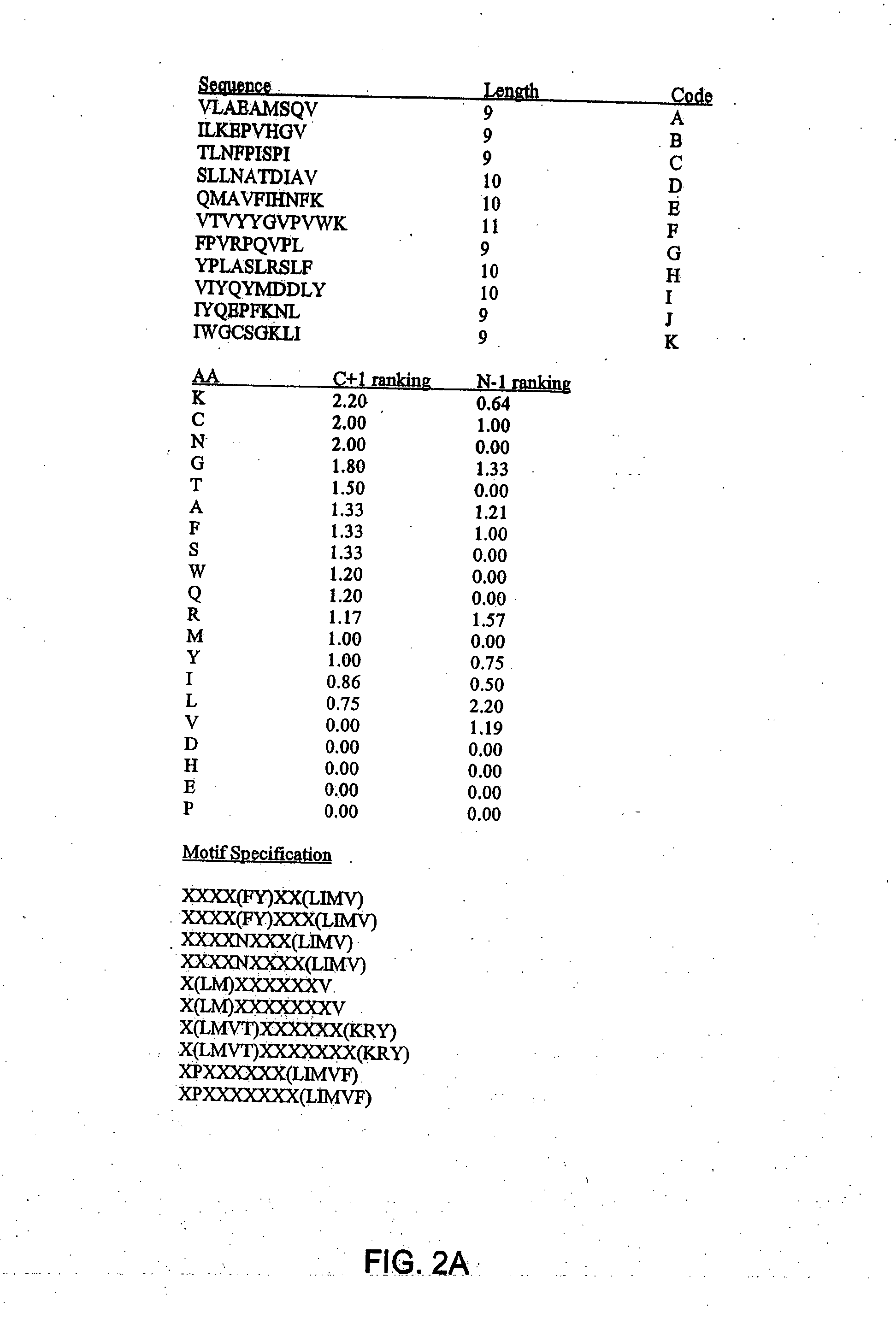
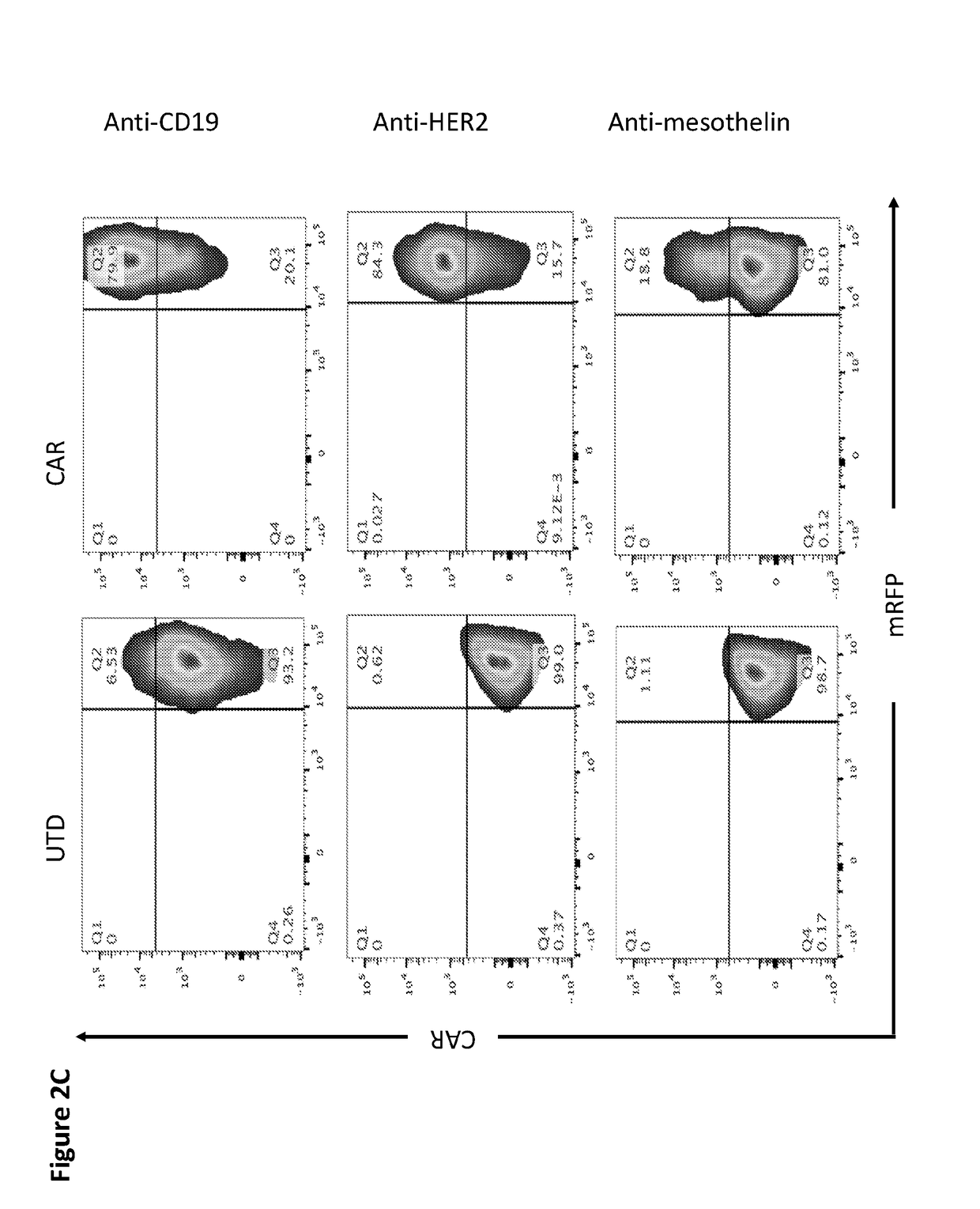
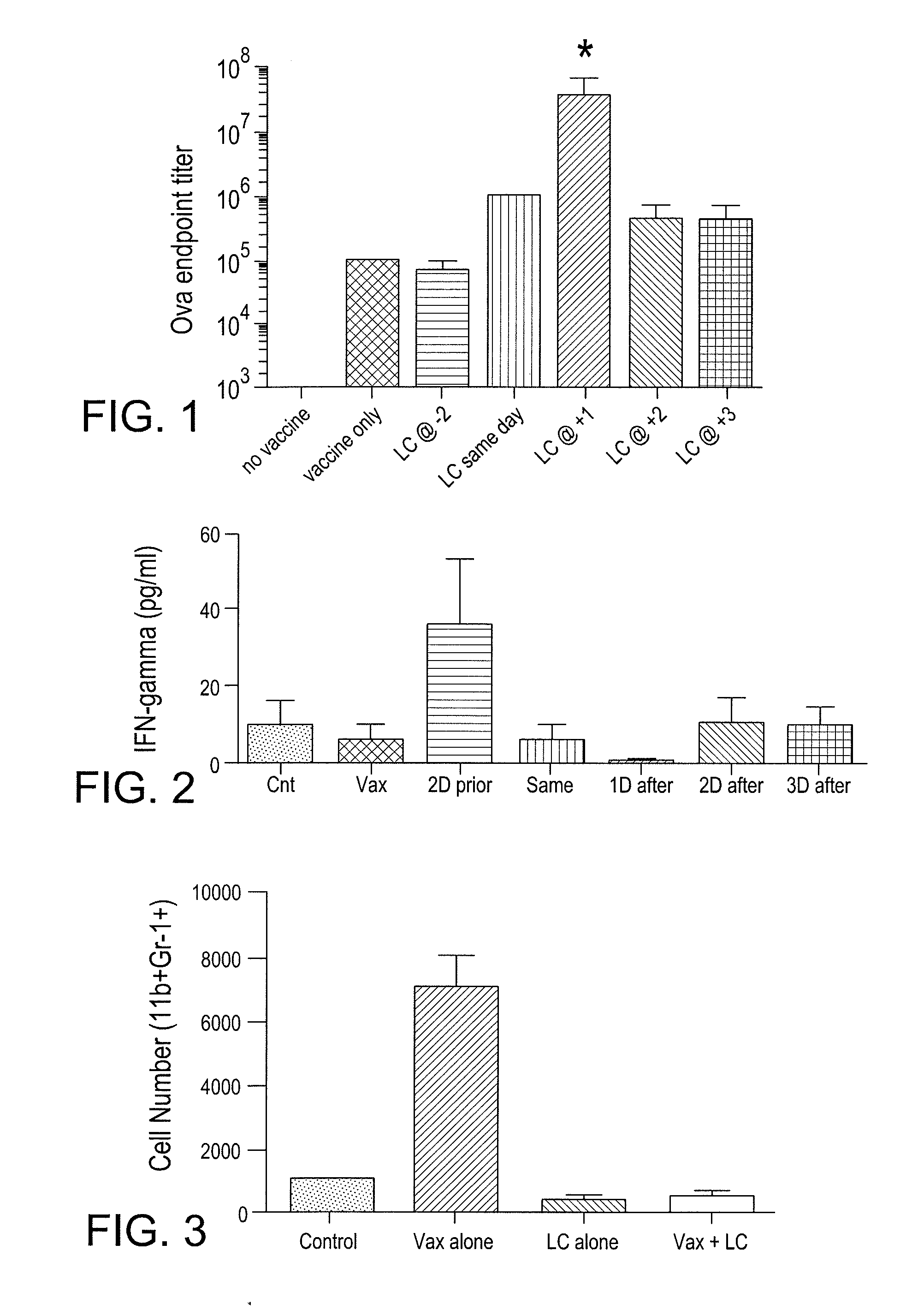
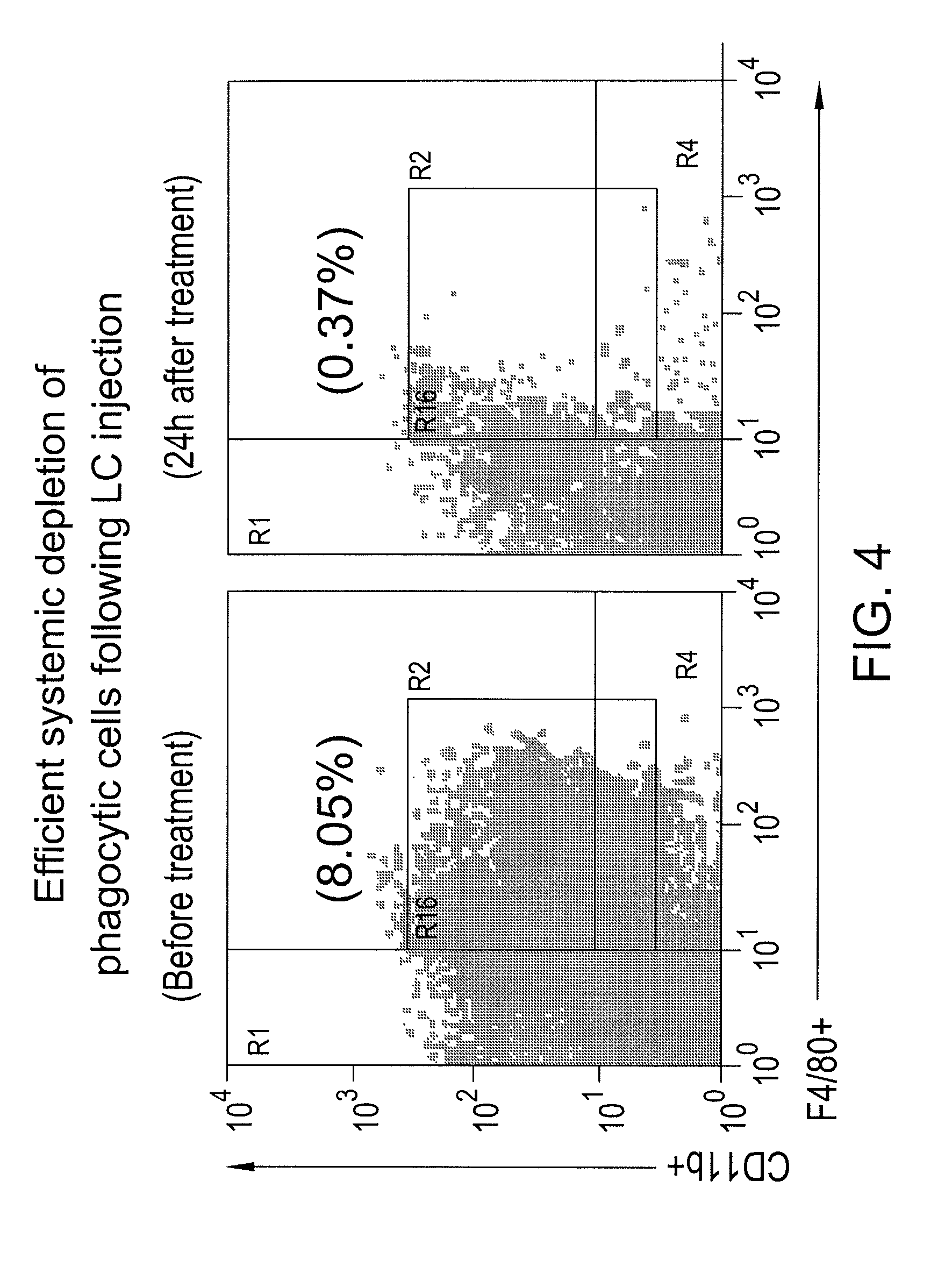
