Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30results about "Intestine cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
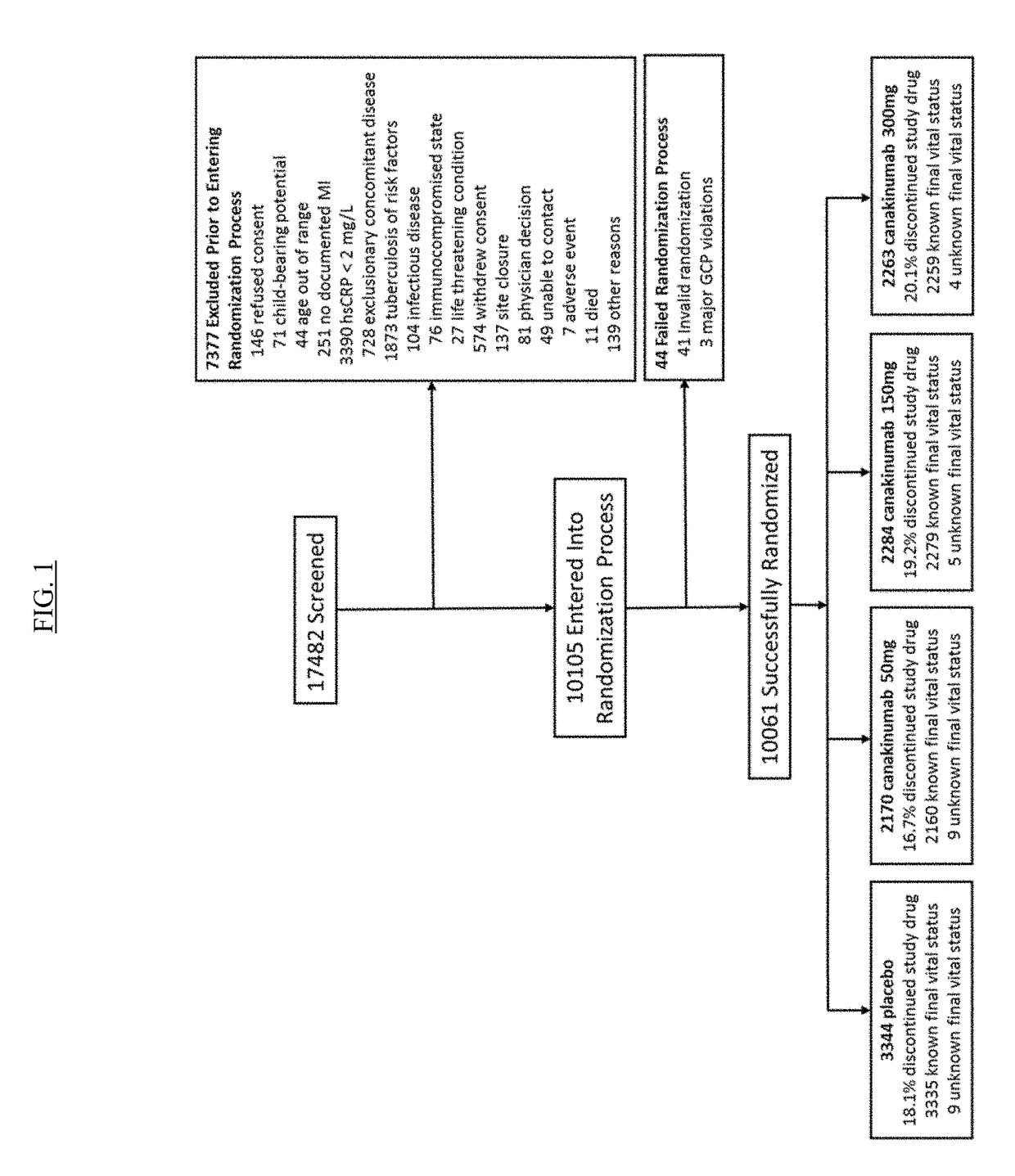
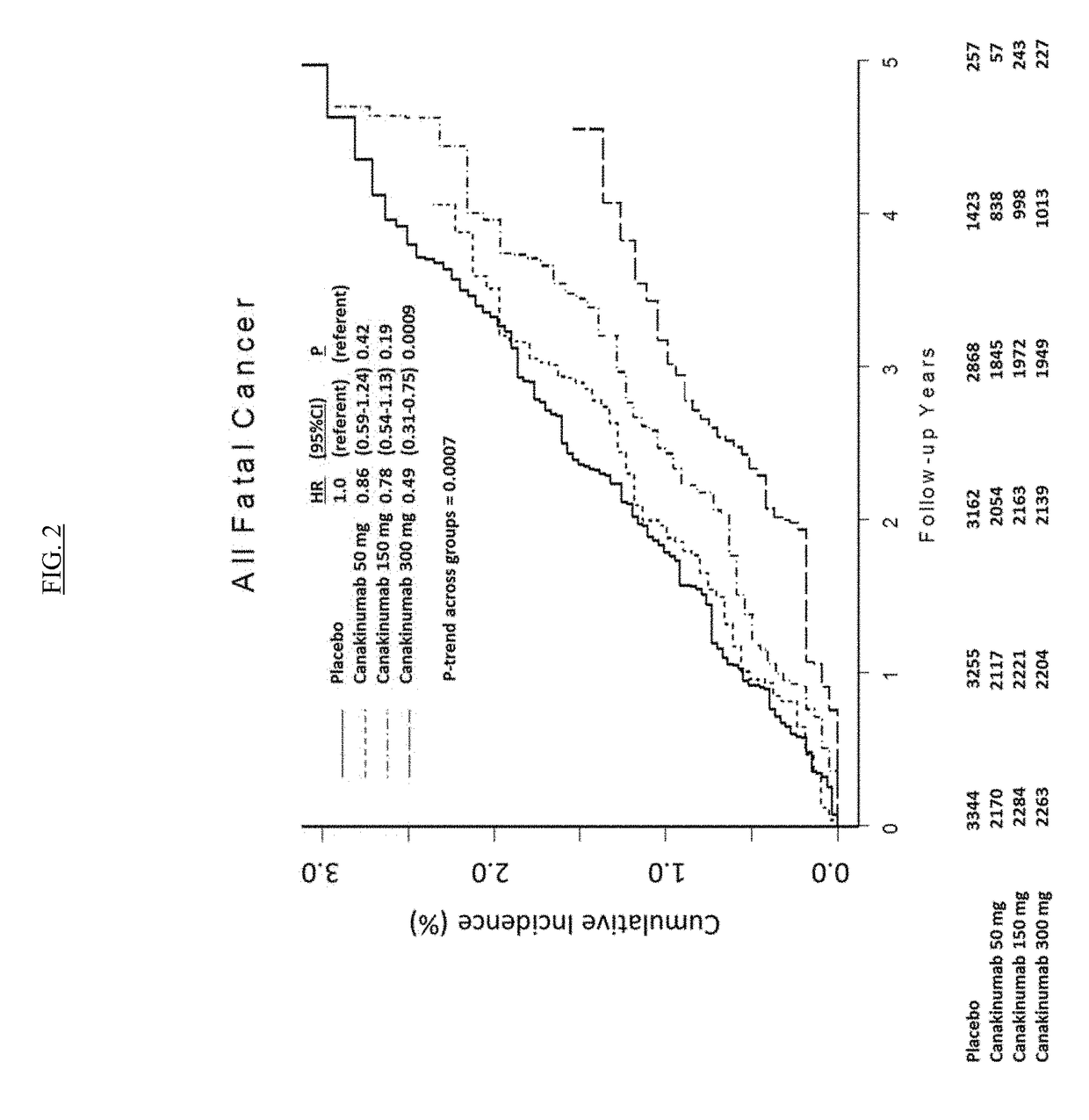
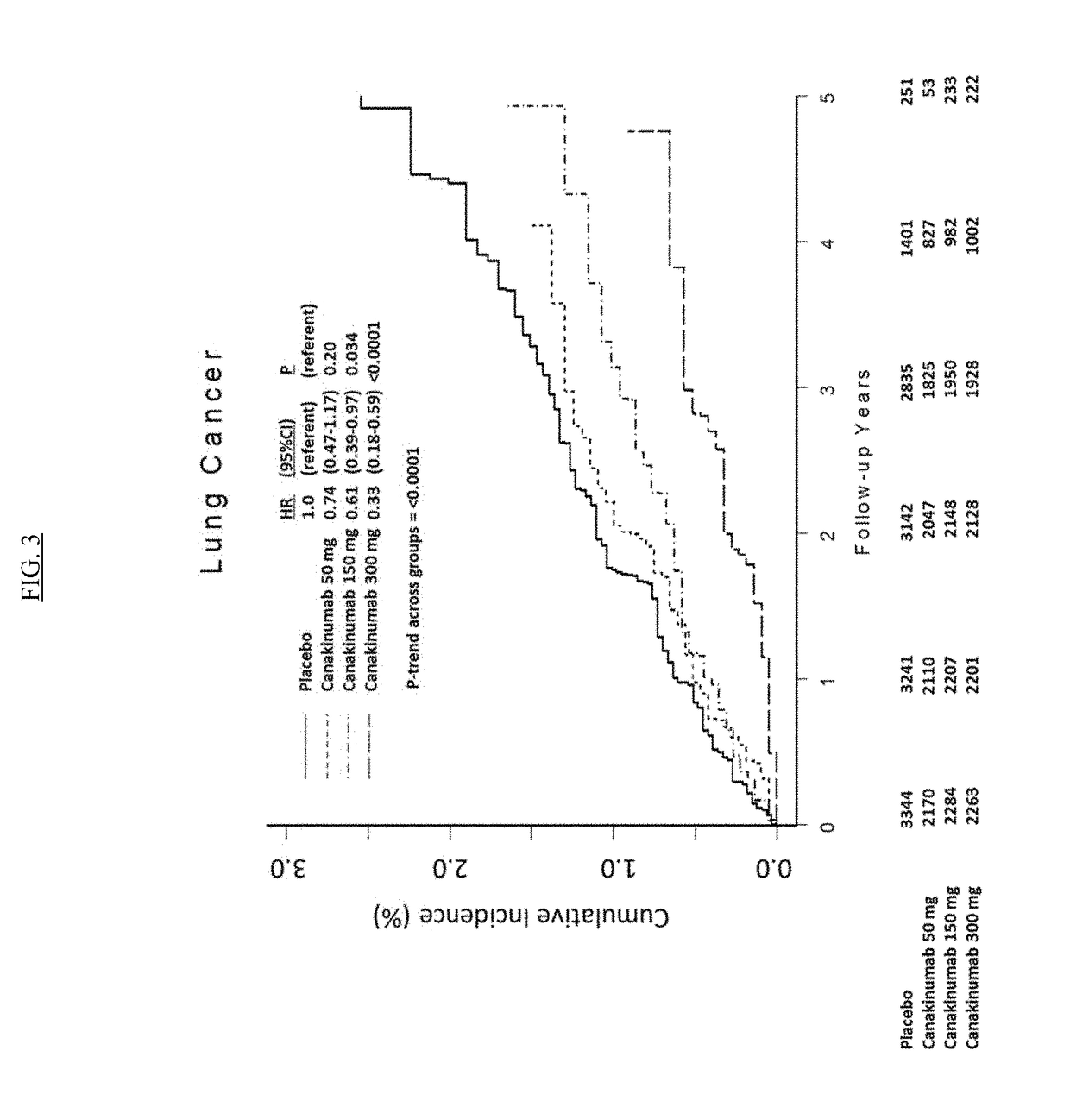
USE OF IL-1beta BINDING ANTIBODIES
InactiveUS20190048072A1Improve treatmentOrganic active ingredientsIntestine cancer vaccineAntigen bindingGevokizumab
Owner:NOVARTIS AG +3
Antibody Constructs for CLDN18.2 and CD3
The present invention relates to an antibody construct comprising a domain which binds to Claudin 18.2 (CLDN18.2) and another domain which binds to CD3. Moreover, the invention provides a polynucleotide encoding the antibody construct, a vector comprising said polynucleotide and a host cell transformed or transfected with said polynucleotide or vector. Furthermore, the invention provides a process for producing the antibody construct of the invention, a medical use of said antibody construct and a kit comprising said antibody construct.
