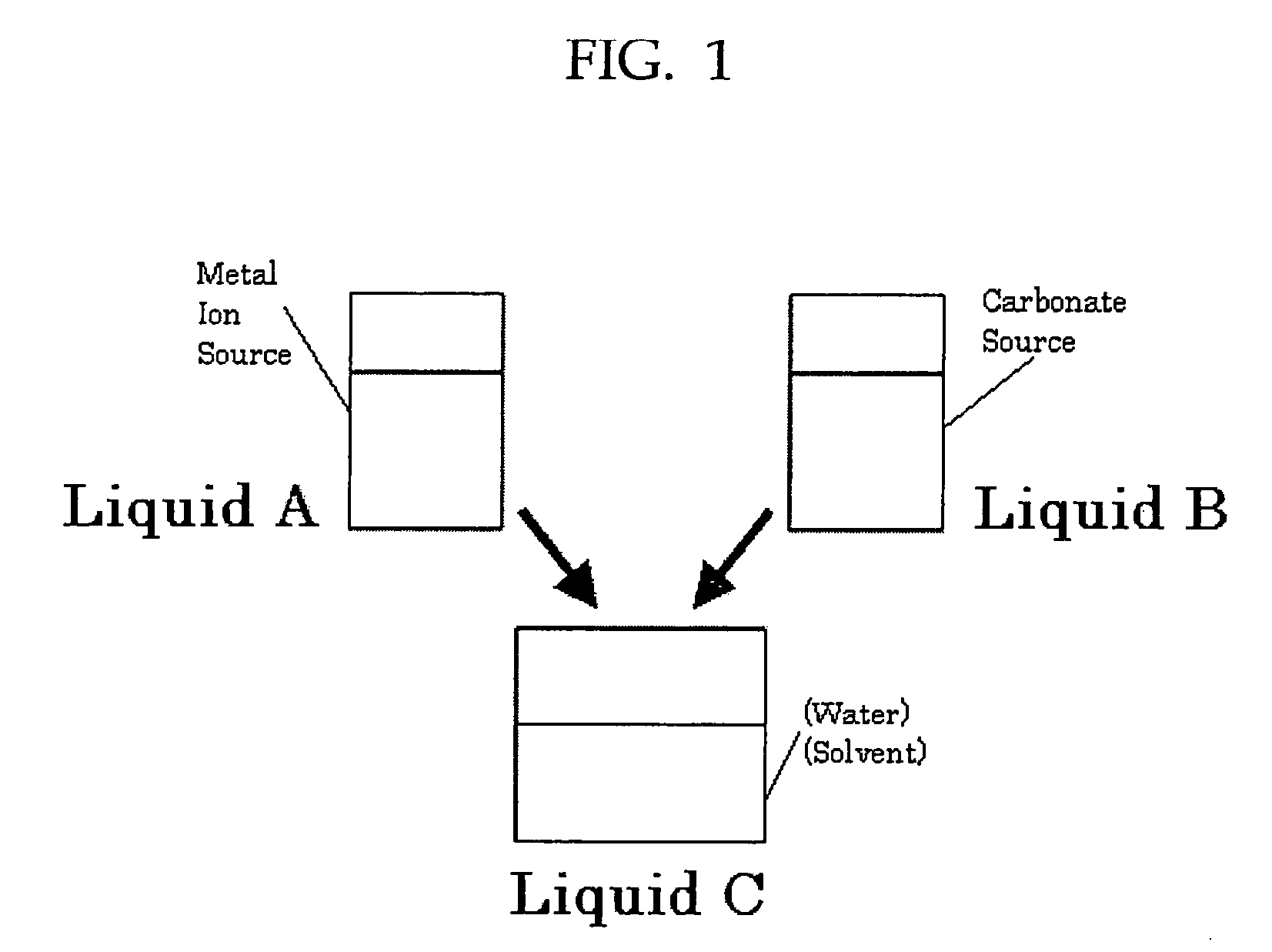
The present invention provides a method for producing carbonates, which includes at least adding an aqueous solution containing a carbonate source into an alcoholic solution containing a metal ion source, wherein an alkaline chemical is added when the carbonate source is reacted with the metal ion source.