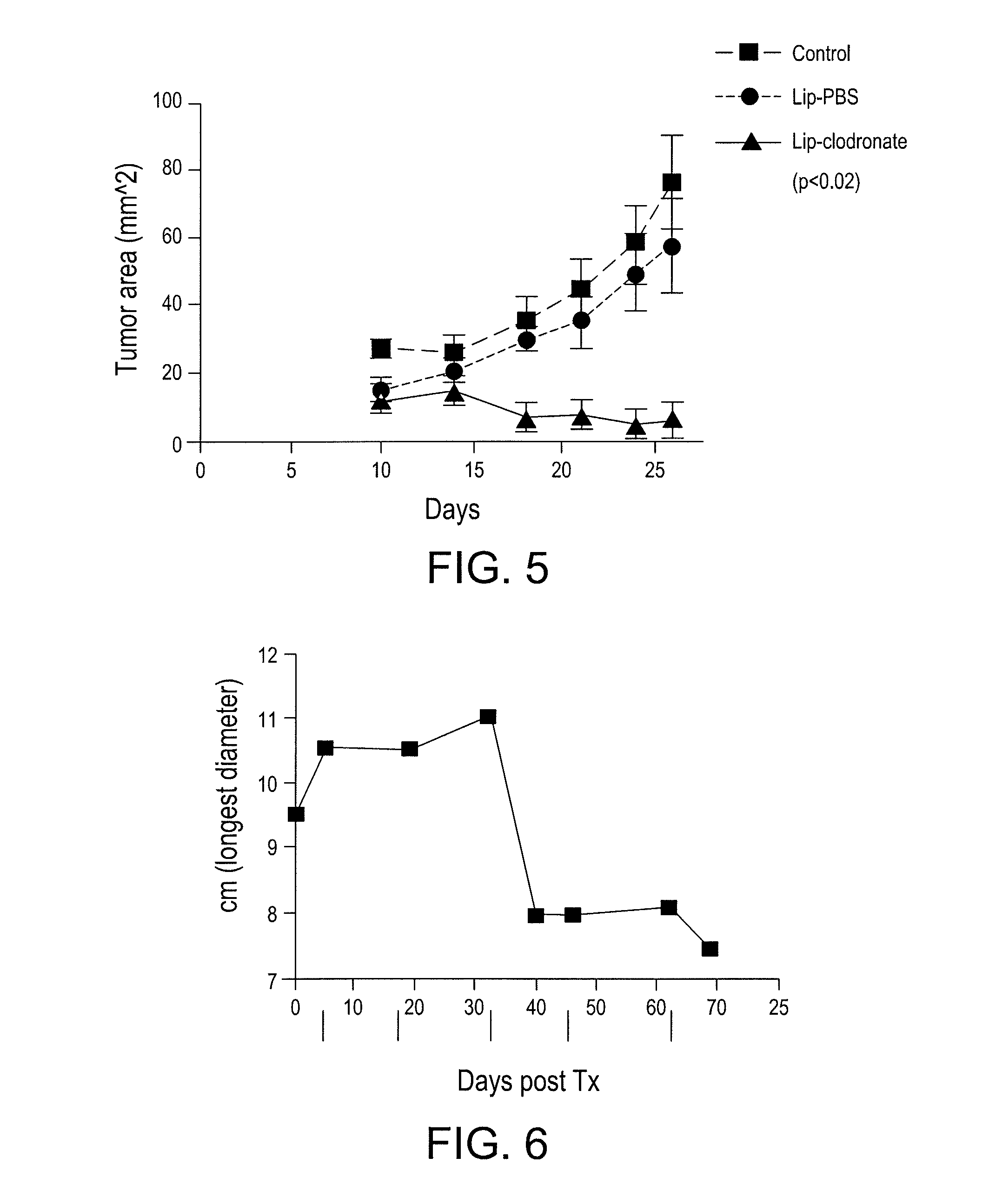
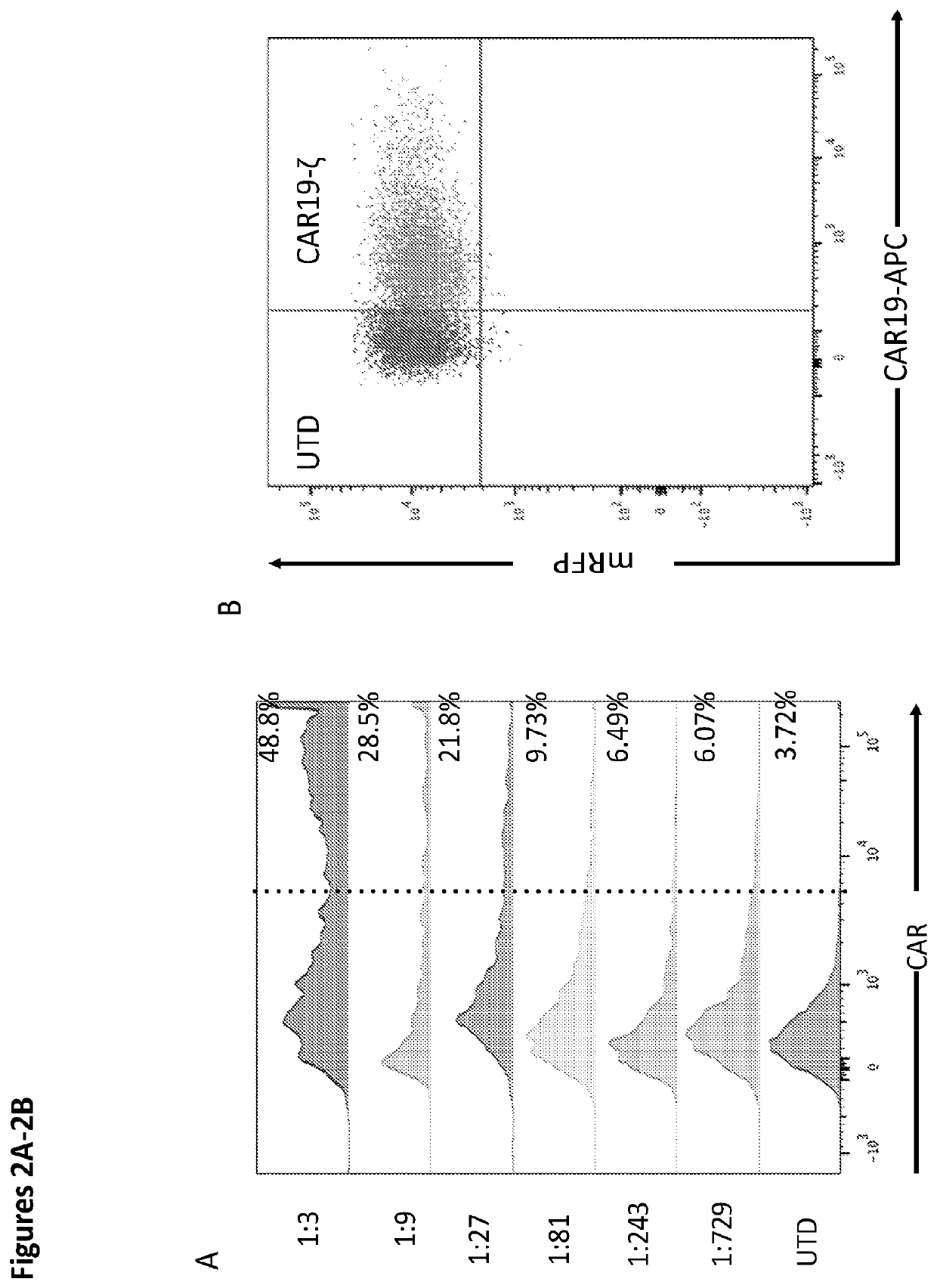
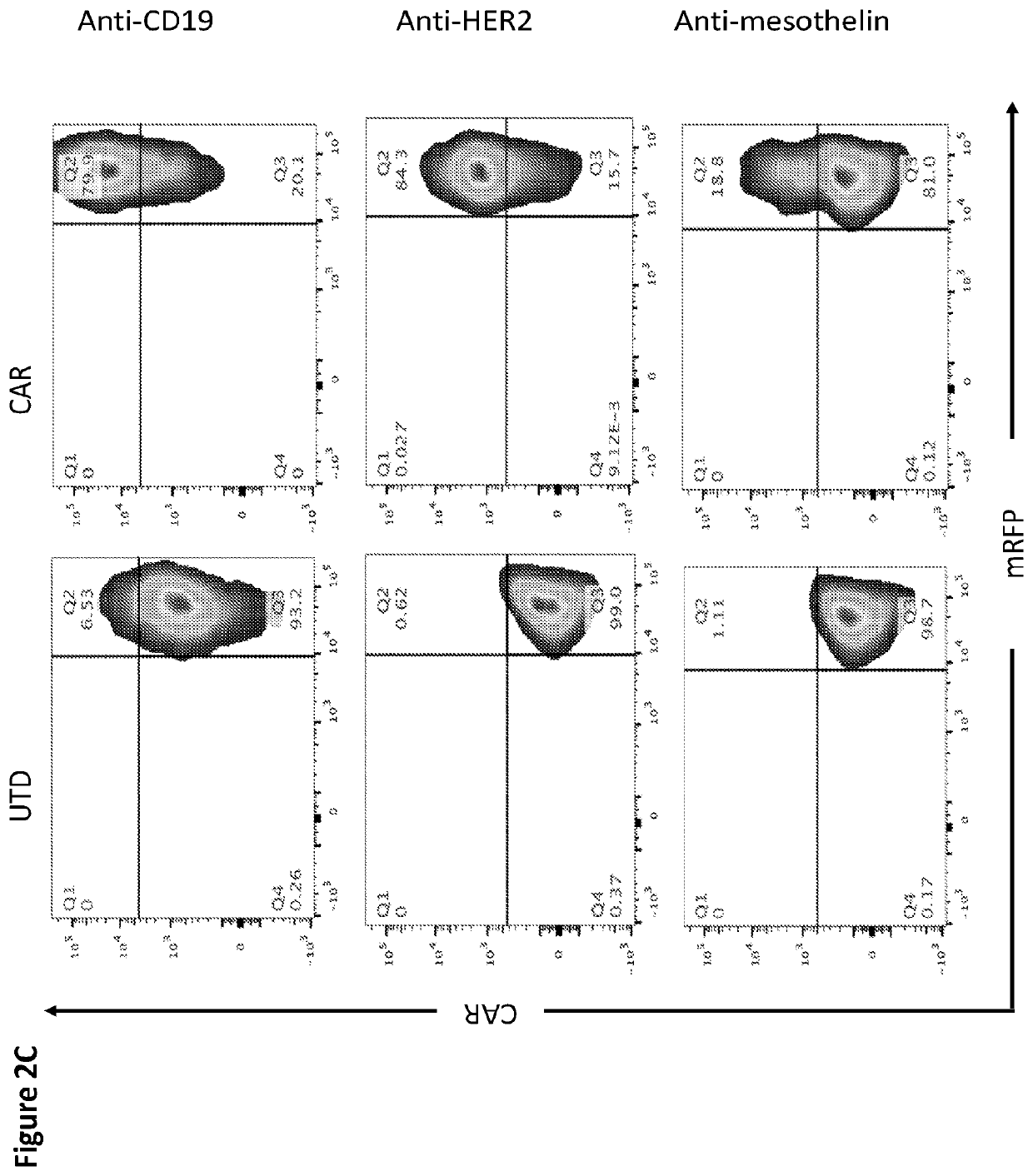
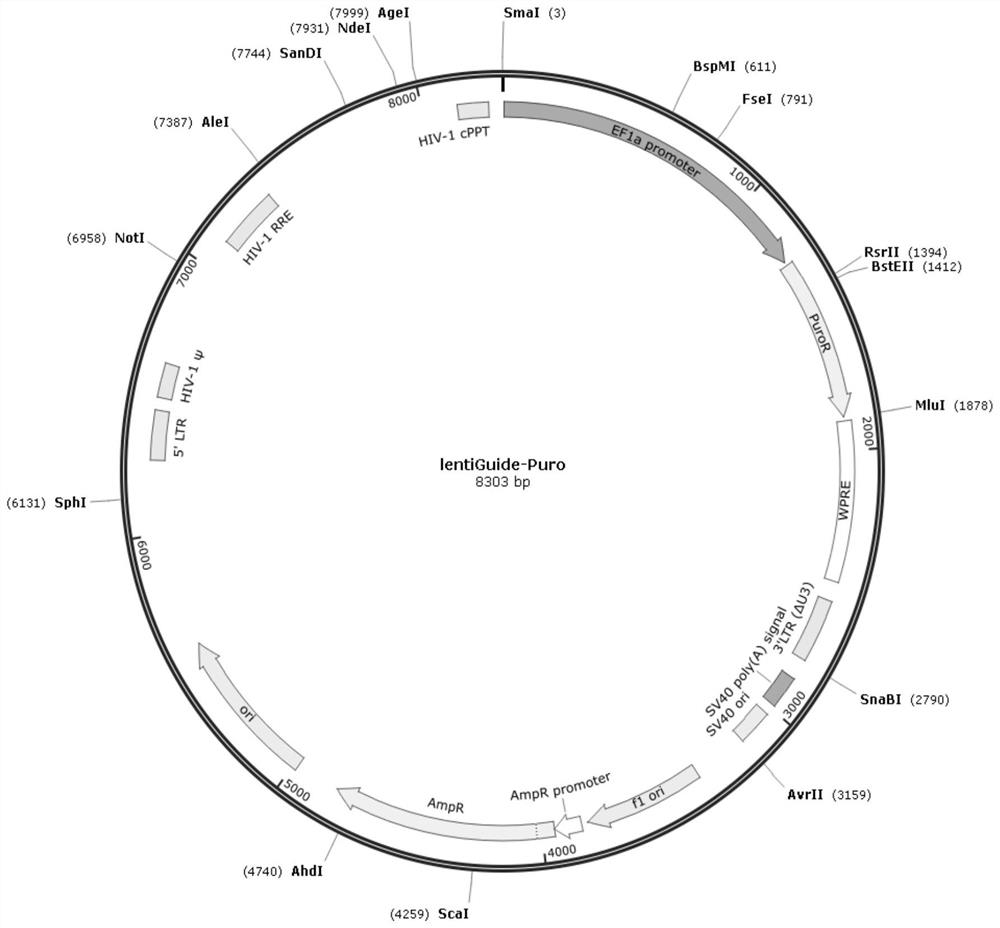