Owner:AMGEN INC +1
Chimeric antigen receptor of cell for targeted expression of Claudin 18.2 and application of chimeric antigen receptor
ActiveCN113354739AEfficient killingStrong killing and cytokine release functionVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
The invention discloses a chimeric antigen receptor of a cell for targeted expression of Claudin 18.2 (CLDN 18.2), and particularly discloses a chimeric antigen receptor with an amino acid sequence as shown in SEQ ID NO.14. The chimeric antigen receptor comprises a Claudin 18.2-targeted single-chain antibody, a hinge region, a transmembrane structural domain and an intracellular signal structural domain. The Claudin 18.2-targeted chimeric antigen receptor disclosed by the invention can achieve effective and specific targeted expression of malignant cells (such as tumor cells) of the Claudin 18.2 surface antigen, so that a more efficient method with fewer side effects and adverse reactions is provided for treating some tumors expressing the Claudin 18.2 surface antigen.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Chimeric antigen receptor T lymphocyte and application thereof to preparation of product for treating solid tumors
ActiveCN111875708ALift specific immune responseEnhance anti-tumor immune responseVirusesAntineoplastic agentsSingle-Chain AntibodiesAntiendomysial antibodies
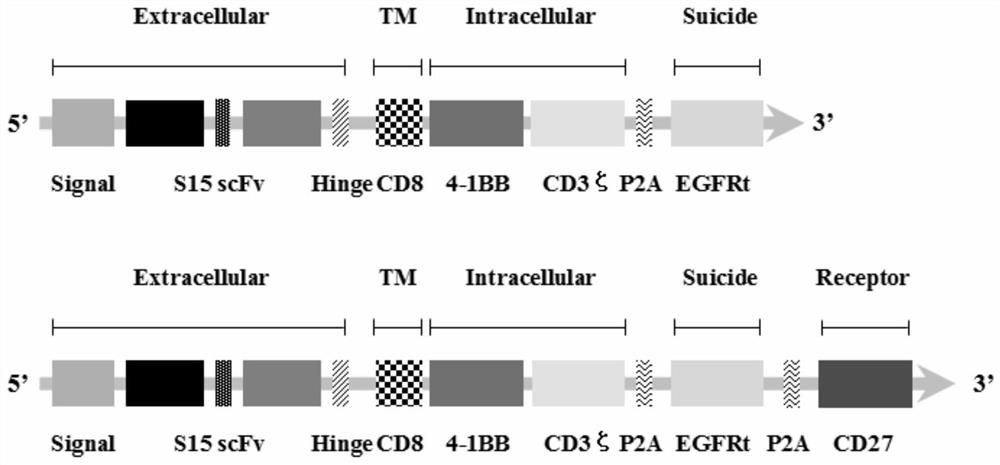
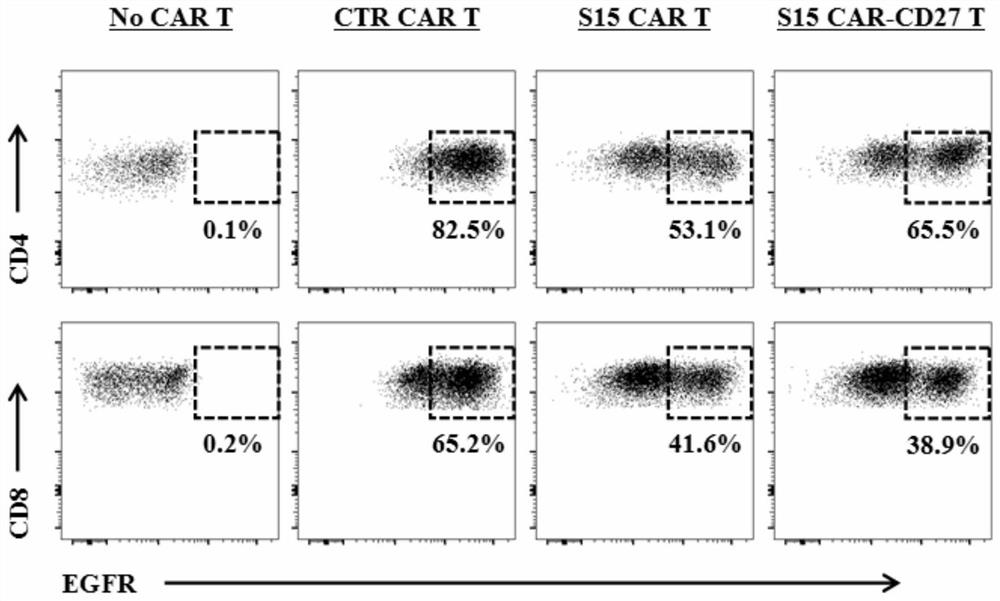
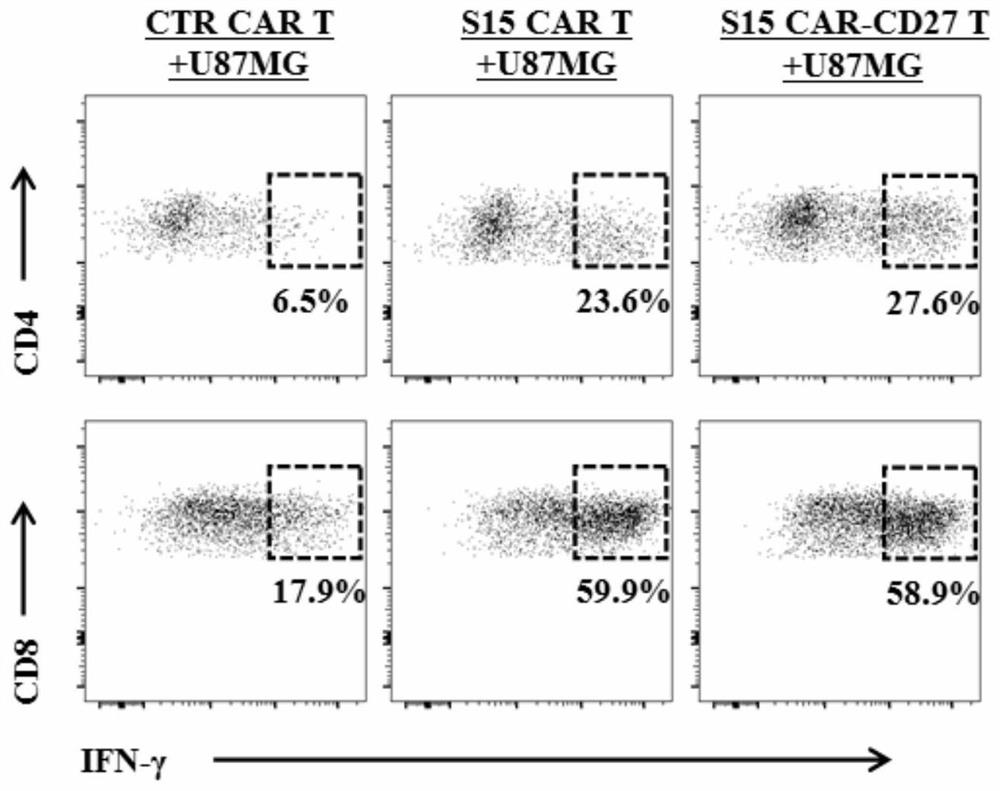
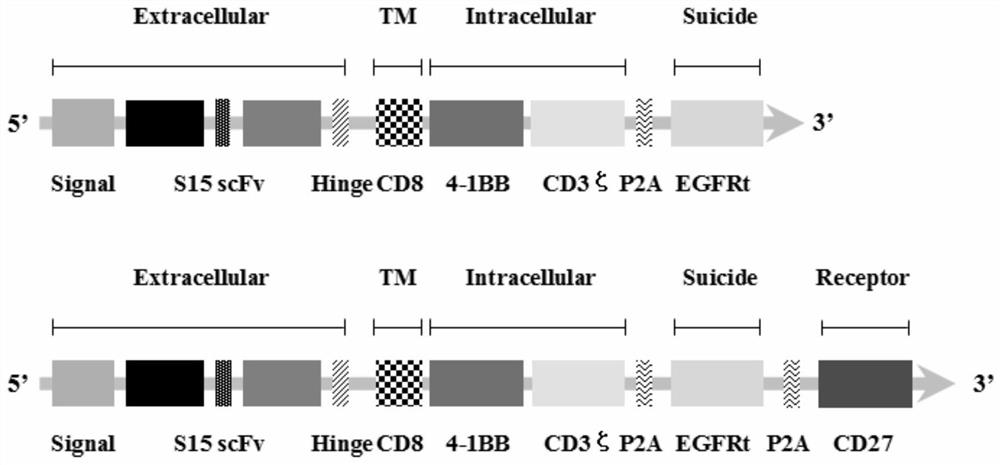
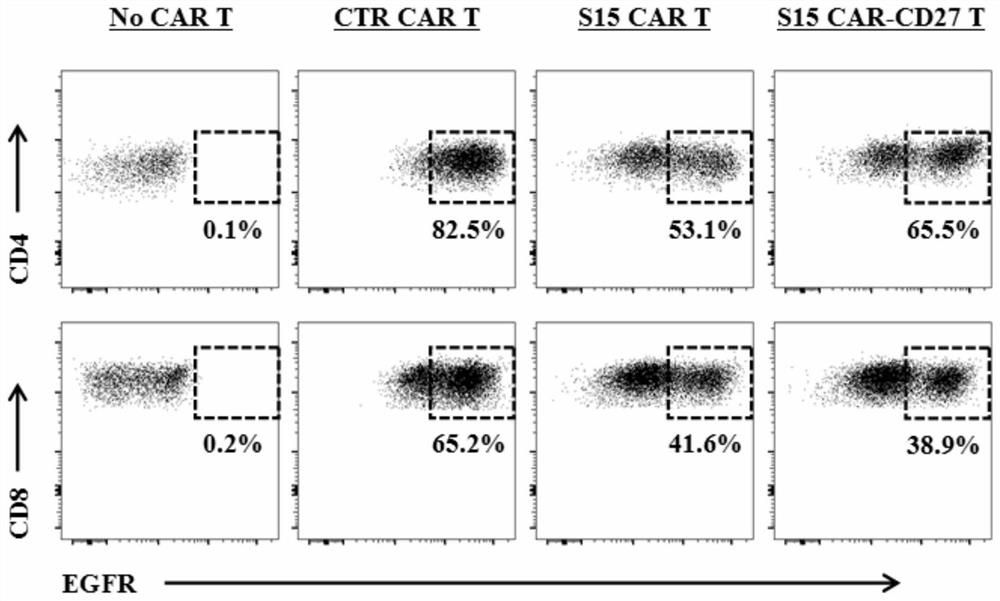
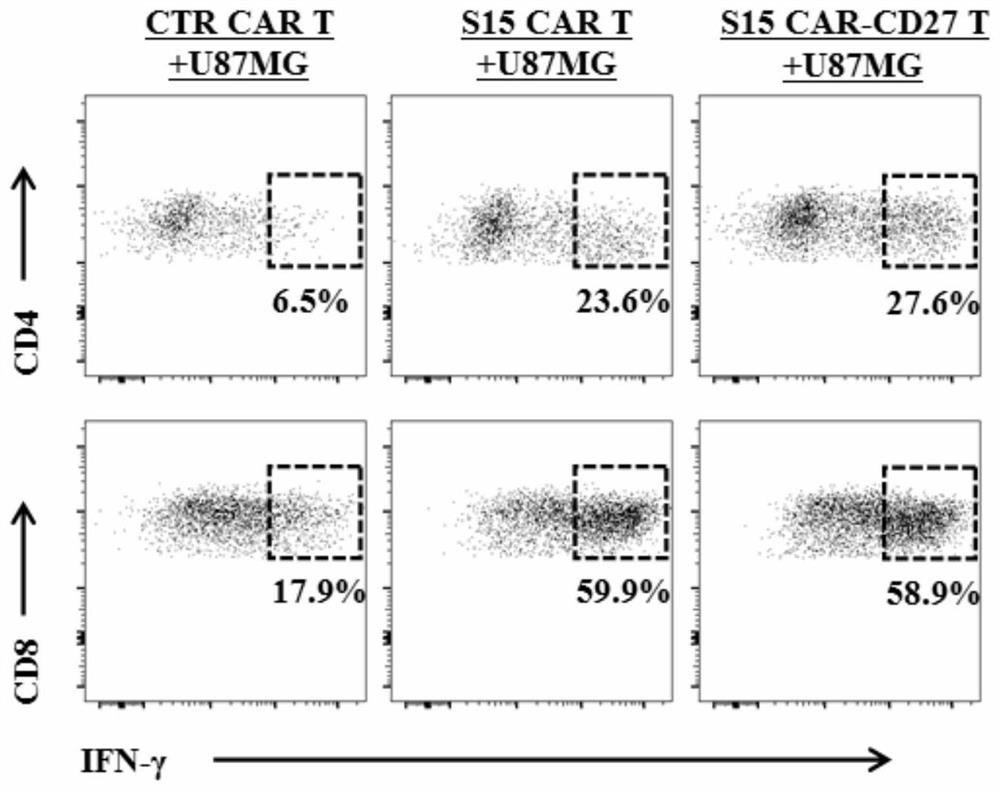
The invention discloses a chimeric antigen receptor T lymphocyte and an application thereof to preparation of a product for treating solid tumors. A chimeric antigen receptor in the chimeric antigen receptor T lymphocyte sequentially comprises a human CD8 lead peptide, an anti-Siglec-15 single-chain antibody, a human CD8 hinge transmembrane region, a human 4-1BB intracellular region, a human CD3 zeta intracellular region, a self-cleavage peptide, a CSF2Ra signal peptide, an EGFRt protein, a self-cleavage peptide and a human CD27. Experiments prove that the chimeric antigen receptor T lymphocyte provided by the invention highly expresses IFN gamma and CD107a, has a strong killing function on Siglec-15 positive tumor cells, and has killing efficiency of more than 80% under the condition thatthe effect-target ratio is 1: 1. A tumor transplantation model experiment shows that the chimeric antigen receptor T lymphocyte also has a strong killing function on Siglec-15 positive tumor cells inan animal body.
Owner:CARBIOGENE THERAPEUTICS CO LTD
Peptides, combination of peptides, and cell based medicaments for use in immunotherapy against urinary bladder cancer and other cancers
InactiveUS20190330274A1Enhance stability and solubilityAid in diagnosisPeptide/protein ingredientsAntibody mimetics/scaffoldsWilms' tumorEx vivo
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
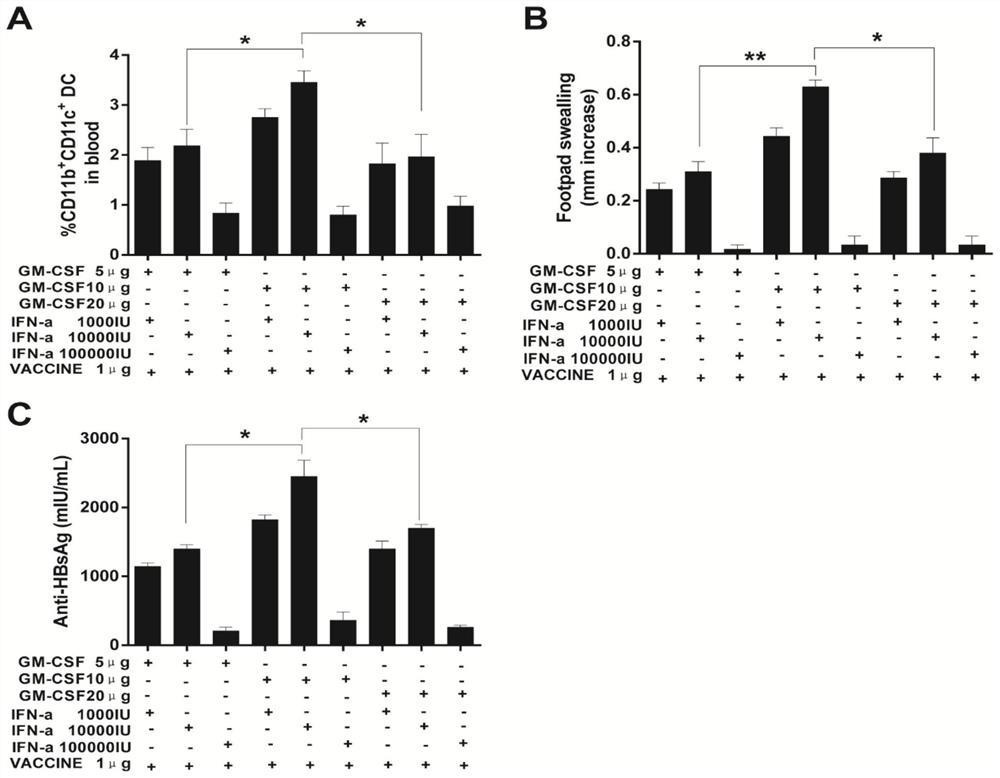
Immunoenhancer, immunotherapy medicine composition, preparation method of composition and application of immunoenhancer and composition
ActiveCN108567977AStrong immune responseImprove immunityVirus peptidesAntiviralsDiseaseGranular cell
The invention discloses an immunoenhancer, an immunotherapy medicine composition, a preparation method of the immunotherapy medicine composition and an application of the immunoenhancer and the immunotherapy medicine composition. The immunoenhancer at least comprises interferon and granular cell-macrophage colony stimulating factors, and the immunotherapy medicine composition at least comprises antigen and the immunoenhancer. The immunoenhancer can remarkably enhance the immunity of a body and improve the antigen presenting efficiency of the body, so that the body can establish effective immune activation and response. Strong antibody and cellular immune protection reaction and pathogen removing capacity can be generated, and the immunoenhancer can be applied to therapy of diseases and tumors caused by microorganisms such as viruses and bacteria.
Owner:FUDAN UNIV
Anti-PD-L1/OX40 bispecific antibody preparation as well as preparation method and application thereof
The present invention relates to formulations comprising an anti-PD-L1 / OX40 bispecific antibody, in particular to pharmaceutical formulations comprising an anti-PD-L1 / OX40 bispecific antibody, a buffer, a stabilizer and a surfactant. In addition, the invention also relates to the use of these formulations for the treatment or prevention of diseases.