Owner:FUJIFILM CORP
Rotary kiln waste heat recovery apparatus
InactiveCN106882818ASimple structureStrontium carbonatesDrying gas arrangementsWaste heat recovery unitSulfite salt
The invention discloses a novel rotary kiln waste heat recovery apparatus. The apparatus is characterized in that the apparatus comprises a rotary kiln body, a circulating pipeline is connected to the rotary kiln body, and the end of the circulating pipeline is connected to a waste heat recovery device; the waste heat recovery device comprises a recovery chamber, and baffle plates are arranged in the recovery chamber in cross arrangement; the recovery chamber is also provided with a heat exchange pipeline which is folded along the baffle plates; the end of the heat exchange pipeline is connected to a sodium sulfite drying case; the end of the recovery chamber is provided with an air outlet, the air outlet is connected to an exhaust gas filtering apparatus, the exhaust gas filtering apparatus comprises a filtering pipeline which is provided with at least three pieces of baffle plates, the baffle plates are arranged in up and down cross arrangement, settlement buckets which are arranged at the bottom of the filtering pipeline and between adjacent two baffle plates are used for communication, and cross section of the filtering pipeline over the settlement bucket is larger than cross section at an inlet of the filtering pipeline. The recovery apparatus has a simple structure, can be used for recycling and reusing heat in the rotary kiln exhaust gas, and the heat can be used for directly drying a sodium sulfite product.
Owner:CHONGQING KINGLONG FINE STRONTIUM CHEM
Strontium carbonate micropowder and process for production
InactiveUS8388749B2Good dispersionAdvantageously employedPigmenting treatmentMaterial nanotechnologyStrontium titanateStrontium carbonate
A fine strontium carbonate powder having a BET specific surface area of 20 to 150 m2 / g, which comprises spherical particles having a mean aspect ratio of 2.0 or less shows high dispersibility in liquid media and is of value for producing dielectric ceramic materials such as strontium titanate.
Owner:UBE IND LTD
Needle-shaped strontium carbonate microparticles and dispersion liquid thereof
ActiveCN106068241AHigh transparencyPigmenting treatmentStrontium carbonatesStrontium carbonateOrganic solvent
[Problem] To provide: a needle-shaped strontium carbonate powder comprising fine needle-shaped strontium carbonate particles; and a dispersion liquid having the fine needle-shaped strontium carbonate particles dispersed in a primary particle or nearly primary microparticle state in an organic solvent. [Solution] A needle-shaped strontium carbonate powder comprising needle-shaped strontium carbonate particles having an average long diameter for the primary particles of 5-50 nm and having an average aspect ratio of 2.2-5.0; and a dispersion liquid having the majority of the needle-shaped strontium carbonate particles dispersed as primary particles in an organic solvent. Ideally a surfactant, including a hydrophilic group and a hydrophobic group and having a group forming anions in water, is attached to the surface of the needle-shaped strontium carbonate particles.
Owner:UBE IND LTD
Method for preparing high-purity strontium carbonate
The invention relates to a method for preparing high-purity strontium carbonate, and belongs to the technical field of preparation of strontium carbonate. The method comprises the steps: firstly, soaking calcined celestite in hydrochloric acid, supplementing with hydrogen peroxide, extracting strontium ions, followed by extracting probiotics in celestite surface soil, screening, culturing, mixing with a strontium ion-containing mixed solution, supplementing with sodium carbonate, under action of probiotics self enzymes, continuously carrying out enzymatic action with surrounding media to generate carbonate ions, and carrying out a reaction with strontium ions to produce strontium carbonate crystals. The prepared strontium carbonate contains low impurity level, has high purity up to more than or equal to 99.5%, has high activity, and cannot produce secondary pollution in the process of preparation.