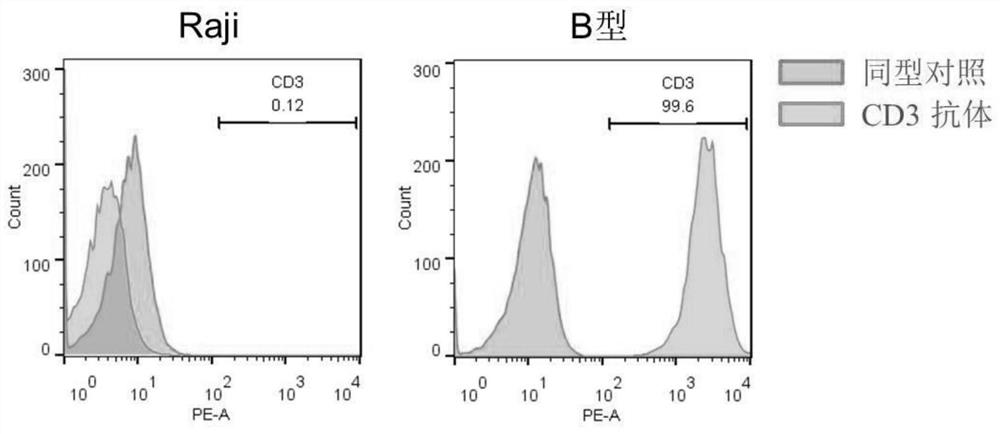
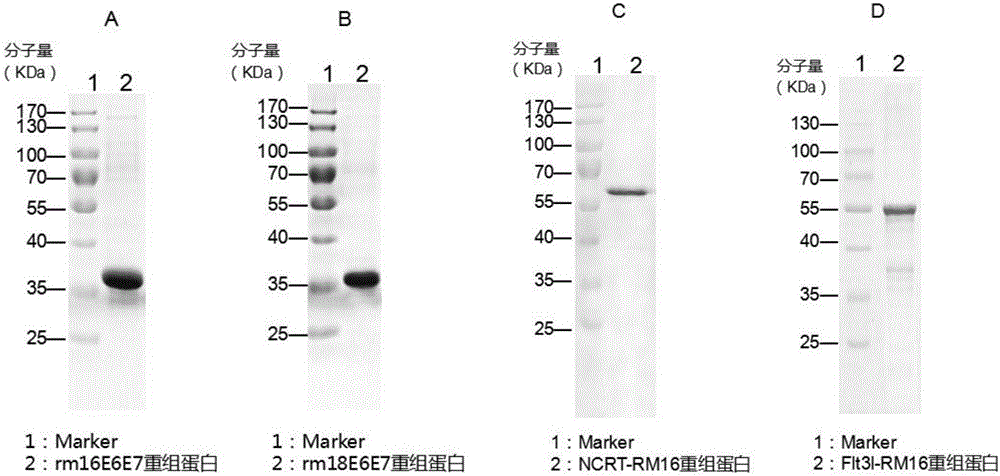
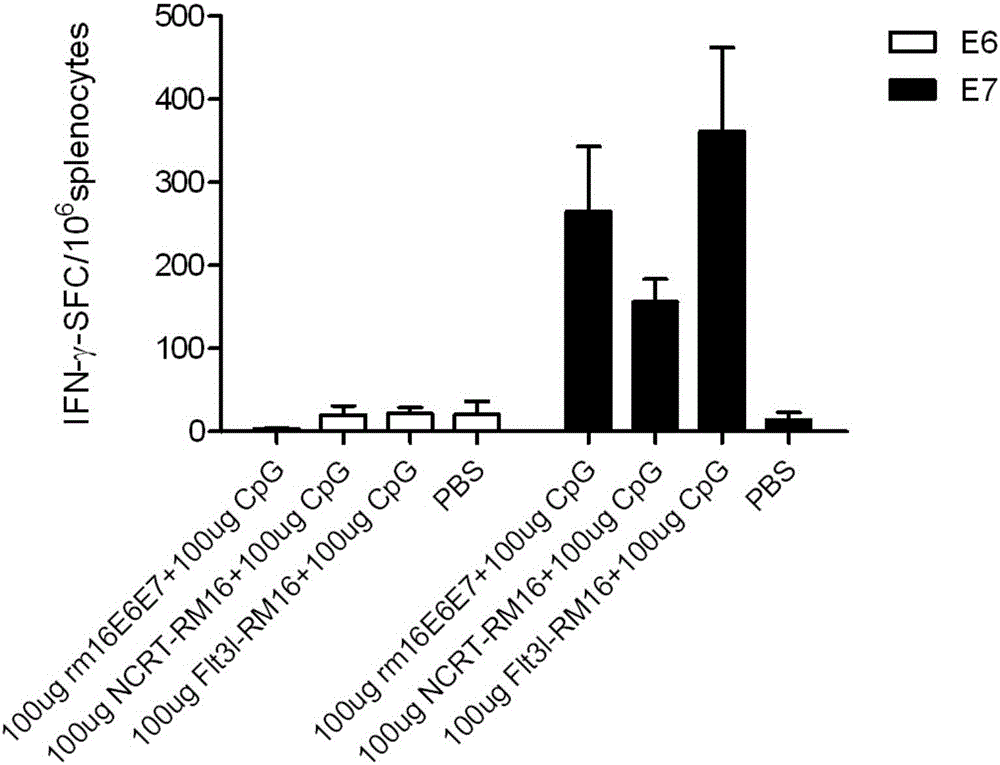
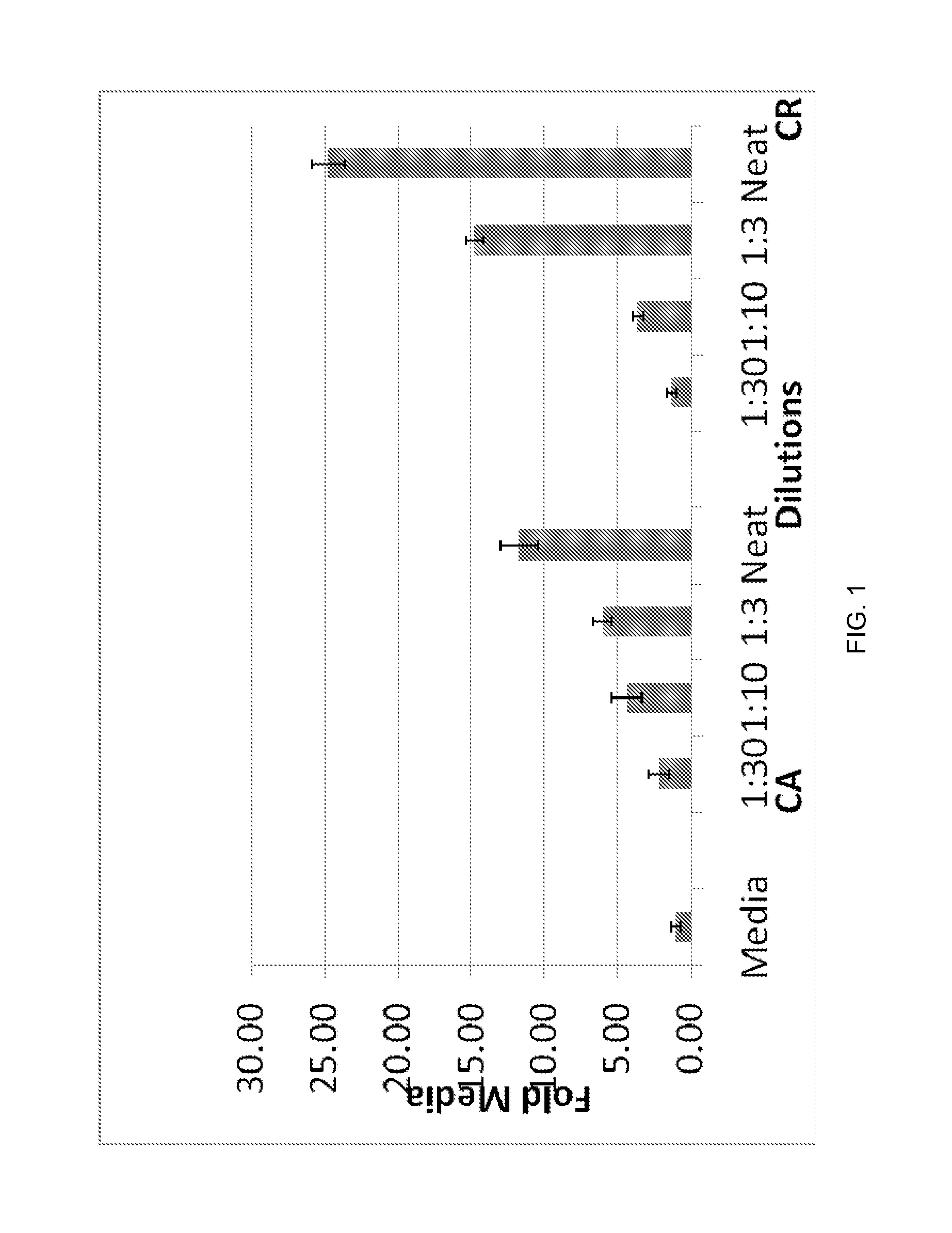
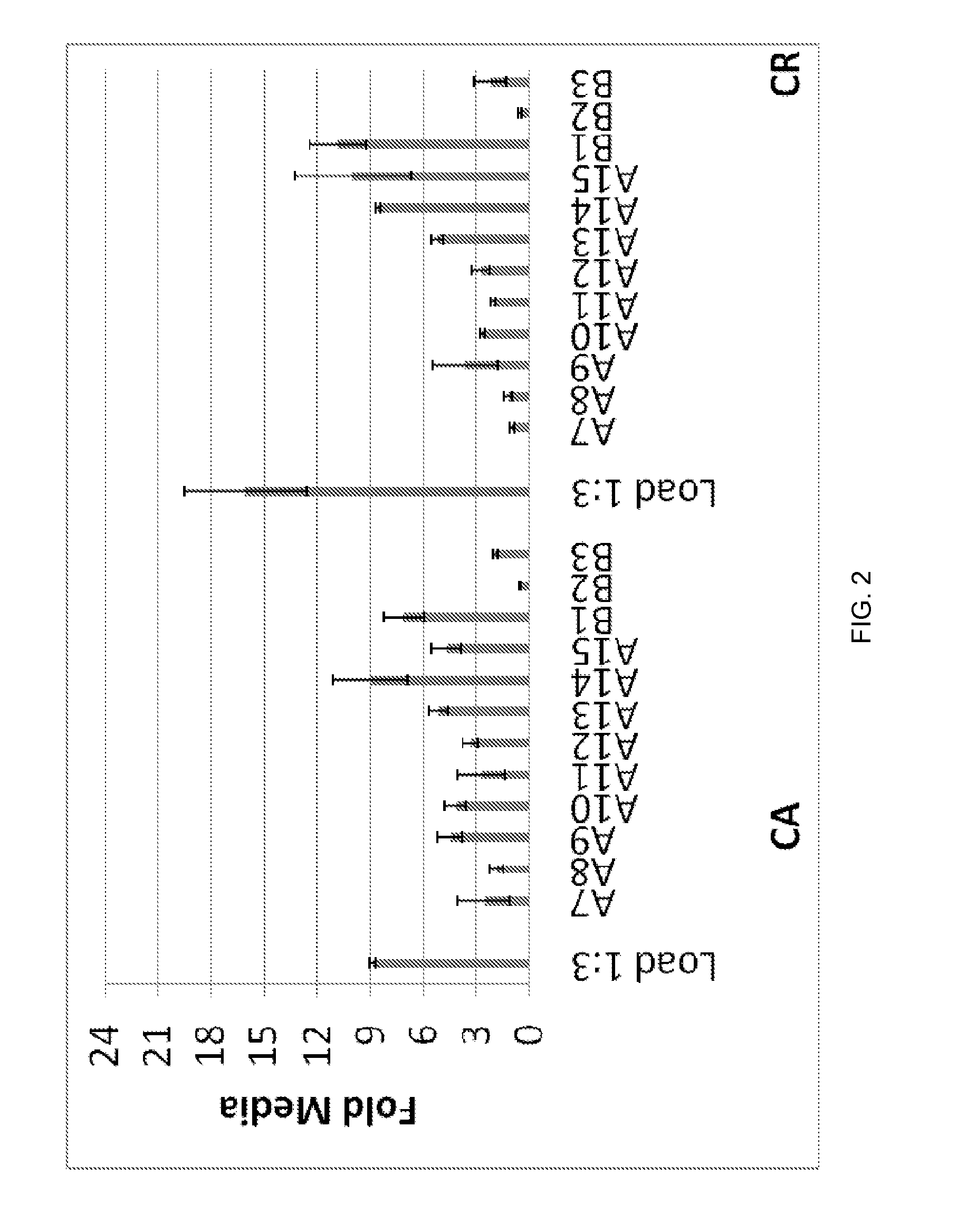