Owner:INNOVENT BIOLOGICS (SUZHOU) CO LTD
Peptides, combination of peptides, and cell based medicaments for use in immunotherapy against urinary bladder cancer and other cancers
ActiveUS20200055898A1Enhance stability and solubilityAid in diagnosisPeptide/protein ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesReceptor
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
IL-1[beta] binding antibodies for use in treating cancer
PendingCN110831967AConvenient treatmentOrganic active ingredientsIntestine cancer vaccineAntiendomysial antibodiesAntigen binding
Owner:NOVARTIS AG
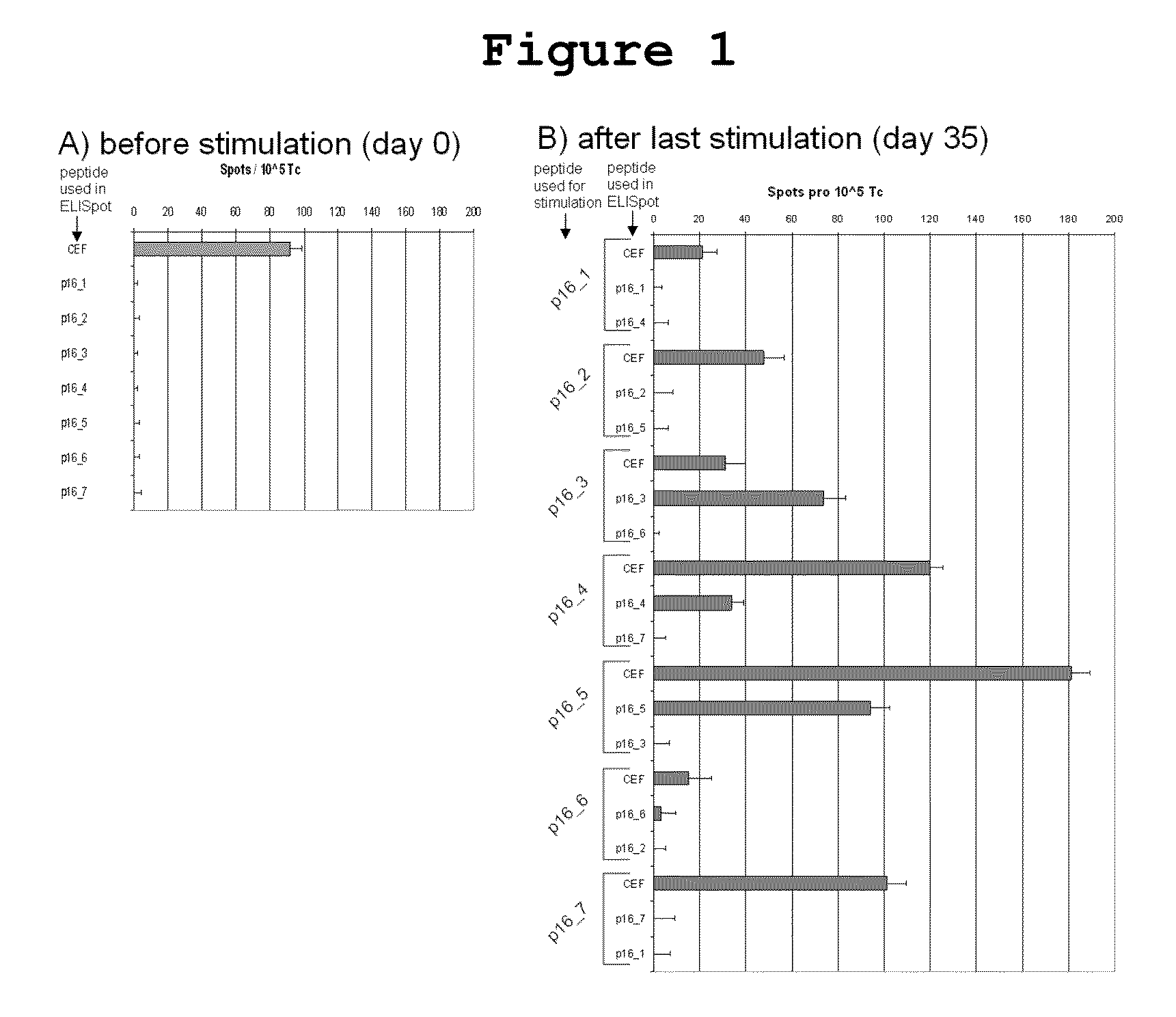
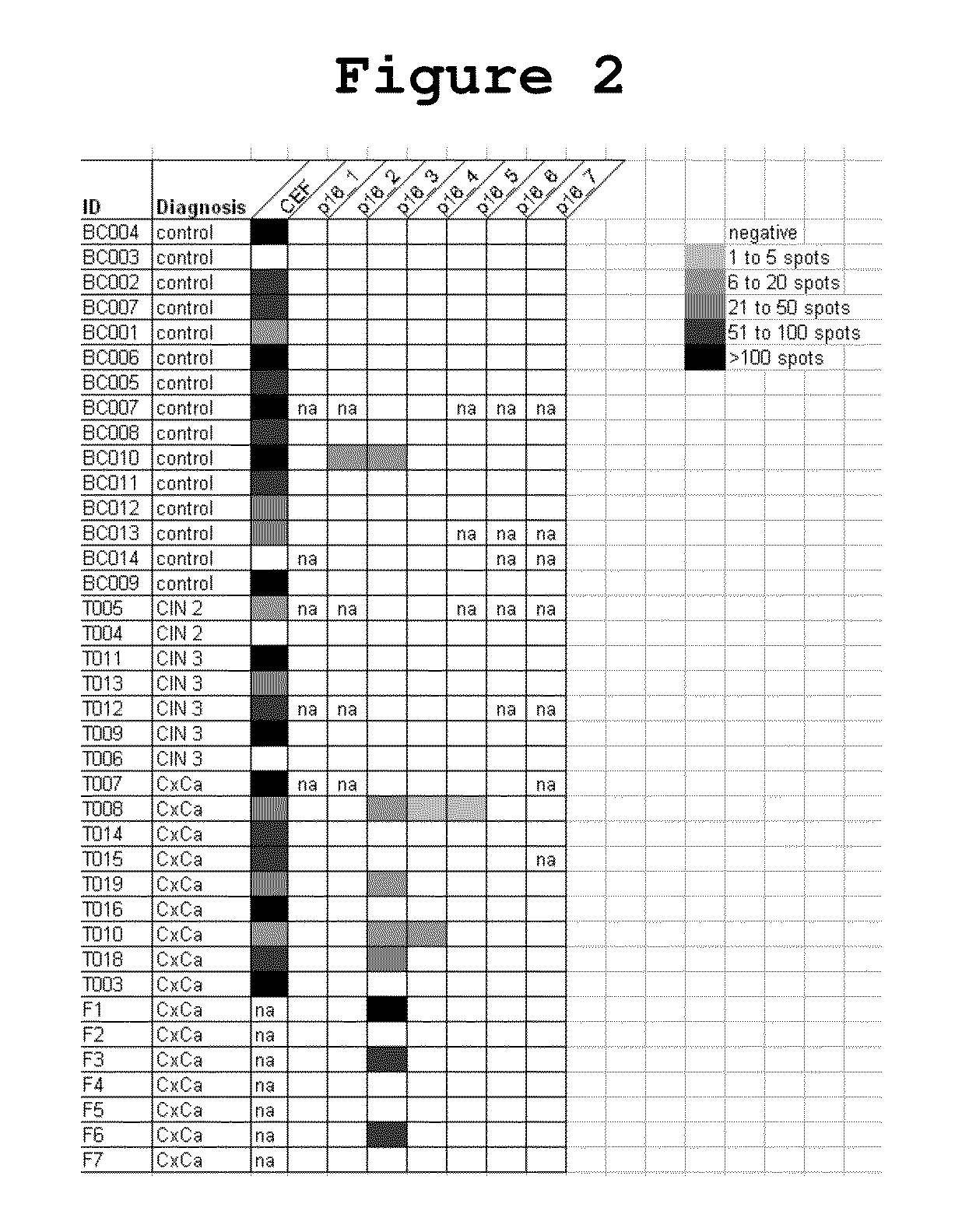
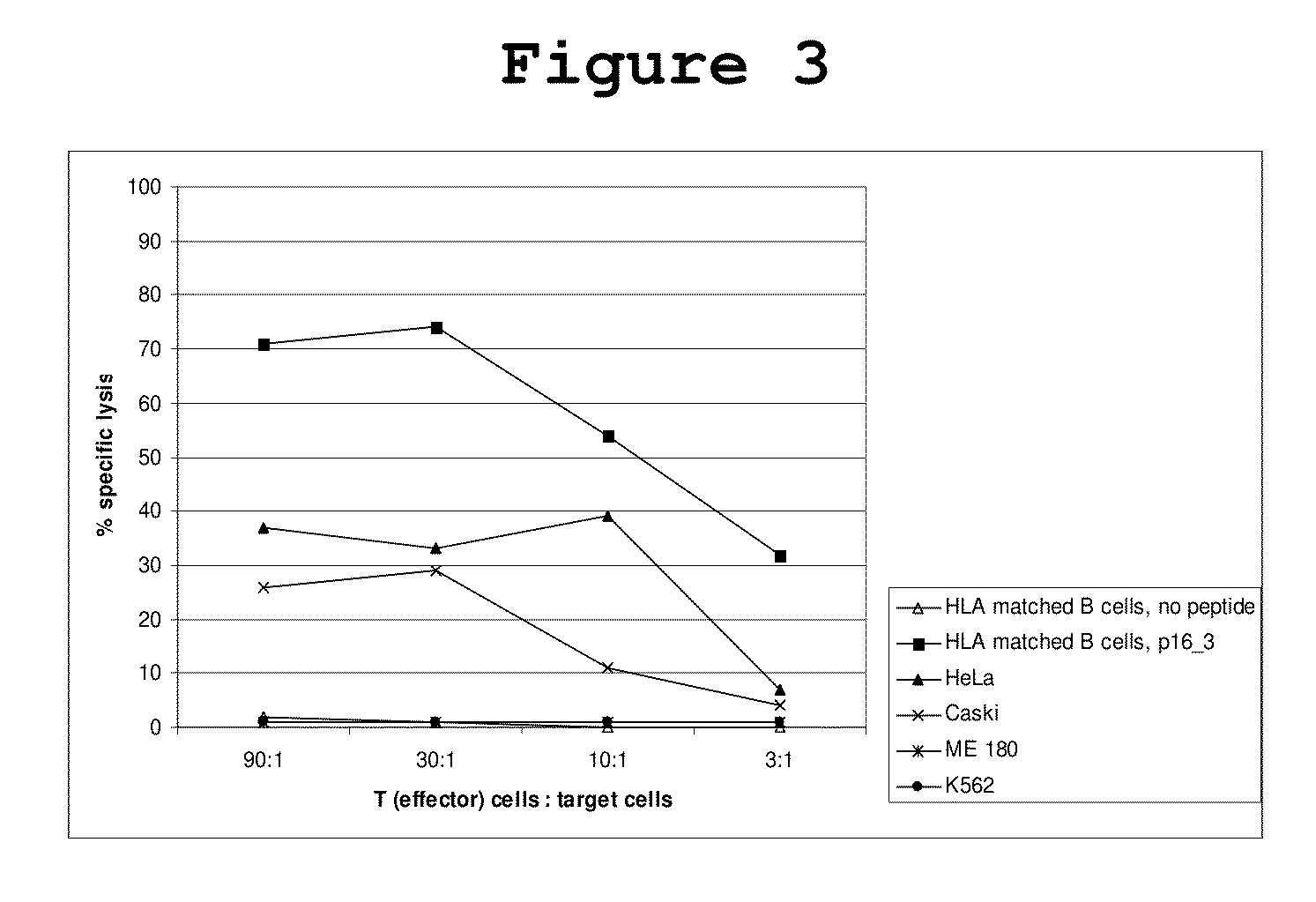
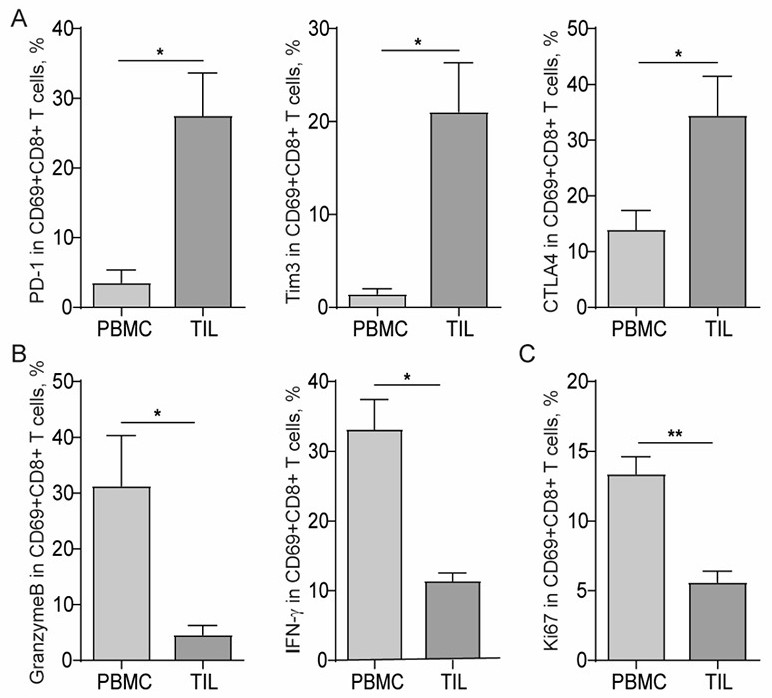
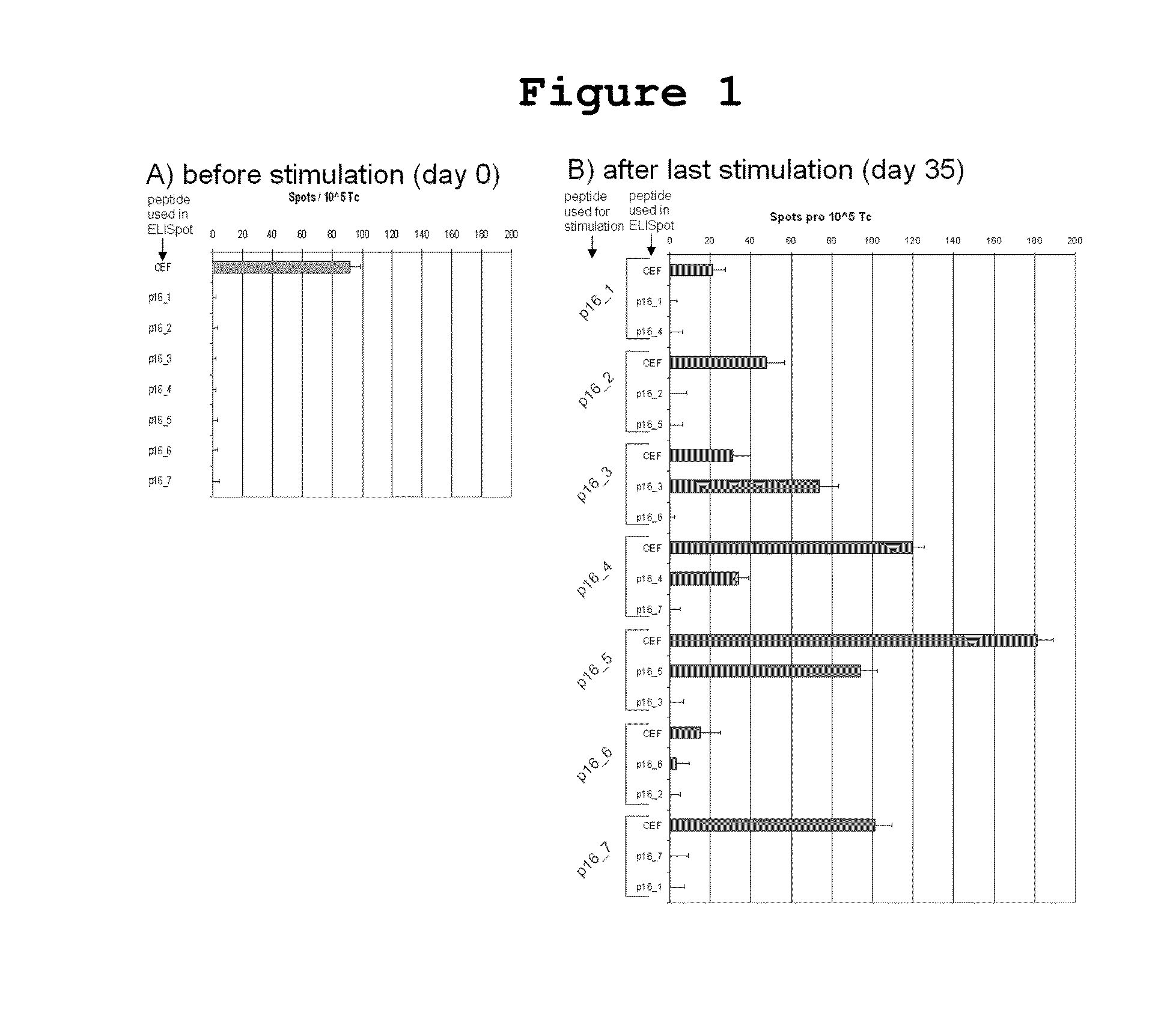
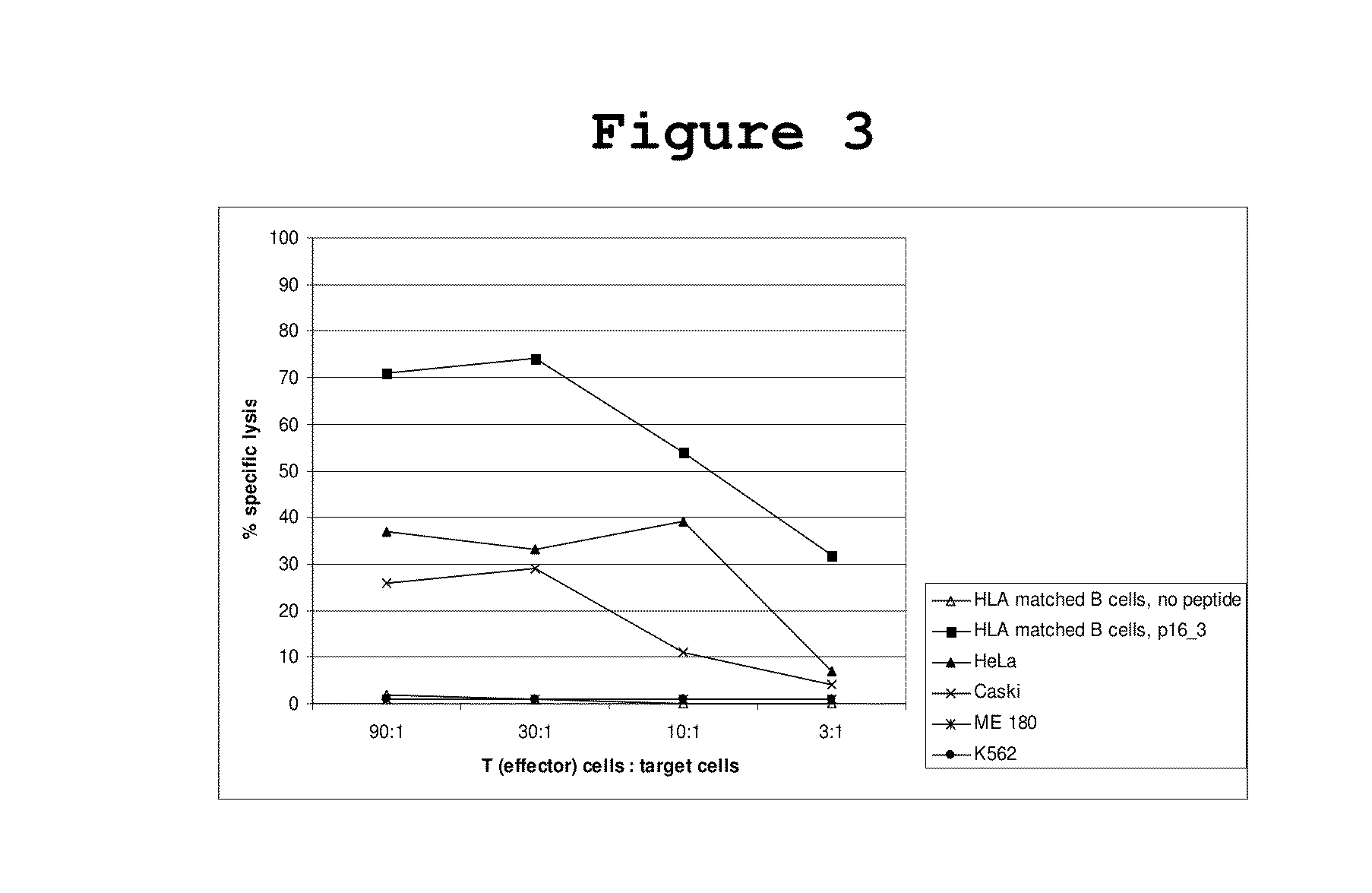
P16INK4A derived peptides for prophylaxis and therapy of HPV-associated tumors and other P16INK4A expressing tumors
Described are particular fragments of the cyclin-dependent kinase inhibitor p16 capable of increasing IFN-γ secretion of T cells or inducing proliferation of T cells and the use of said fragments for immunizing an individual against HPV-associated or other p16INK4a expressing carcinomas, preferably advanced carcinomas.
Owner:UNIVERSITY OF HEIDELBERG
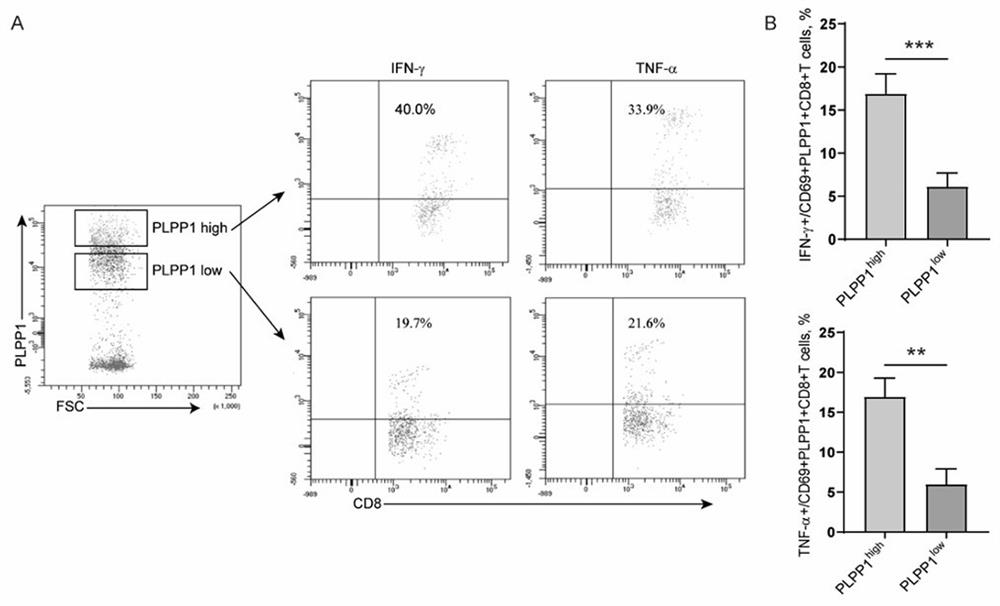
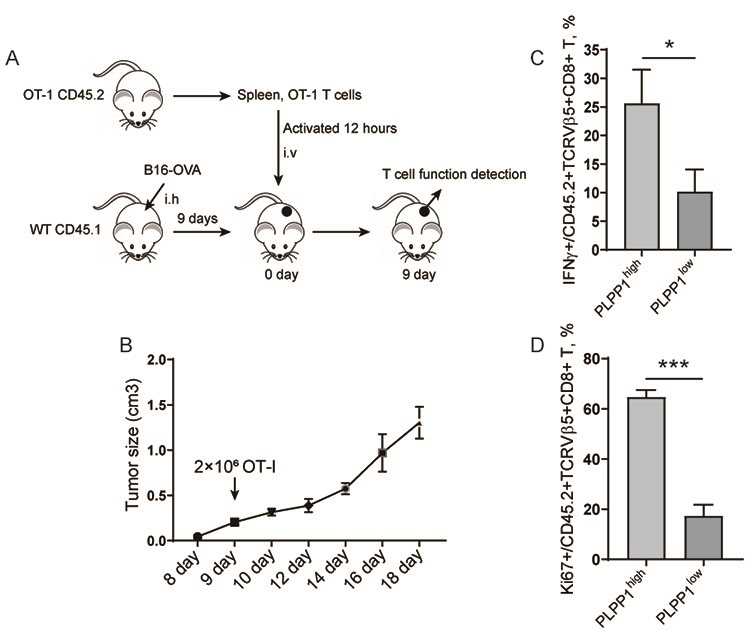
Application of PLPP1 in preparation of T cell immune tumor related medicament
PendingCN111773380AInhibition of proliferative abilityIntestine cancer vaccineSkin cancer vaccinePhospholipinAbnormal T cells
The invention belongs to the technical field of cellular immune biology, and particularly relates to application of PLPP1 in preparation of a T cell immune tumor related medicament. The PLPP1 is a keyenzyme for regulating and controlling T cell phospholipid metabolism; in a tumor microenvironment, the reduction of the expression of the metabolism key enzyme PLPP1 causes abnormal CD8+ T cell phospholipid metabolism, then causes abnormal CD8+ T cell metabolism, and finally causes abnormal T cell functions; and that is, the expression of the PLPP1 has a positive correlation relationship with activation, infiltration and functions of CD8+ T cells in the tumor