Owner:TRUSYN CHEM TECH
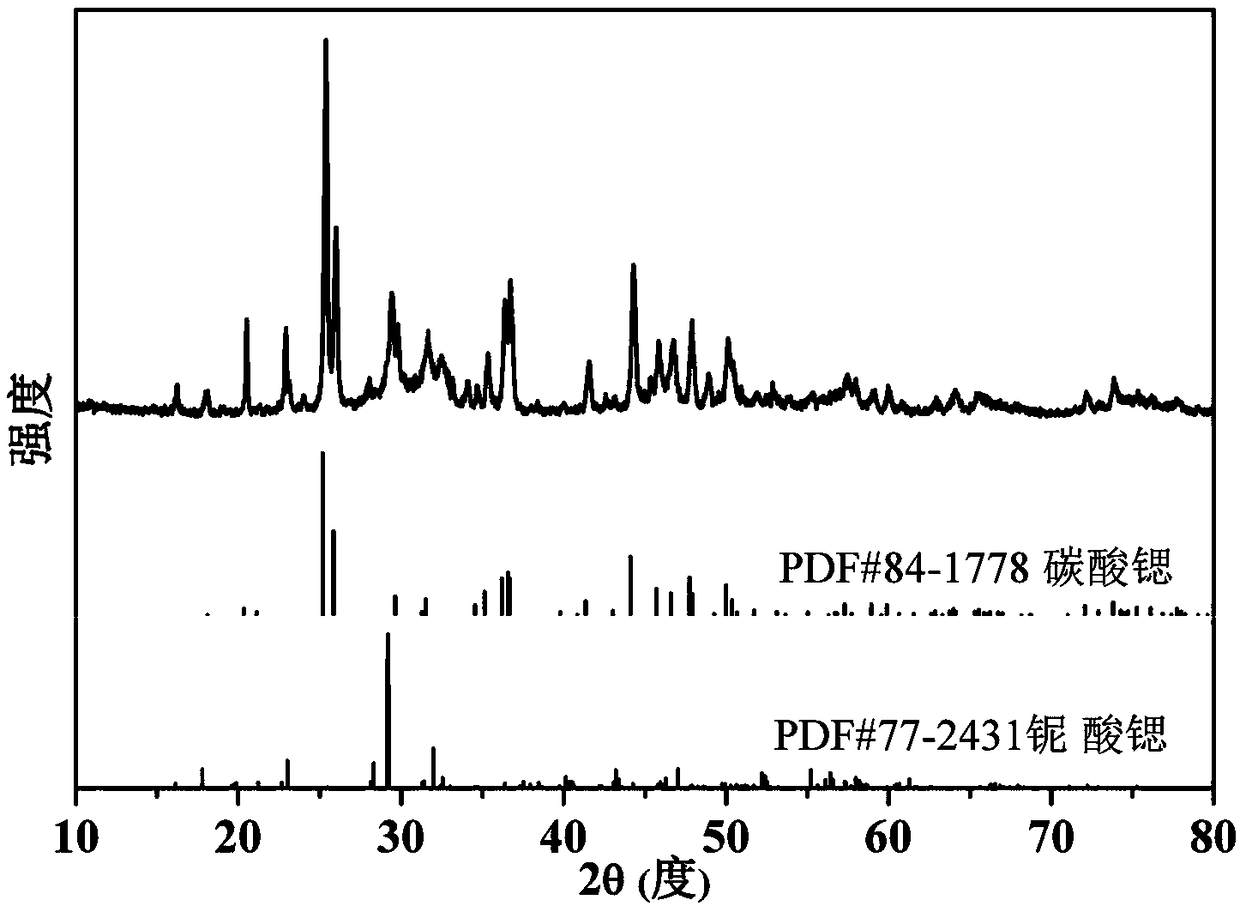
Method for preparing SrNb2O6 (strontium niobate)/SrCO3 (strontium carbonate) composite nanometer material
ActiveCN109319840AQuality improvementPromote Applied ResearchPhysical/chemical process catalystsStrontium carbonatesStrontium carbonateStable state
The invention provides a method for preparing a SrNb2O6 (strontium niobate) / SrCO3 (strontium carbonate) composite nanometer material. The method comprises the following steps of using a SnNb2O6 nanometer material as a raw material; performing hydrothermal reaction in a mixed alkaline solution of potassium hydroxide and strontium hydroxide under the condition of existence of CO2 (carbon dioxide). The method provided by the invention has the advantages that the repeatability is good, the operation is simple, and the method is suitable for quantity production; the cost is greatly reduced, and theefficiency is improved. The prepared strontium niobate / strontium carbonate composite nanometer material has the advantages that the quality is stable; a better application prospect is realized in thefield of photocatalytic water treatment; the development of semi-stable state niobate crystallizing method is promoted, and the practical application study of the niobate is promoted.
Owner:BEIJING UNIV OF TECH +1
Strontium carbonate micropowder and process for production
InactiveUS20120214927A1Good dispersionAdvantageously employedPigmenting treatmentMaterial nanotechnologyStrontium titanateStrontium carbonate
A fine strontium carbonate powder having a BET specific surface area of 20 to 150 m2 / g, which comprises spherical particles having a mean aspect ratio of 2.0 or less shows high dispersibility in liquid media and is of value for producing dielectric ceramic materials such as strontium titanate.