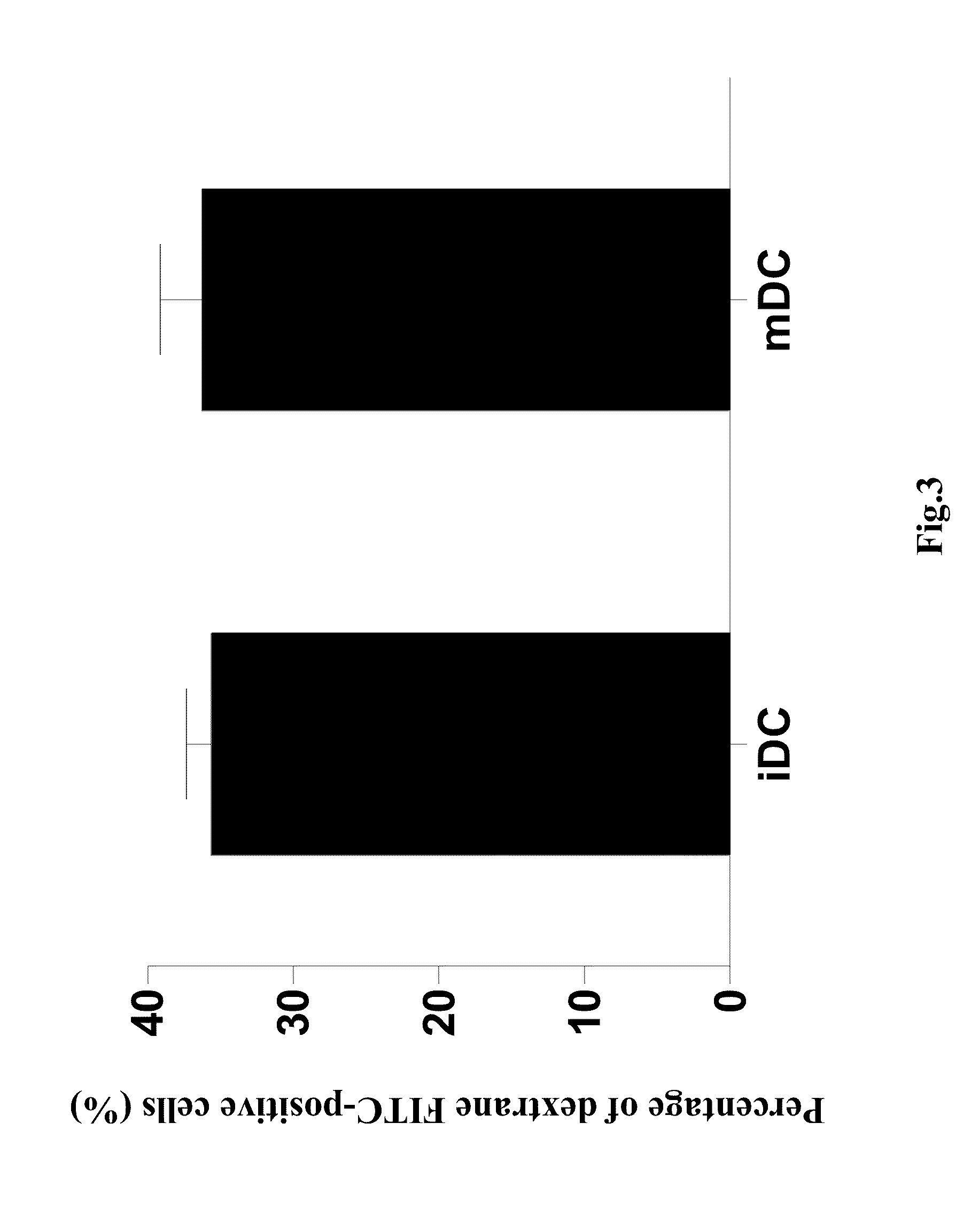
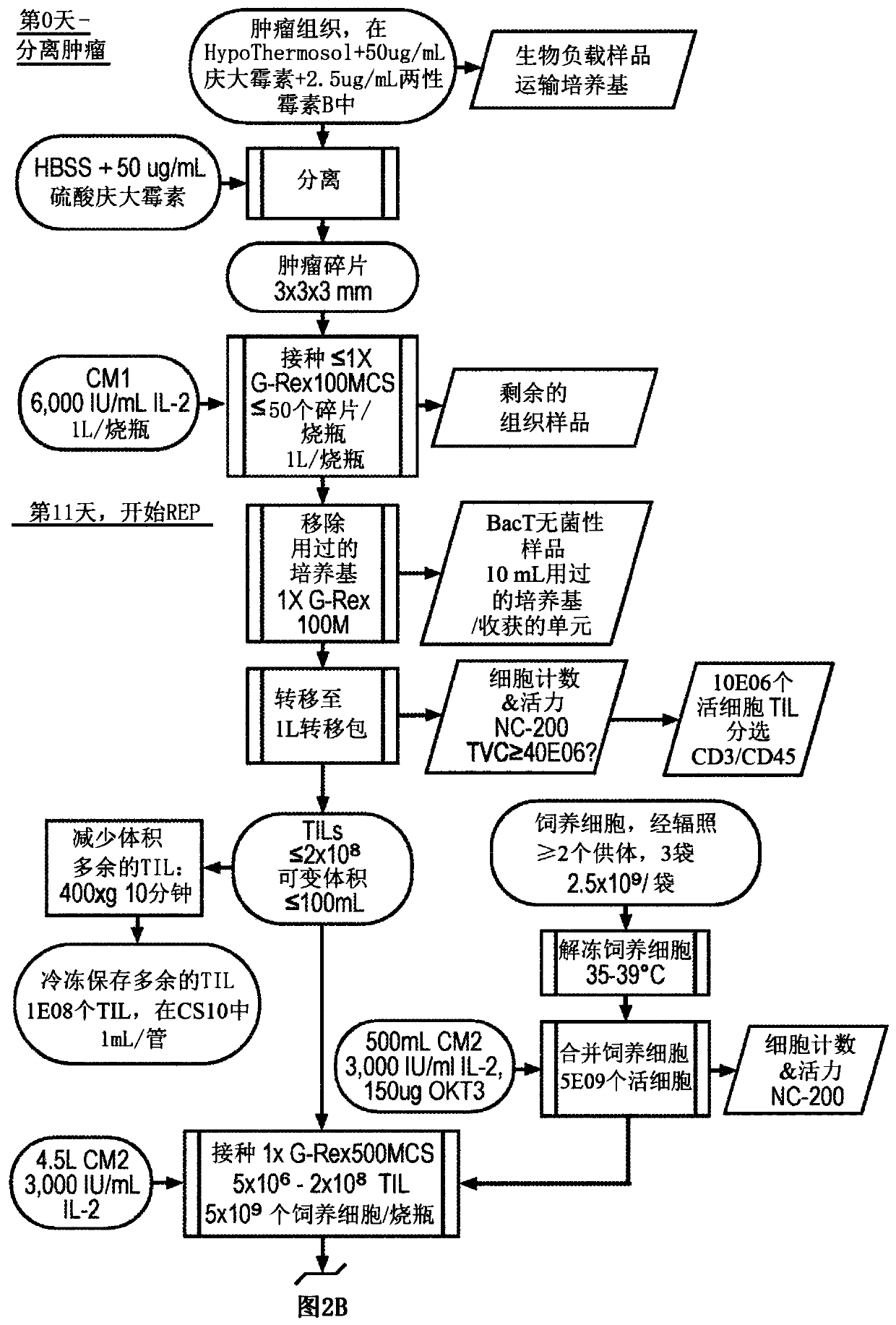
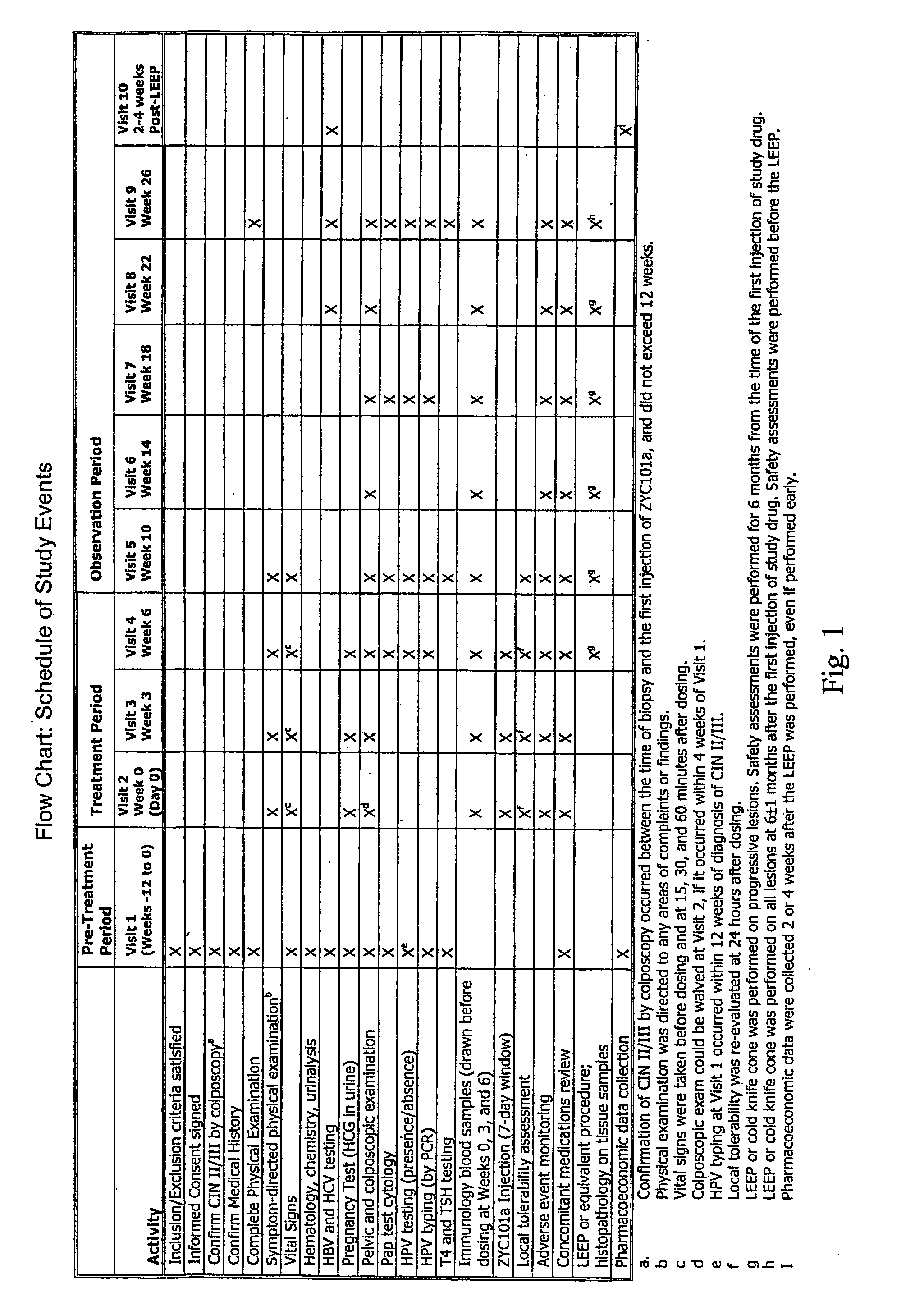
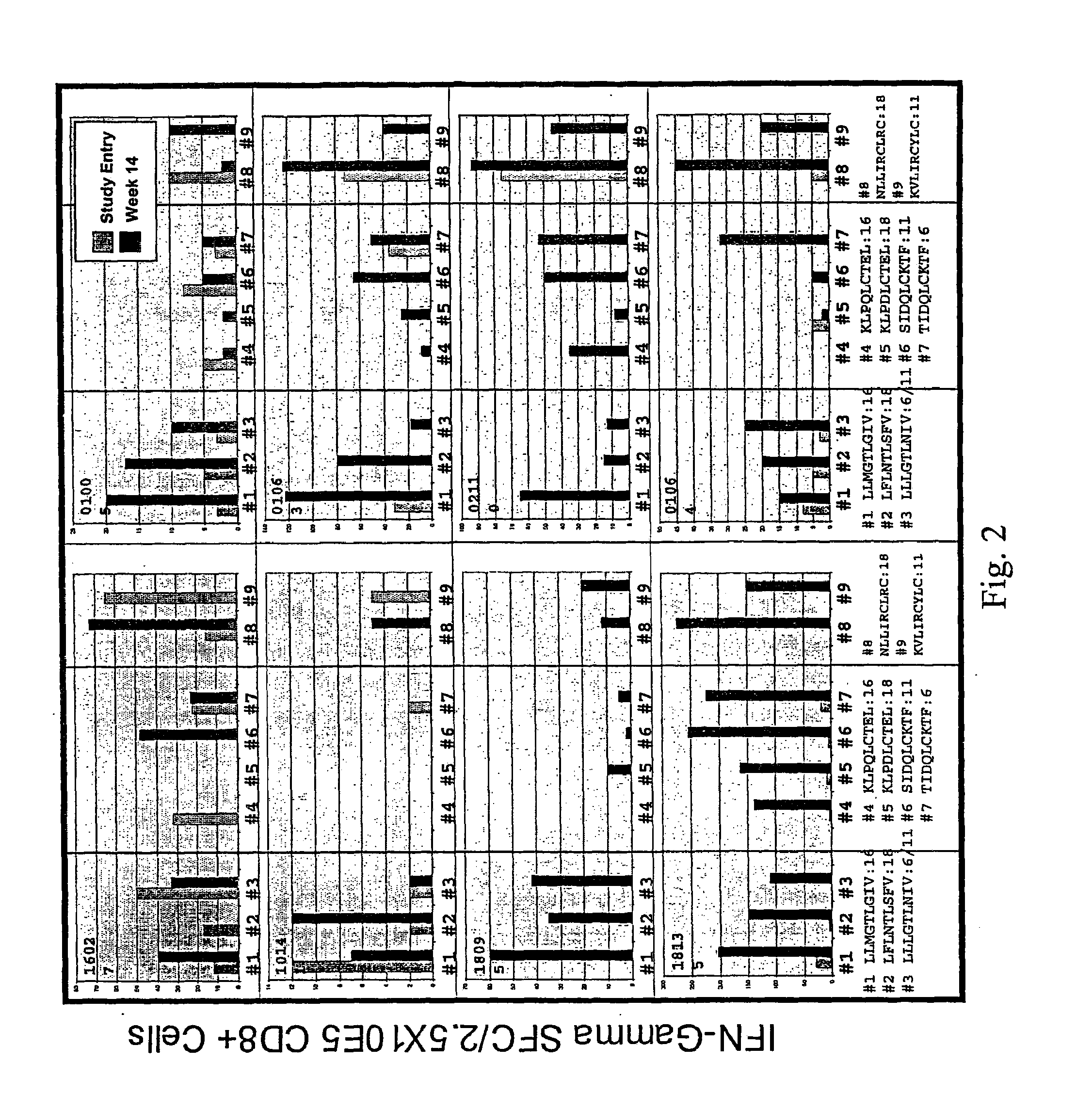
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
101results about "Reproductive system cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions
InactiveUS20070014810A1Reduce the possibilityImproving immunogenicitySugar derivativesViral antigen ingredientsEpitopeT cell
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to identify and prepare human papillomavirus (HPV) epitopes, and to develop epitope-based vaccines directed towards HPV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HPV infection.