microenvironment. In-depth studies show that after a PDL1 / PD-1 signal path is activated, an AKT / mTOR signal path is further activated, the expression of the T cell phospholipid metabolism key enzyme PLPP1 is regulated and controlled by a transcription factor GATA1, and finally the T cell function adjustment is achieved by the change of the T cell metabolism function.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
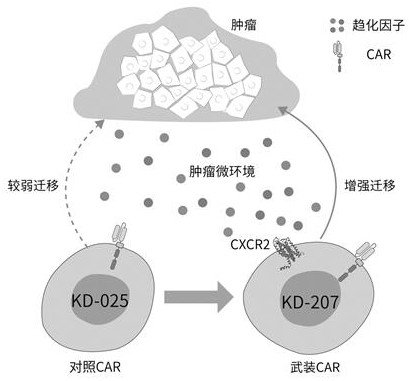
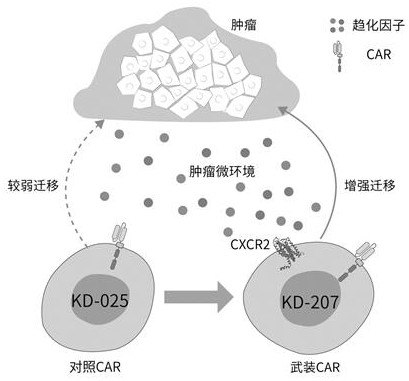
Specific chimeric antigen receptor cell armed with targeted CXCR2 ligand as well as preparation method and application thereof
ActiveCN111607006AEasy to migrateEffective targeted attackImmunoglobulins against cytokines/lymphokines/interferonsMammal material medical ingredientsAntigen receptorOncology
The invention relates to a specific chimeric antigen receptor cell armed with a targeted CXCR2 ligand as well as a preparation method and application thereof. Through a method, which uses a recombinant carrier of a chimeric antigen receptor that specifically targets human NKG2DL armed with a targeting CXCR2 ligand to modify a immune response cell, the obtained novel functionalized immune responsecell can effectively attack multiple tumors in a targeted mode; and the functionalized immune response cell can be used to prepare preparations for treating malignant tumors. The engineering immune cells modified by a chimeric antigen receptor that is armed with a targeting CXCR2 ligand and specifically targets human NKG2DL can more easily migrate to a tumor position and continuously kill the tumors in a targeted mode.
Owner:NANJING KAEDI BIOTHERAPEUTICS LTD
Inflammasome activators and methods of use to treat tumors
Owner:LOYOLA UNIV OF CHICAGO
Inflammasome activators and methods of use to treat tumors
ActiveUS20180243390A1Effective anti-tumor immune responseIntestine cancer vaccineViral antigen ingredientsTumor regressionWilms' tumor
Compositions and treatments for inducing tumor regression by activating inflammasomes in tumor cells and tumor-associated cells. A tumor in a subject is treated by administering a composition to the subject that activates inflammasomes in cells of the tumor and thereby causes tumor cell pyroptosis and tumor regression.