Owner:UBE IND LTD
Process for the preparation of micron/nano sized inorganic material
The invention discloses methods for making micron / nano meter sized particles of various inorganic materials such as minerals / oxides / sulphides / metals / ceramics at a steadily expanding liquid-liquid interface populated by suitable surfactant molecules that spontaneously organize themselves into superstructures varying over large length-scales. This experiment is realized in a radial Hele-Shaw cell where the liquid-liquid interfacial growth rate and consequently time scales such as arrival of surfactant molecules to the interface, the hydrodynamic flow effect to modulate the material organization into super structures at the dynamic charged interface.
Owner:COUNCIL OF SCI & IND RES
Production method of high-purity strontium carbonate special for light-emitting material
ActiveCN105712389ASmall particle sizeIncrease supersaturationStrontium carbonatesStrontium carbonateStrontium hydroxide
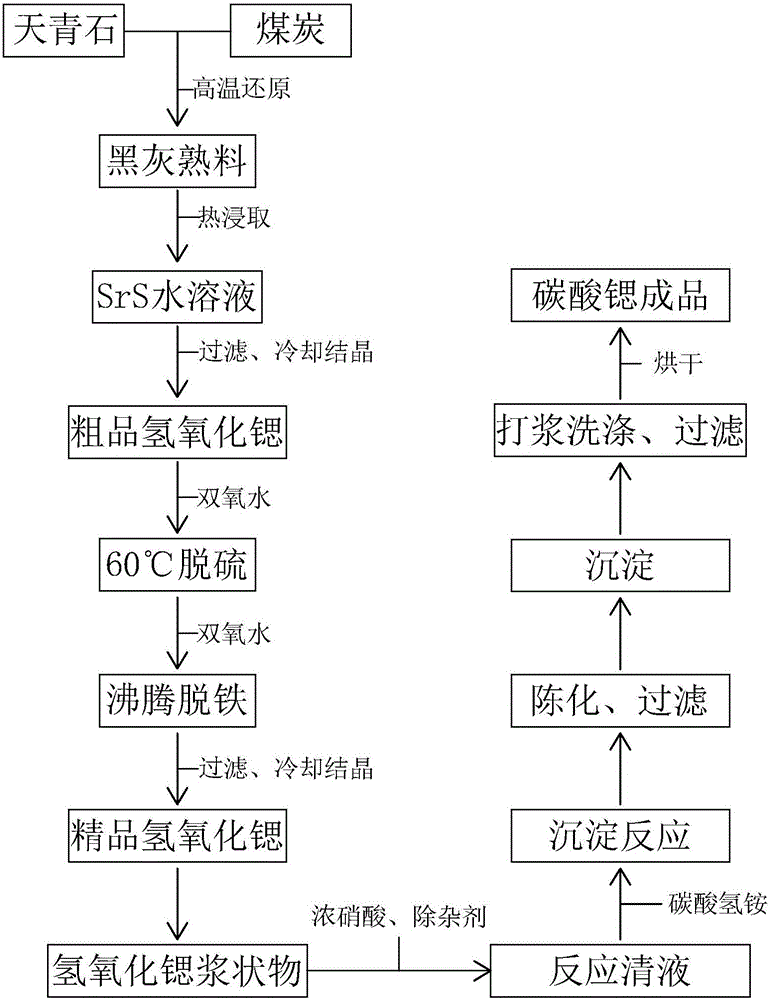
The invention discloses a production method of high-purity strontium carbonate special for a light-emitting material. The method comprises the following steps that 1, strontium hydroxide and water are mixed and prepared into a strontium hydroxide slurry; 2, concentrated nitric acid is slowly and dropwise added to the strontium hydroxide slurry, and the reaction endpoint is controlled to meet the condition that the pH is equal to 7.5; 3, ZnO2 is added, sufficient stirring and aging are conducted, filtering is conducted, and clear reaction liquid is obtained; 4, proportionable NH4HCO3 solution is slowly added to the clear reaction liquid, stirring and heat preservation precipitation reaction are conducted, and then sufficient aging is conducted; 5, the material sufficiently aged in the step 4 is subjected to primary filtering, beating and washing are conducted with water, solid-liquid separation is conducted, a filter cake is obtained, a strontium carbonate finished product is obtained after the filter cake is dried, and then packing is conducted. According to the production method of high-purity strontium carbonate special for the light-emitting material, the purity of the prepared strontium carbonate product is larger than or equal to 99.5%, has the spherical micro morphology and has a narrow particle size range distribution, and the particle size is about 20 micrometers.
Owner:CHONGQING DAZU HONGDIE STRONTIUM IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com