Owner:LOYOLA UNIV OF CHICAGO
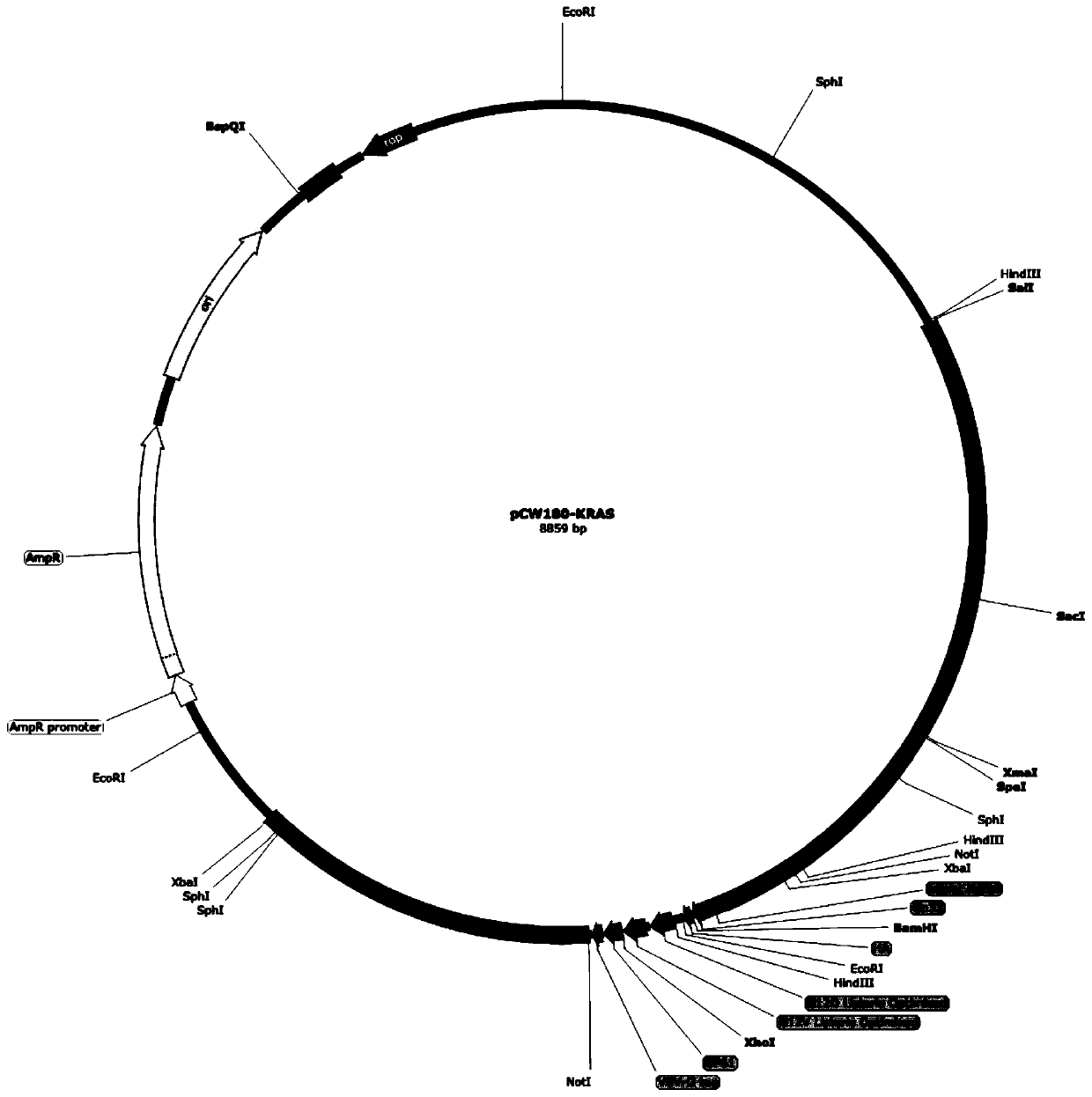
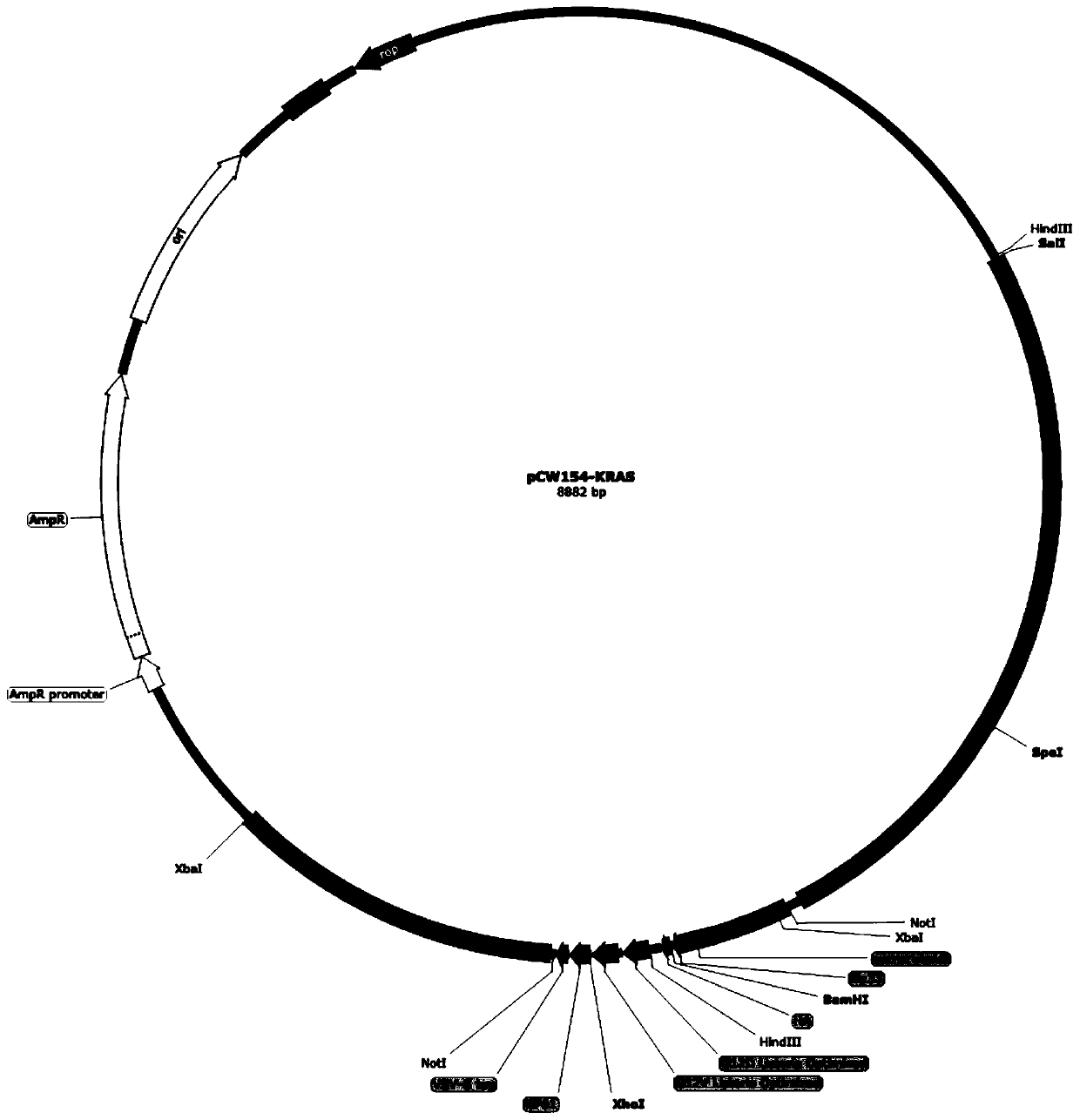
KRAS high-expression cancer vaccine based on recombinant attenuated Listeria monocytogenes and preparation method and application method thereof
PendingCN111388658AImprove immune activityHigh immune activityIntestine cancer vaccineTumor rejection antigen precursorsAdjuvantSpecific immunity
The invention discloses a KRAS high-expression cancer vaccine based on recombinant attenuated Listeria monocytogenes and a preparation method and application method thereof. The cancer vaccine is bacterial liquid of an Lm delta actA / plcB-KRAS strain; the Lm delta actA / plcB-KRAS strain is a gene recombinant bacterium carrying a KRAS protein gene by taking the attenuated Listeria monocytogenes asa carrier; the attenuated Listeria monocytogenes is obtained by completely knocking out two virulence genes of actA and plcB from the Listeria monocytogenes; and the KRAS protein gene is a DNA sequence obtained by synthesizing a KRAS protein amino acid sequence after being optimized by a Listeria codon, adding a Hind III restriction enzyme cutting site at the upstream and adding an Xho I restriction enzyme cutting site at the downstream. According to the KRAS high-expression cancer vaccine disclosed by the invention, the attenuated Listeria monocytogenes is used as the carrier, and the specific immune response in a tumor microenvironment is effectively improved by utilizing the characteristic that the Listeria monocytogenes can uniquely grow in a host phagocyte and is a natural T cell immune activation adjuvant, so that the immune tolerance of an organism is broken, and further the development of tumors can be inhibited.
Owner:IMMORNA (HANGZHOU) BIOTECHNOLOGY CO LTD
Immunopotentiator, immunotherapy pharmaceutical composition and its preparation and application
ActiveCN108567977BStrong immune responseImprove immunityVirus peptidesAntiviralsDiseaseImmunocompetence
The invention discloses an immune enhancer, which at least includes interferon and granulocyte-macrophage colony-stimulating factor, and an immunotherapy pharmaceutical composition, which at least includes an antigen and the above-mentioned immune enhancer. The invention also discloses the preparation method of the immunotherapy pharmaceutical composition, the application of the above immune enhancer and immunotherapy pharmaceutical composition. The immunopotentiator provided by the invention can significantly improve the immune ability of the body, improve the antigen presentation efficiency of the body, and enable the body to establish effective immune activation and response; produce stronger antibodies and cellular immune protection responses and the ability to remove pathogens, can It is used in the treatment of diseases and tumors caused by microorganisms such as viruses and bacteria.
Owner:FUDAN UNIV
Dosage regimen for combination therapy using pd-1 axis binding antagonists and gpc3 targeting agent
PendingUS20200216542A1Reduce functionIncreased activationIntestine cancer vaccineLiver cancer vaccineDosing regimenRegimen
Provided are dosage regimens for combination therapy using PD-1 axis binding antagonists and GPC3 targeting agent. For example, the dosage regimens comprise (i) a loading period within which the GPC3 targeting agent is administered, followed by (ii) a maintenance period within which the PD-1 axis binding antagonist and the GPC3 targeting agent are administered.
Owner:F HOFFMANN LA ROCHE & CO AG
Armed specific chimeric antigen receptor cell targeting cxcr2 ligand and its preparation method and application
ActiveCN111607006BEasy to migrateEffective targeted attackImmunoglobulins against cytokines/lymphokines/interferonsMammal material medical ingredientsAntigen receptorCancer research
Owner:NANJING KAEDI BIOTHERAPEUTICS LTD
Cancer vaccines for kidney cancer
PendingUS20210213116A1Reduce riskIncrease opportunitiesIntestine cancer vaccineTumor rejection antigen precursorsTumor reductionVaccination
The invention relates to the field of cancer, in particular kidney cancer. In particular, it relates to the field of immune system directed approaches for tumor reduction and control. Some aspects of the invention relate to vaccines, vaccinations and other means of stimulating an antigen specific immune response against a tumor in individuals. Such vaccines comprise neoantigens resulting from frameshift mutations that bring out-of-frame sequences of the BAP, PBRM1, SETD2, and VHL genes in-frame. Such vaccines are also useful for ‘off the shelf’ use.
Owner:CUREVAC NETHERLANDS BV
Viral vector constructs for delivery of nucleic acids encoding cytokines and uses thereof for treating cancer
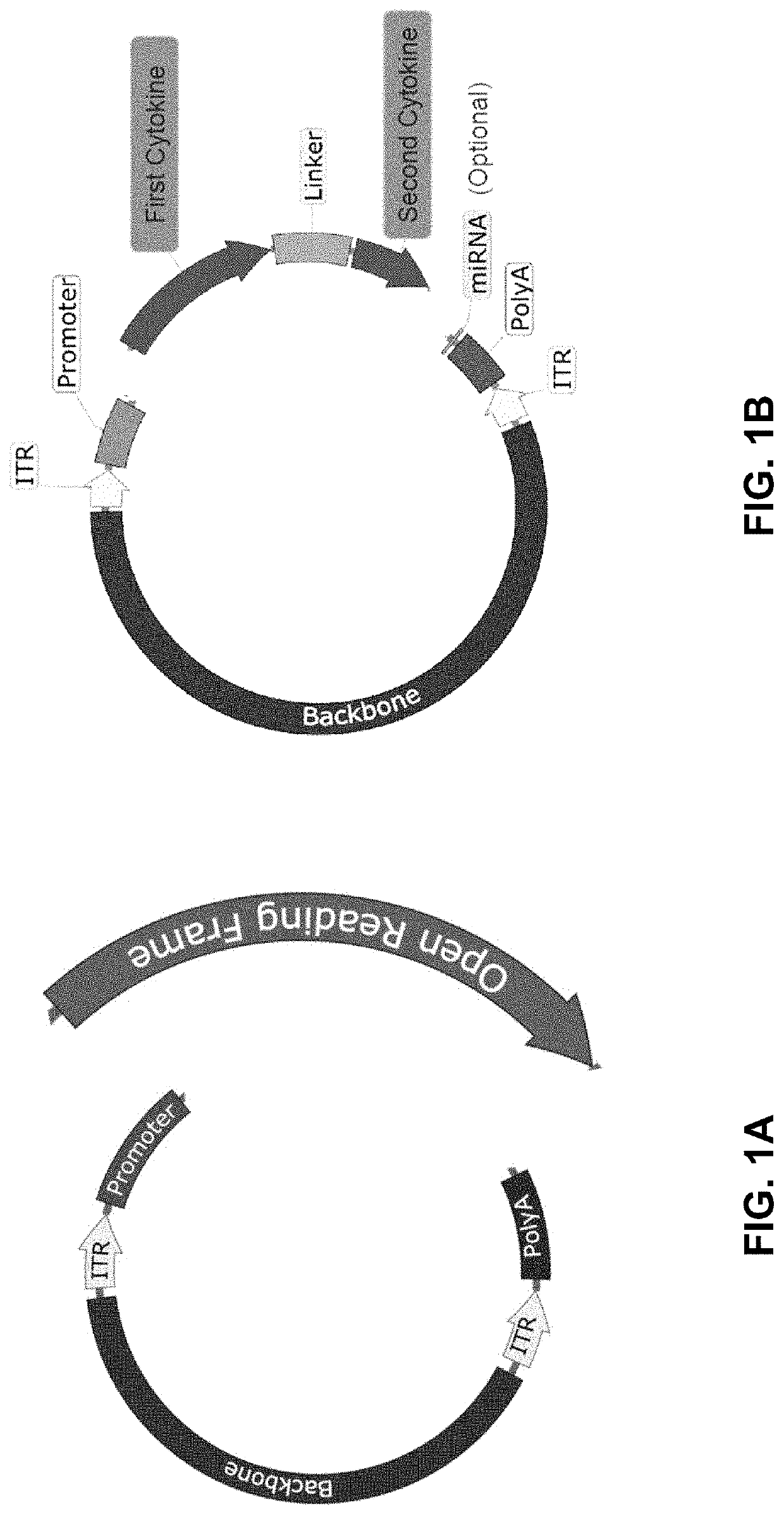
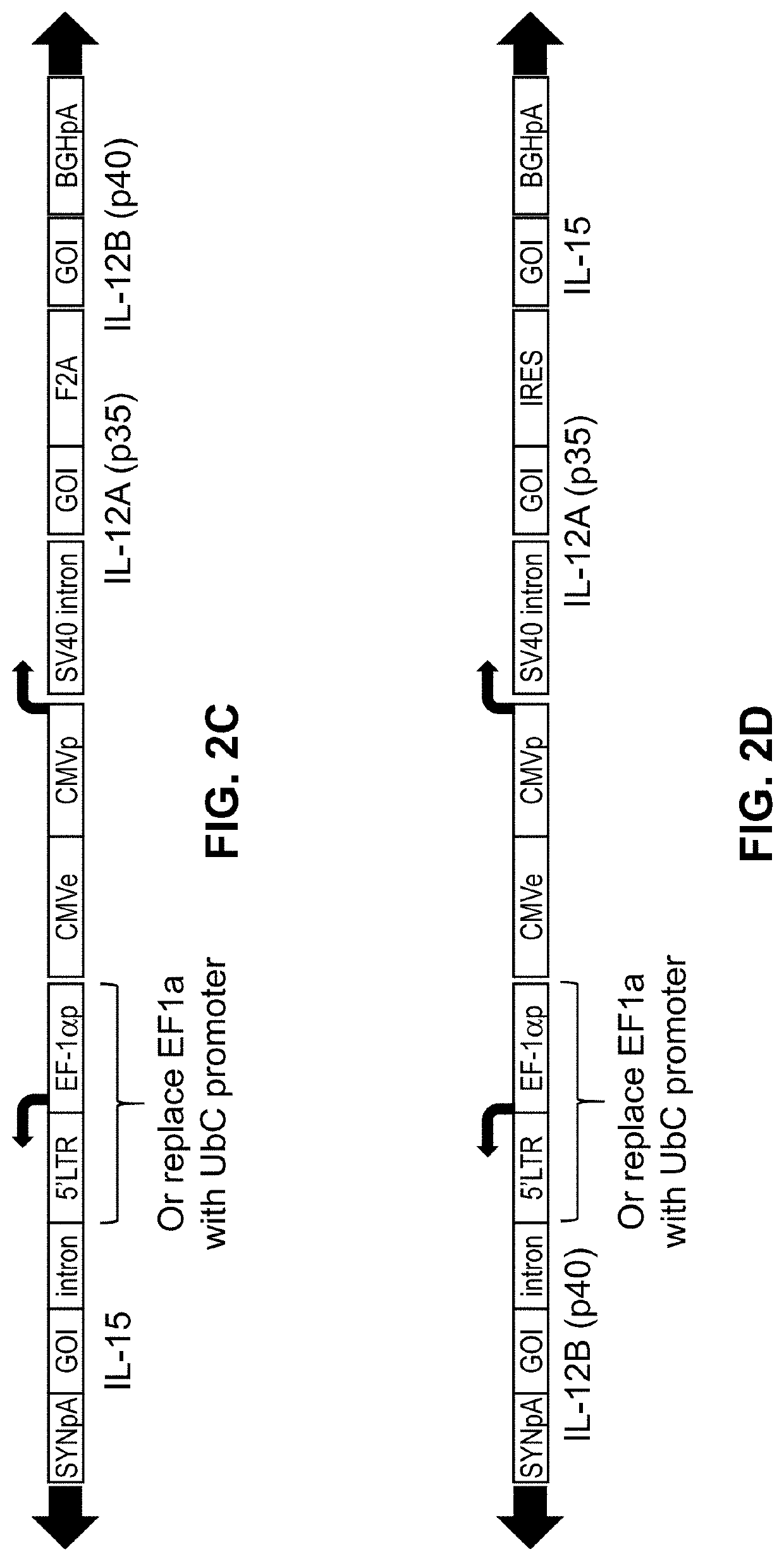
The present disclosure provides the gene therapy compositions comprising vectors (e.g., viral vectors) suitable for delivery of nucleic acids encoding immunomodulatory proteins or functional fragments thereof, and methods of using the same. Certain aspects of the disclosure are directed to an adeno-viral vector (AAV) delivery of nucleic acids encoding two or more immunomodulatory proteins or functional fragments thereof to a tumor.
Owner:KRIYA THERAPEUTICS INC
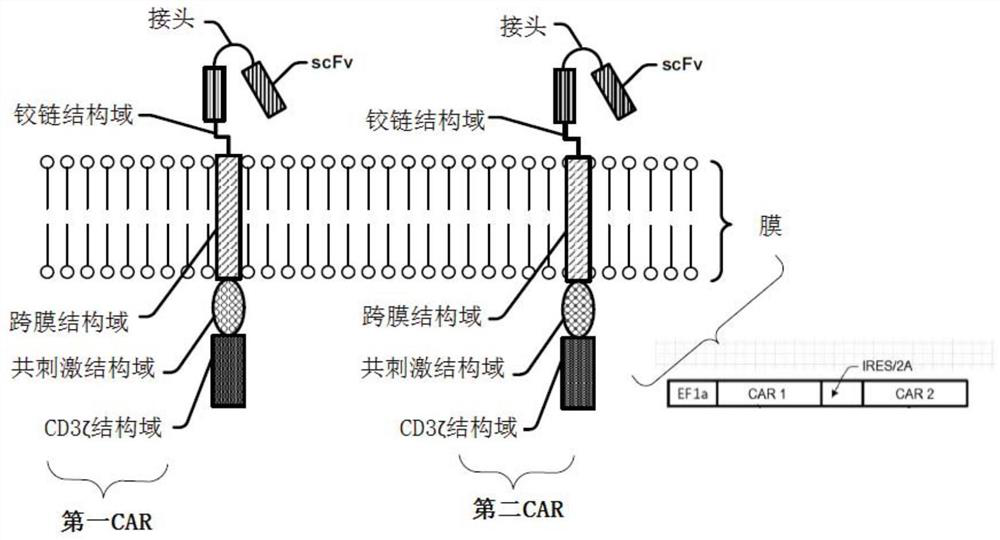
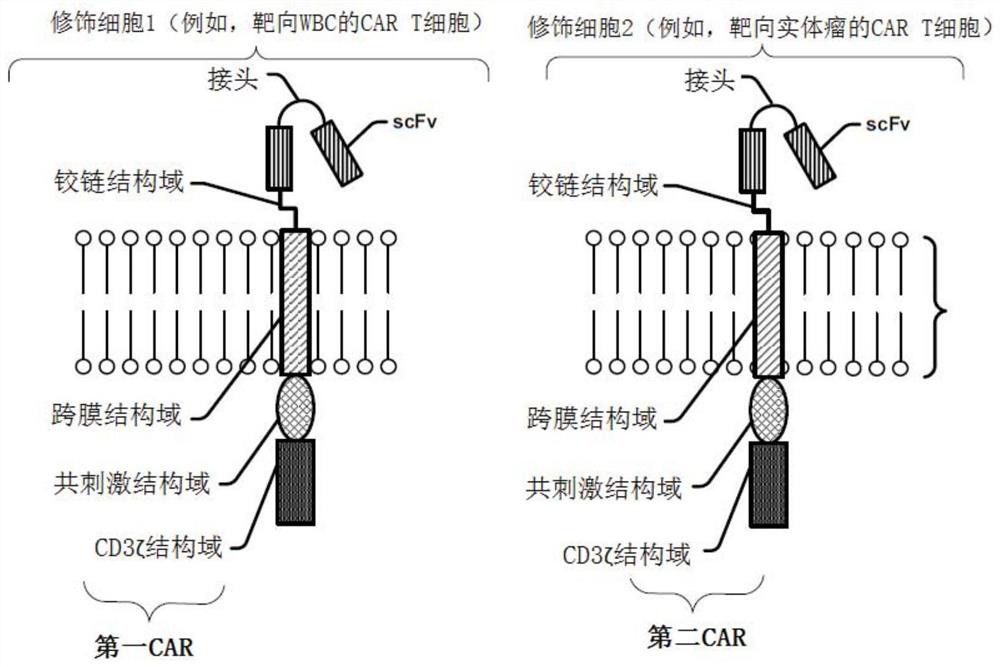
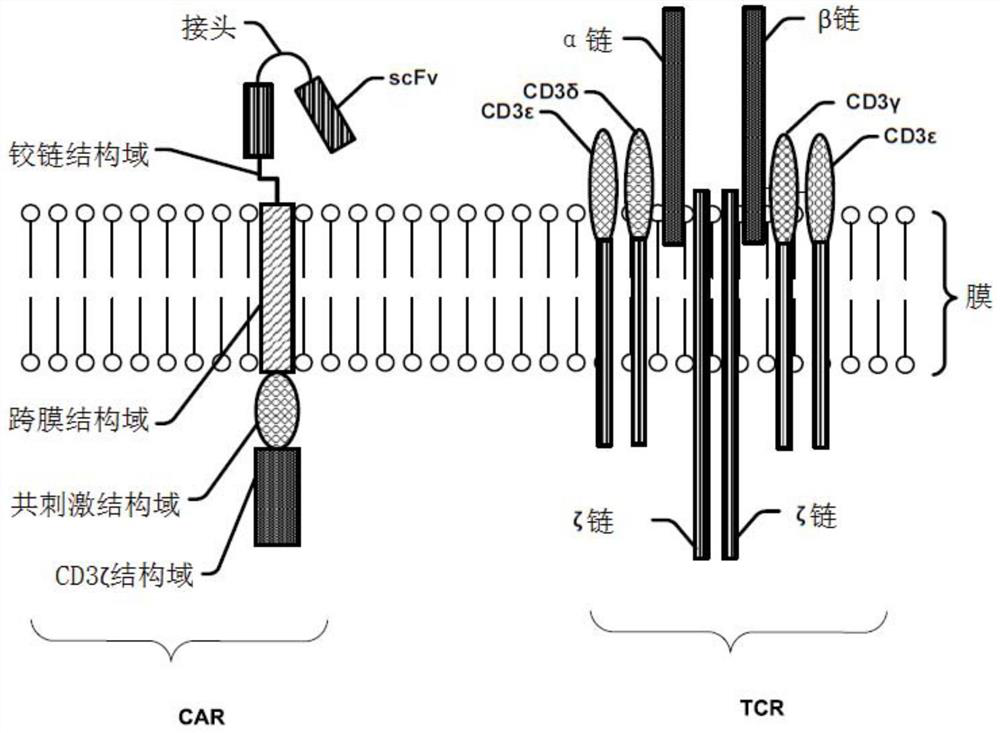
Modified cell expansion and uses thereof
The present disclosure relates to compositions and methods for enhancing T cell response and / or CAR cell expansion and / or maintenance in vivo and / or in vitro. For example, a method of enhancing T cell-based therapy comprises administering a mixed population of T cells comprising modified T cells comprising a first chimeric antigen receptor (CAR) and modified T cells comprising a second CAR, wherein a binding domain of the first CAR binds a first antigen, and a binding domain of the second CAR binds a second antigen. The first antigen is different from the second antigen. In embodiments, the first CAR binds a surface molecule or antigen of a white blood cell.
Owner:斯丹赛控股有限公司
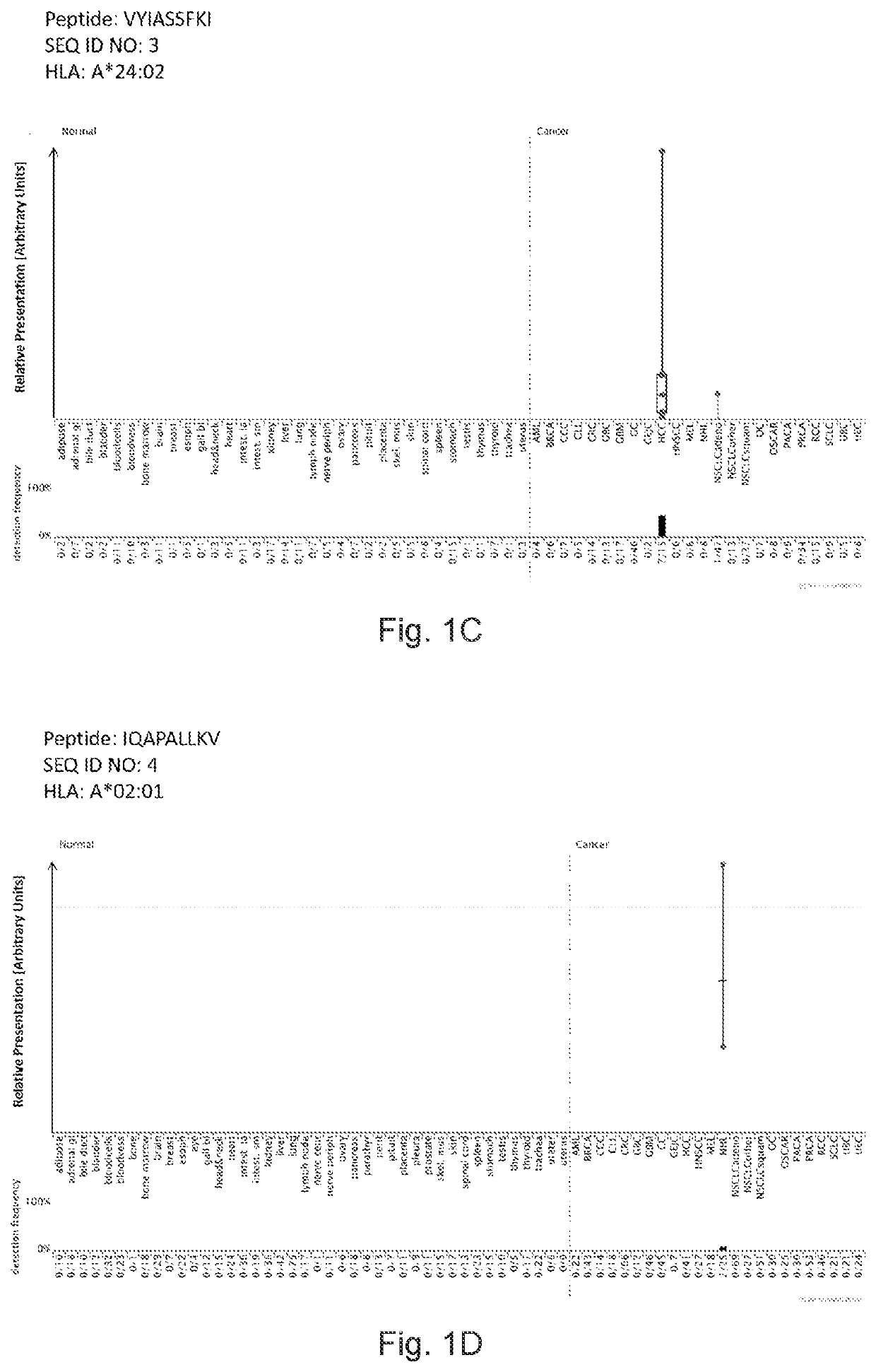
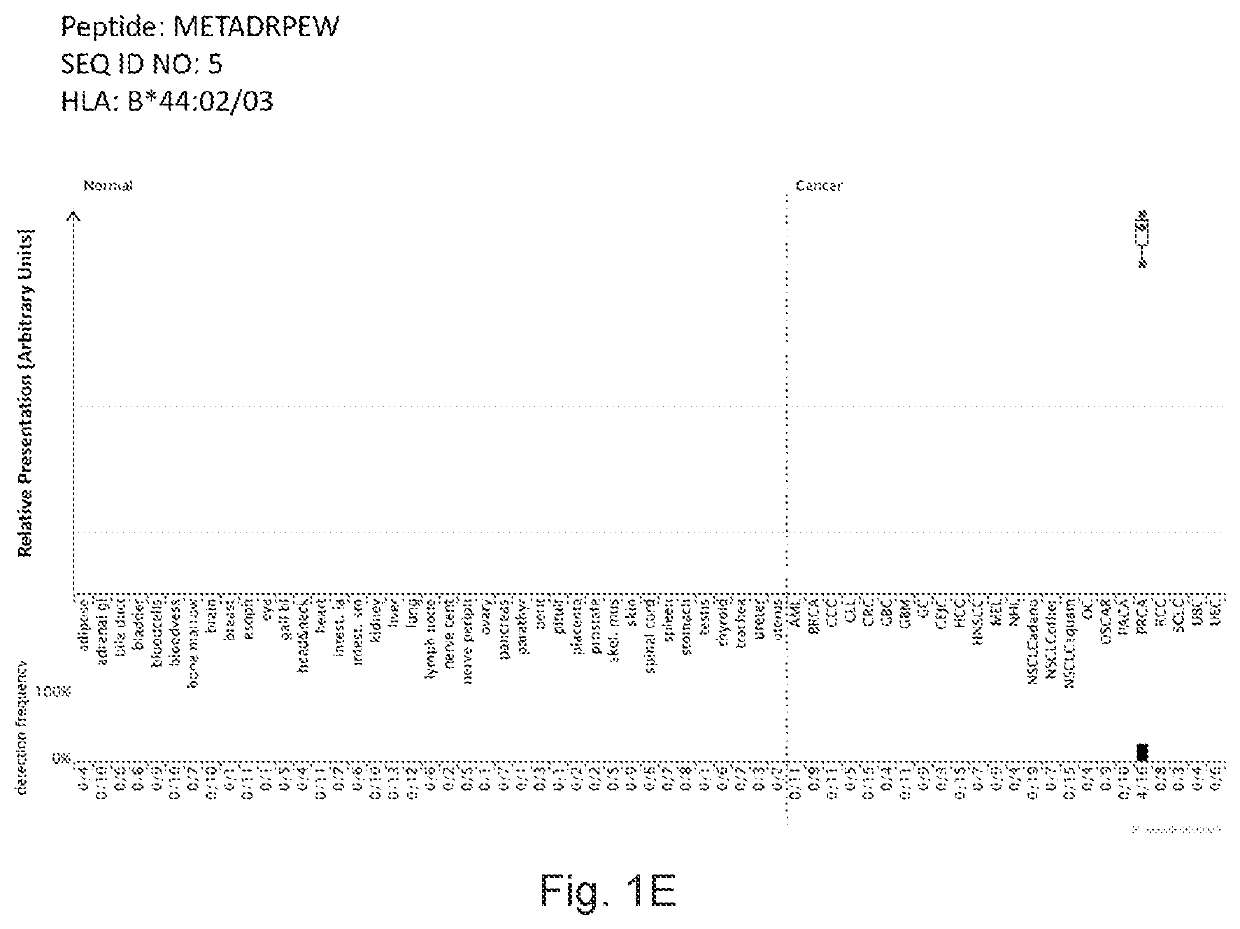
Peptides displayed by HLA for use in immunotherapy against different types of cancers
PendingUS20220226376A1Enhance stability and solubilityAid in diagnosisTumor rejection antigen precursorsTumor specific antigensPeptide displayInducer Cells
The invention relates to a peptide comprising an amino acid sequence selected from the group consisting of (i) SEQ ID NO: 1 to SEQ ID NO: 216, and (ii) a variant sequence thereof which maintains capacity to bind to MHC molecule(s) and / or induce T cells cross-reacting with said variant peptide, or a pharmaceutically acceptable salt thereof.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
P16ink4a derived peptides for prophylaxis and therapy of hpv-associated tumors and other p16ink4a expressing tumors
Described are particular fragments of the cyclin-dependent kinase inhibitor p16 capable of increasing IFN-γ secretion of T cells or inducing proliferation of T cells and the use of said fragments for immunizing an individual against HPV-associated or other p16INK4a expressing carcinomas, preferably advanced carcinomas.
Owner:UNIVERSITY OF HEIDELBERG
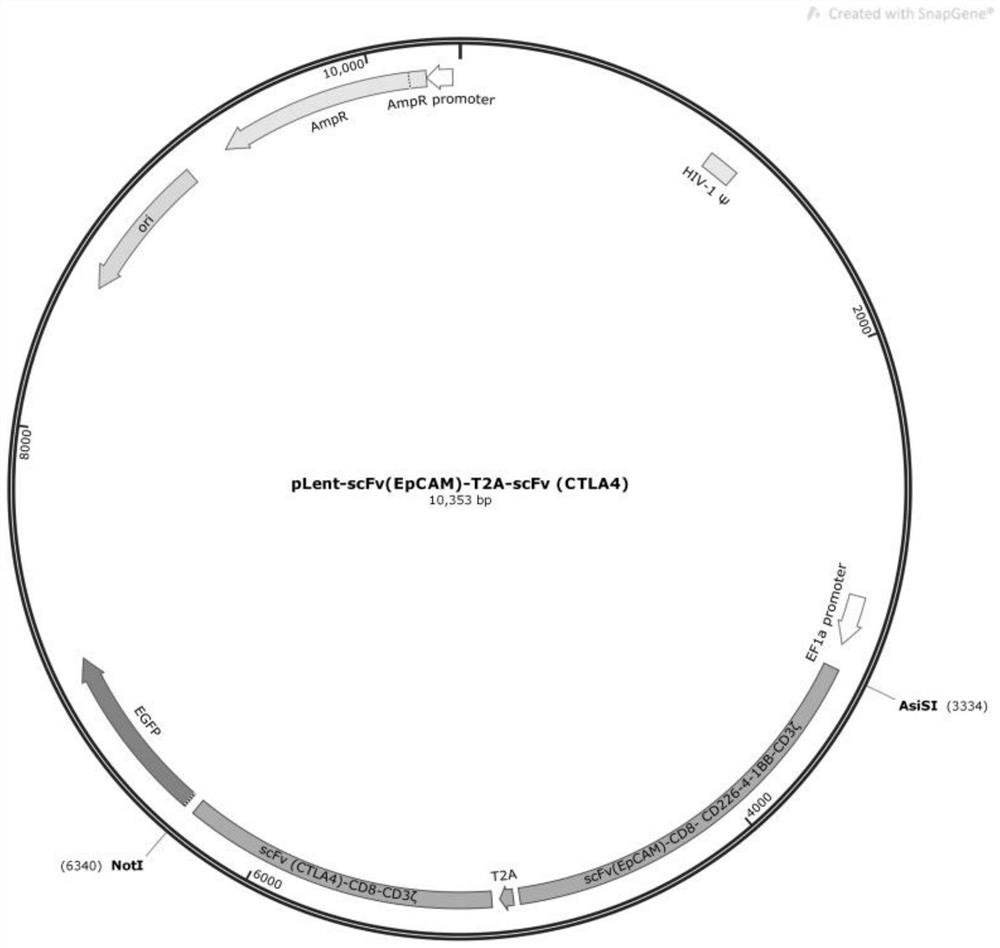
Encoding gene of anti-EpCAM chimeric antigen receptor, preparation method of encoding gene, plasmid with gene, immune cell and application of encoding gene
ActiveCN112359052ADecreased killing activityGood treatment effectVirusesAntibody mimetics/scaffoldsGenetic enhancementAntigen receptors
The invention discloses an encoding gene of an anti-EpCAM chimeric antigen receptor, a preparation method of the encoding gene, a plasmid with the gene, an immune cell and an application of the encoding gene, wherein the encoding gene at least comprises an EpCAM binding region and a CTLA4 binding region and also comprises a T2A nucleic acid artificial sequence located between the EpCAM binding region and the CTLA4 binding region, and the encoding gene of the anti-EpCAM chimeric antigen receptor further comprises a transmembrane-stimulation structural domain; the transmembrane-stimulation structural domain is selected from all or part of fragments of CD8, CD27, CD28, CD226, 4-1BB, OX40 and ICOS molecules. The encoding gene of the anti-EpCAM chimeric antigen receptor provided by the invention enhances the killing effect of T cells on tumor cells.
Owner:SHANDONG XINRUI BIOTECH CO LTD
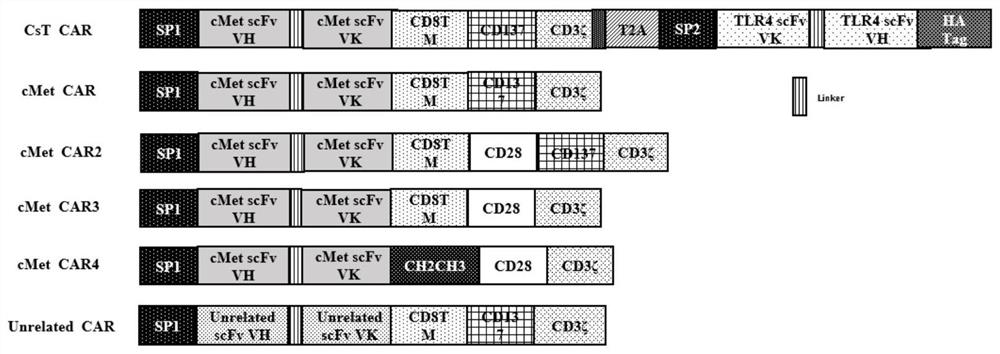
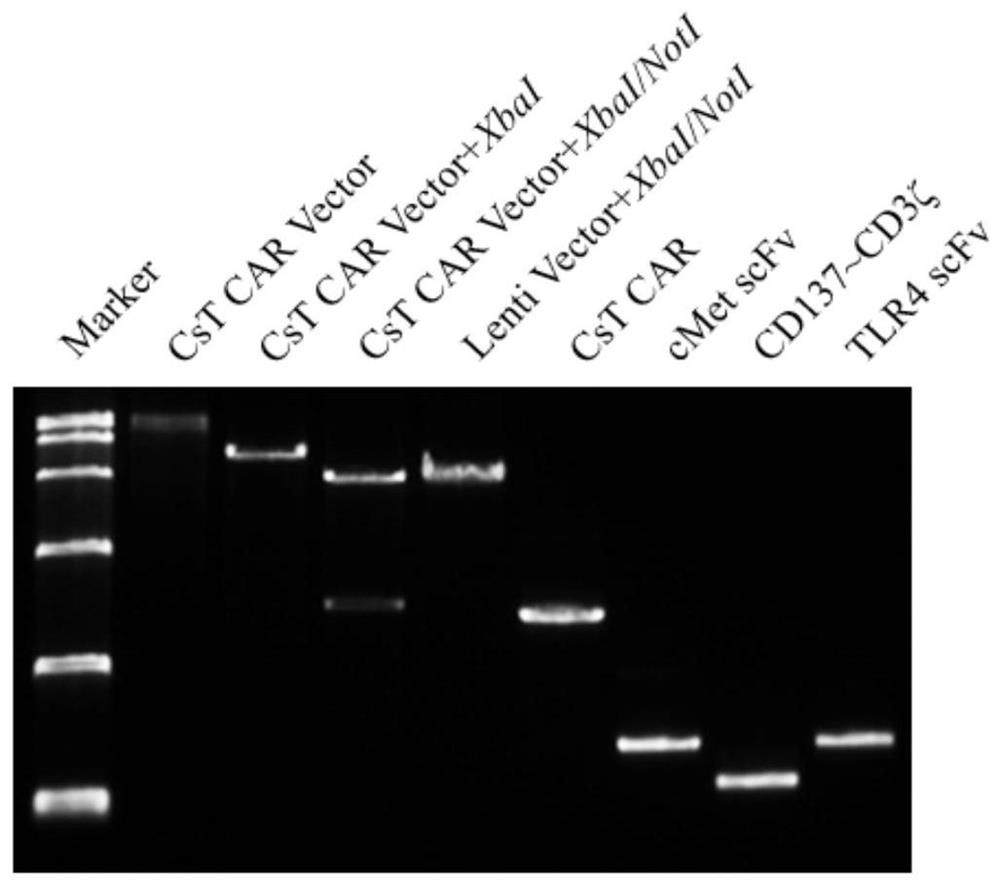
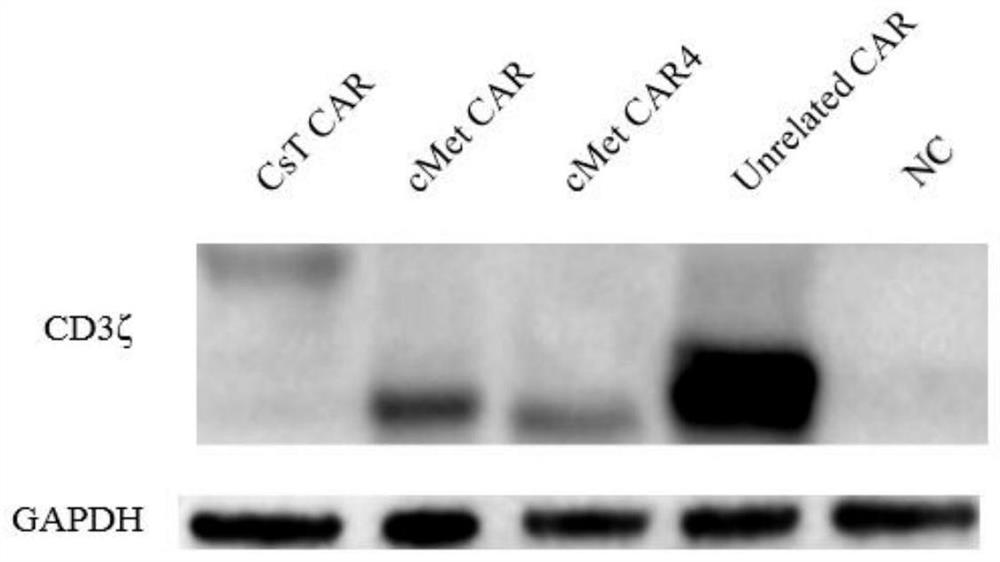
A chimeric antigen receptor modified T cell that can autocrine tlr4 scFv and targets cMet and its application
ActiveCN111533808BIncrease lethalityReduce the chance of escapeVirusesReproductive system cancer vaccineScFv AntibodiesCD8
The invention provides a chimeric antigen receptor (CAR) modified T cell capable of secreting TLR4 scFv and targeting cMet as well as application of the T cell. The T cell contains a coding sequence of a CAR recognizing cMet and a coding sequence of a TLR4 scFv antibody secreting a signal peptide. The CAR modified T cell can be activated while specifically targeting tumor cells with high expression of cMet, secret the TLR4 scFv antibody, weaken the immunosuppressive action of a tumor microenvironment, reduce expression of Treg cells and promote the killing effect of CD8<+>T cells on tumor.
Owner:苏州福济达细胞工程有限公司
Combined pharmaceutical preparation for treating melanoma, lung cancers or colorectal cancers
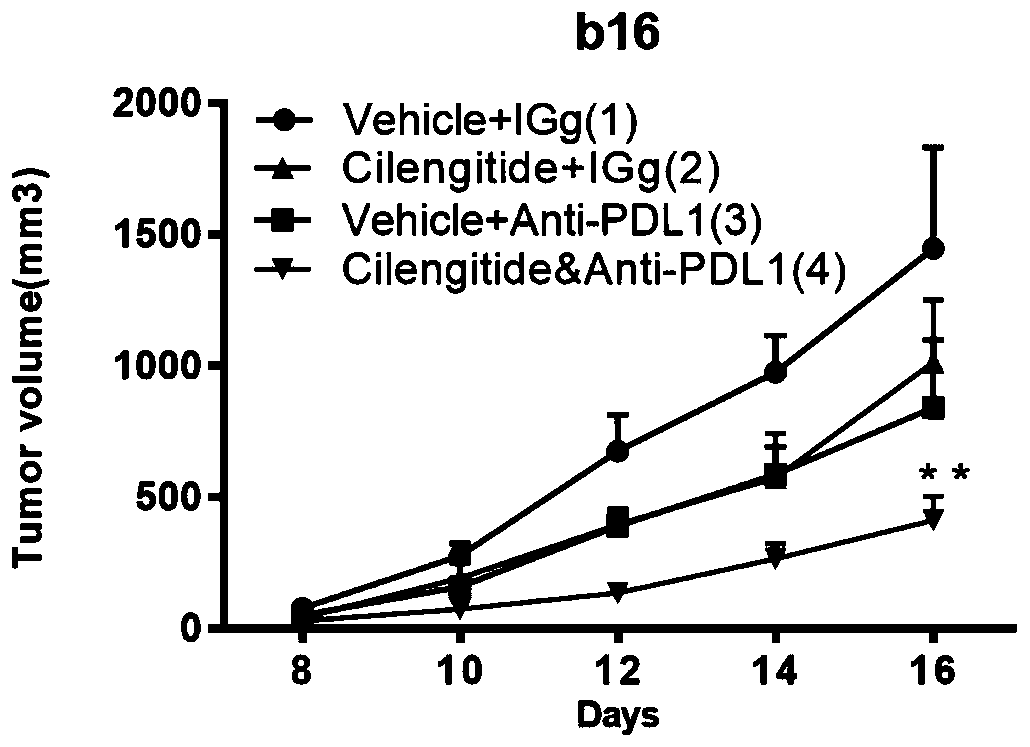
PendingCN110179977APrevent proliferationSuppress generationIntestine cancer vaccineColon cancer vaccineAbnormal tissue growthPD-L1 inhibitor
The present invention discloses a combined pharmaceutical preparation for treating melanoma, lung cancers or colorectal cancers. The pharmaceutical preparation comprises an effective amount of an antibody of a programmed death receptor ligand 1 (PD-L1) and cilengitide. The combined pharmaceutical preparation combines the integrin inhibitor cilengitide and the PD-L1 inhibitor to inhibit tumor cellproliferation while inhibiting formation of new blood vessels in a tumor microenvironment and enhancing infiltration and activation of T cells to improve an immune microenvironment. Compared with single drug use, the inhibition effects of the cilengitide and PD-L1 inhibitors are relatively clear, side effects are relatively low, and risks are relatively low. After the combined use, an objective remission rate of the tumors is higher than that of the single drug use, total survival time is increased and no lethal side effects are observed.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
A chimeric antigen receptor T-lymphocyte and its application in the preparation of products for treating solid tumors
ActiveCN111875708BLift specific immune responseEnhance anti-tumor immune responseVirusesAntineoplastic agentsSingle-Chain AntibodiesT lymphocyte
The invention discloses a chimeric antigen receptor T lymphocyte and its application in preparing products for treating solid tumors. The chimeric antigen receptors in the chimeric antigen receptor T lymphocytes sequentially include human CD8 leader peptide, anti-Siglec-15 single-chain antibody, human CD8 hinge transmembrane region, human 4-1BB intracellular region, human CD3ζ cell Inner region, self-cleaving peptide, CSF2Ra signal peptide, EGFRt protein, self-cleaving peptide and human CD27. It is proved by experiments that the chimeric antigen receptor T lymphocytes of the present invention highly express IFNγ and CD107a, have a strong killing function on Siglec-15 positive tumor cells, and the killing efficiency exceeds 1 when the effect-target ratio is 1:1. 80%. Tumor transplantation model experiments show that the chimeric antigen receptor T lymphocytes of the present invention also have a strong killing function against Siglec‑15 positive tumor cells in animals.
Owner:CARBIOGENE THERAPEUTICS CO LTD
Immunopotentiator, immunotherapeutic pharmaceutical composition and its preparation and use
The present invention provides an immune enhancer comprising at least an interferon and a granulocyte-macrophage colony-stimulating factor, and an immunotherapeutic pharmaceutical composition comprising at least an antigen and the above-mentioned immune enhancers. The present invention further discloses a preparation method of the immunotherapeutic pharmaceutical composition, the use of the immune enhancer and the immunotherapeutic pharmaceutical composition. The immune enhancer can be applied to disease and tumor treatments caused by viruses, bacteria, and other microorganisms.
Owner:FUDAN UNIV
Application of double-antibody coupling anti-cancer drug in colorectal cancer tumor treatment
PendingCN114681622AEnhanced inhibitory effectOrganic active ingredientsIntestine cancer vaccineEndothelial Growth FactorsAntiendomysial antibodies
A colorectal cancer cell sw837 and a cell endothelial growth factor VEGF-B186 are respectively used for immunizing a chicken to obtain two IgY antibodies, and the IgY (sw837) and an anti-cancer drug 5 are mixed; according to the present invention, the two IgY antibodies are subjected to coupling to obtain the antibody conjugate, the two IgY antibodies are mixed and drenched into the experimental mouse, and the test result shows that the obvious tumor inhibition effect is provided;
Owner:BEIJING WANHUA BIOENG
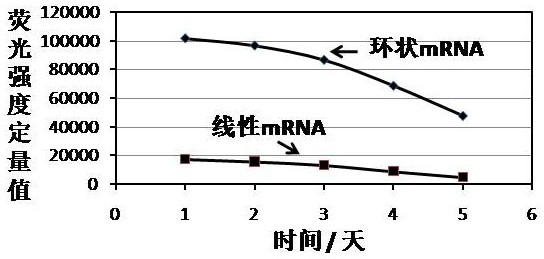
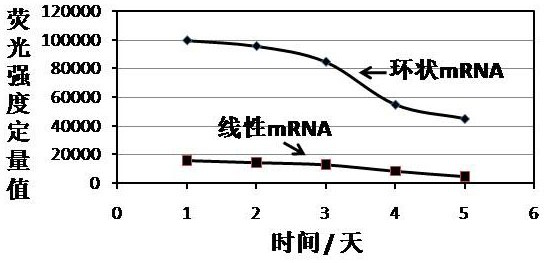
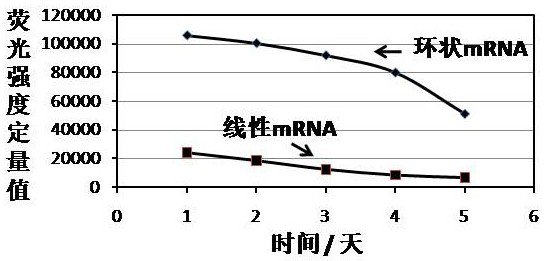
Cyclic mRNA (messenger ribonucleic acid) tumor immune drug for colorectal cancer
PendingCN114409760AReduce pressure on social securityIntestine cancer vaccineTumor rejection antigen precursorsAntigenOncology
The invention discloses a potential colorectal cancer tumor immune drug. The colorectal cancer tumor immune drug is based on a colorectal cancer tumor neoantigen and a circular mRNA molecule with a specific IRES sequence. The colorectal cancer tumor immune drug is expected to expand the benefit range of colorectal cancer treatment patients, improve the survival rate of the colorectal cancer patients, and bring more colorectal cancer treatment benefits to the colorectal cancer patients, patient families and society.
Owner:奥明(杭州)生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
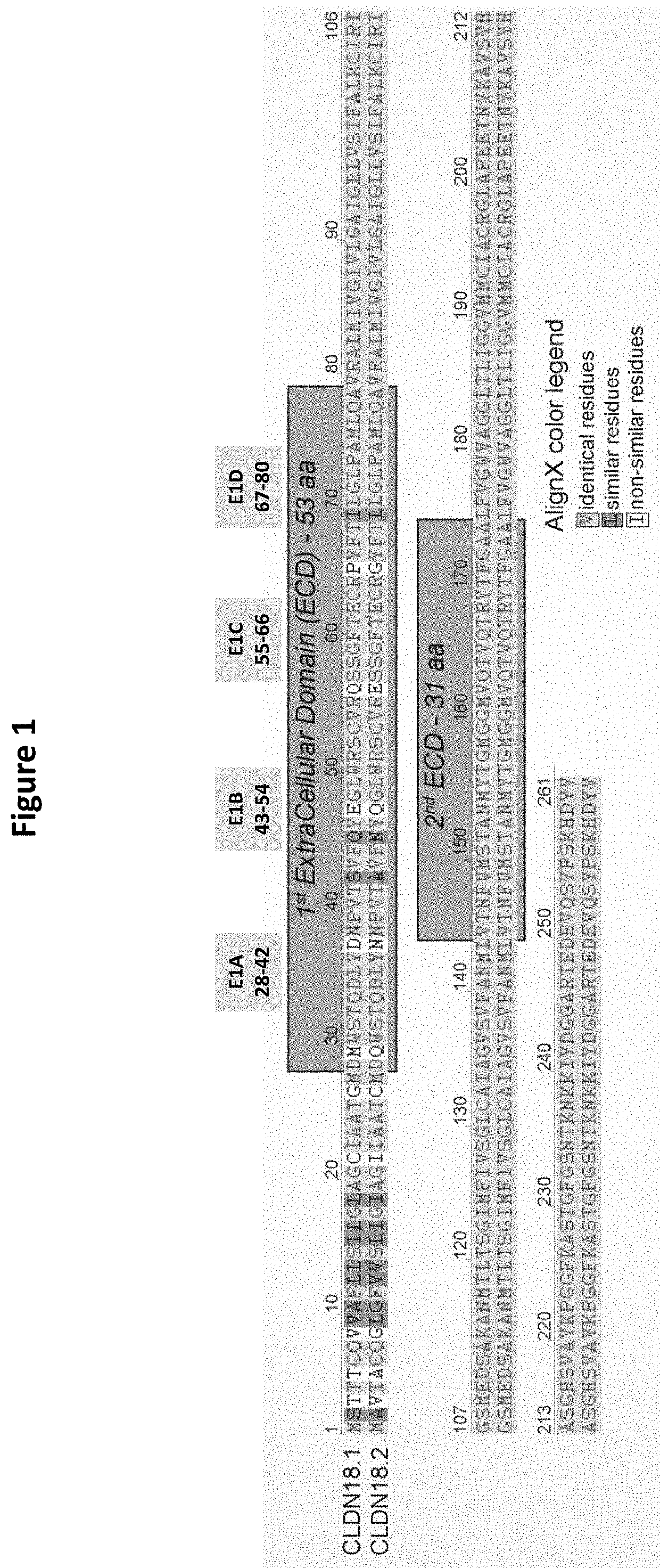











![IL-1[beta] binding antibodies for use in treating cancer IL-1[beta] binding antibodies for use in treating cancer](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d284657-23c7-4a72-af64-d4f0cdde0f9e/HDA0002328181950000011.png)
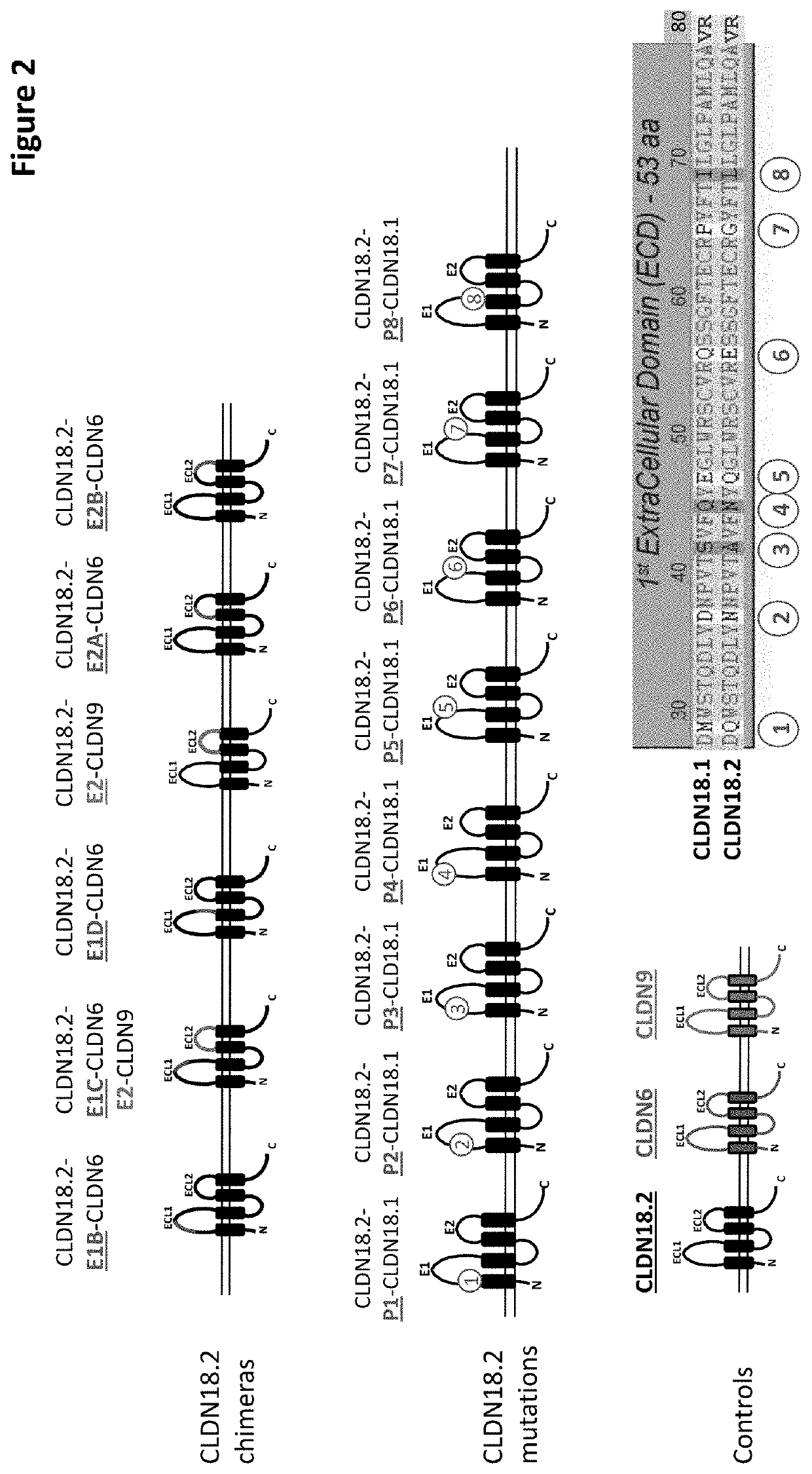
![IL-1[beta] binding antibodies for use in treating cancer IL-1[beta] binding antibodies for use in treating cancer](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d284657-23c7-4a72-af64-d4f0cdde0f9e/HDA0002328181950000021.png)
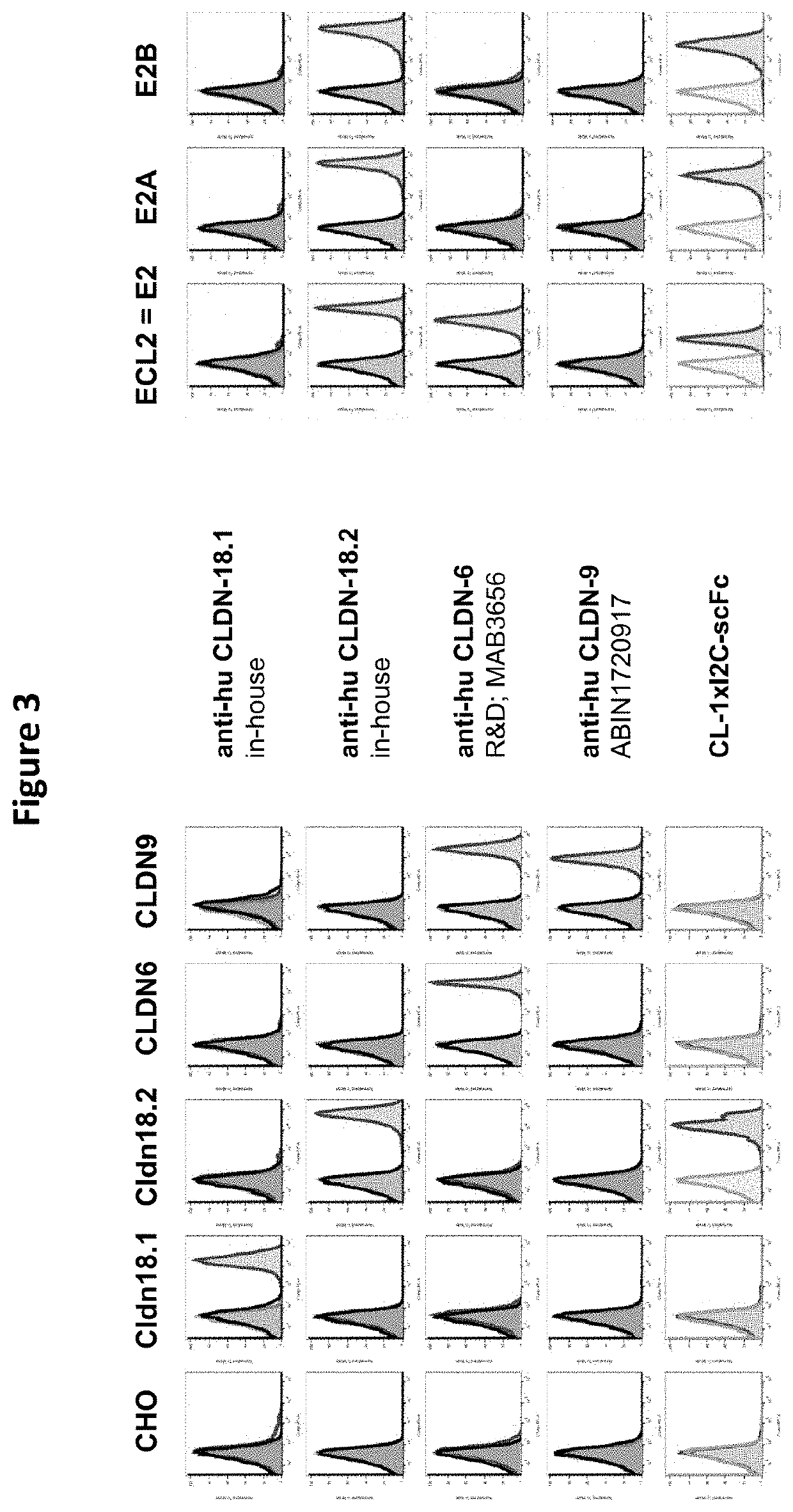
![IL-1[beta] binding antibodies for use in treating cancer IL-1[beta] binding antibodies for use in treating cancer](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d284657-23c7-4a72-af64-d4f0cdde0f9e/HDA0002328181950000031.png)