Owner:GENIMMUNE NV +1
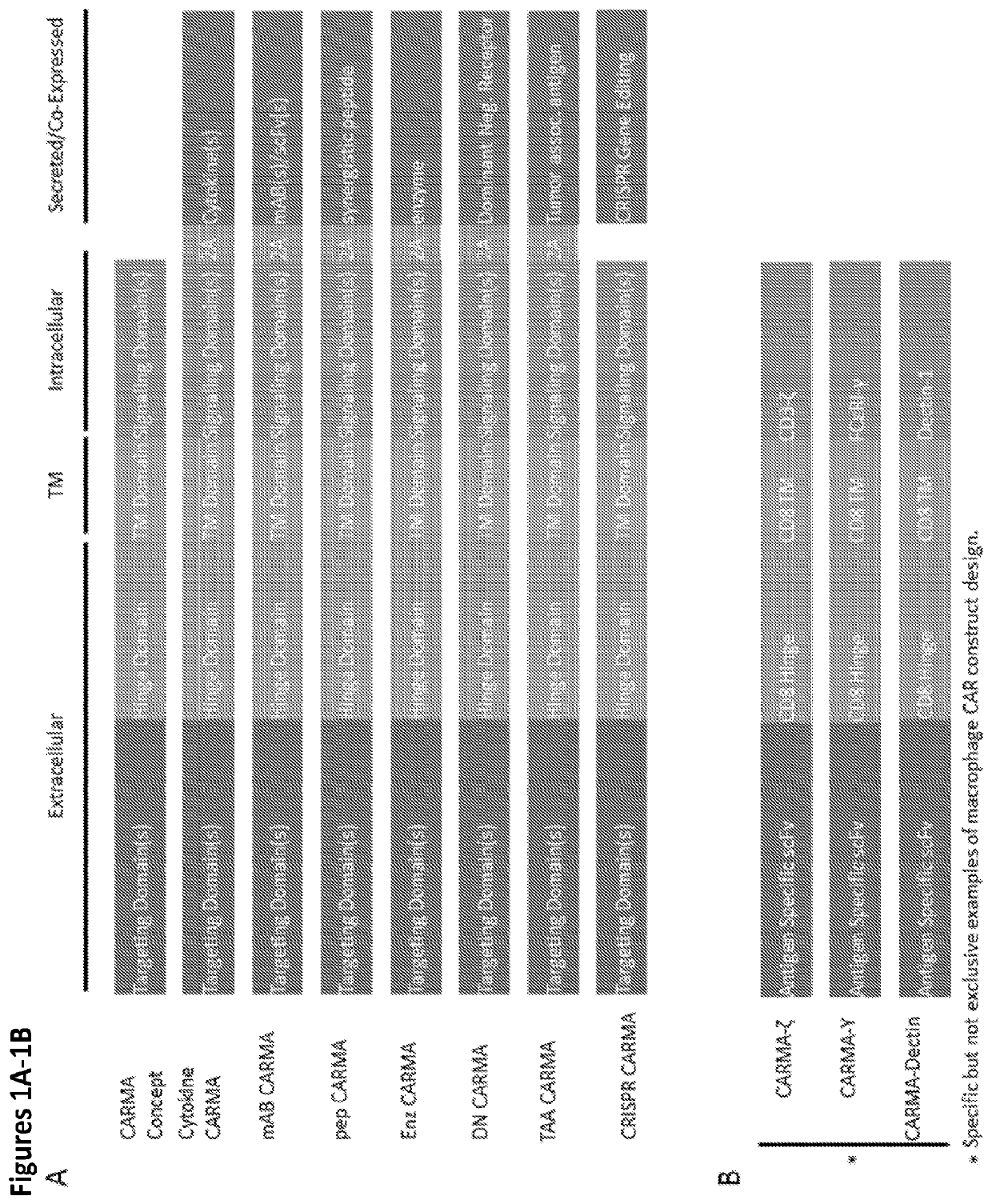
Modified Monocytes/Macrophage Expressing Chimeric Antigen Receptors and Uses Thereof
ActiveUS20180244748A1Stimulate immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsDendritic cellGackstroemia
The present invention includes methods and compositions for treating cancer, whether a solid tumor or a hematologic malignancy. By expressing a chimeric antigen receptor in a monocyte, macrophage or dendritic cell, the modified cell is recruited to the tumor microenvironment where it acts as a potent immune effector by infiltrating the tumor and killing the target cells. One aspect includes a modified cell and pharmaceutical compositions comprising the modified cell for adoptive cell therapy and treating a disease or condition associated with immunosuppression.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Myeloid derived suppressor cell inhibiting agents
ActiveUS20120156280A1Improve immune activityImproved therapeutic preparationSnake antigen ingredientsAntibody ingredientsAdjuvantCcr2 antagonist
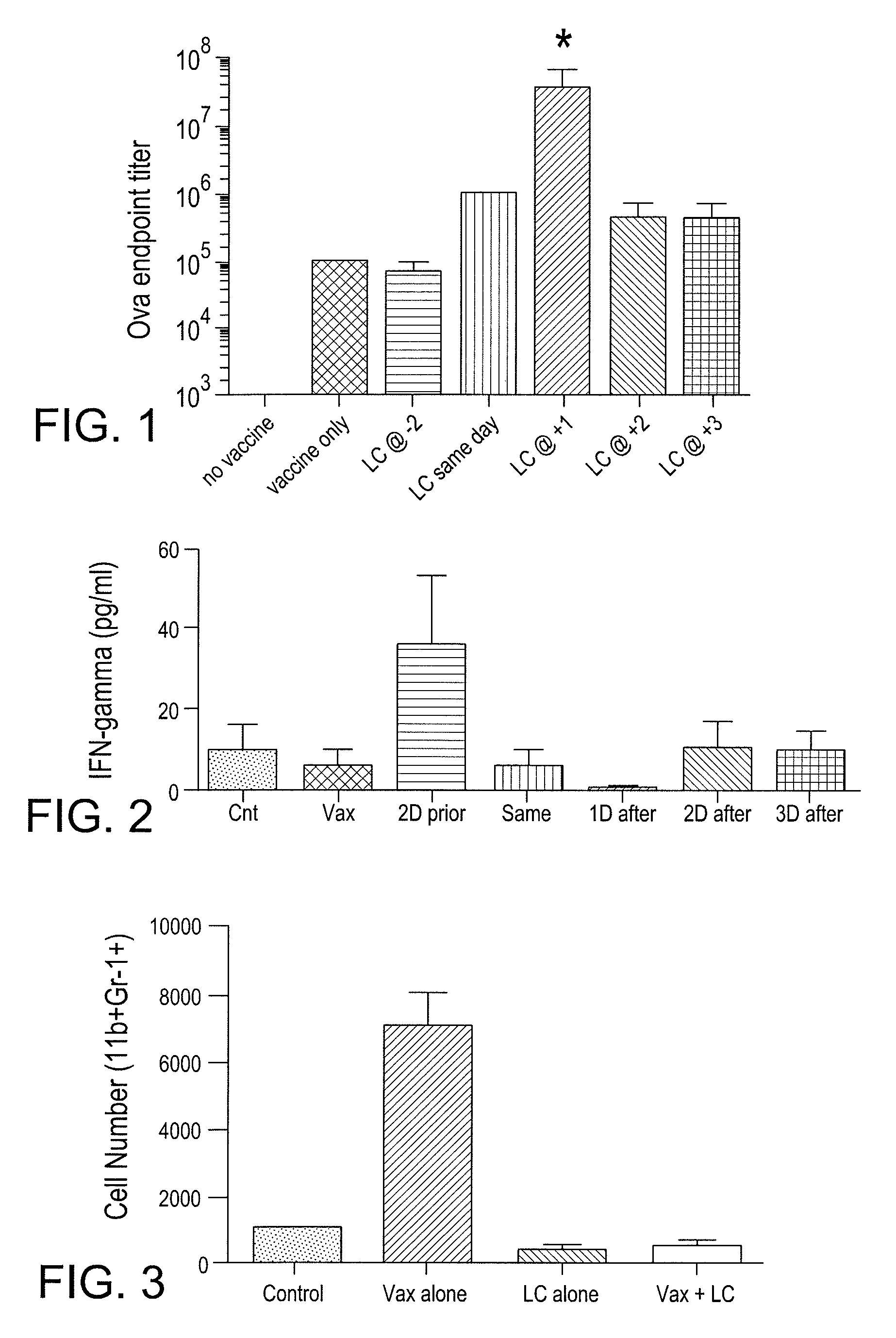
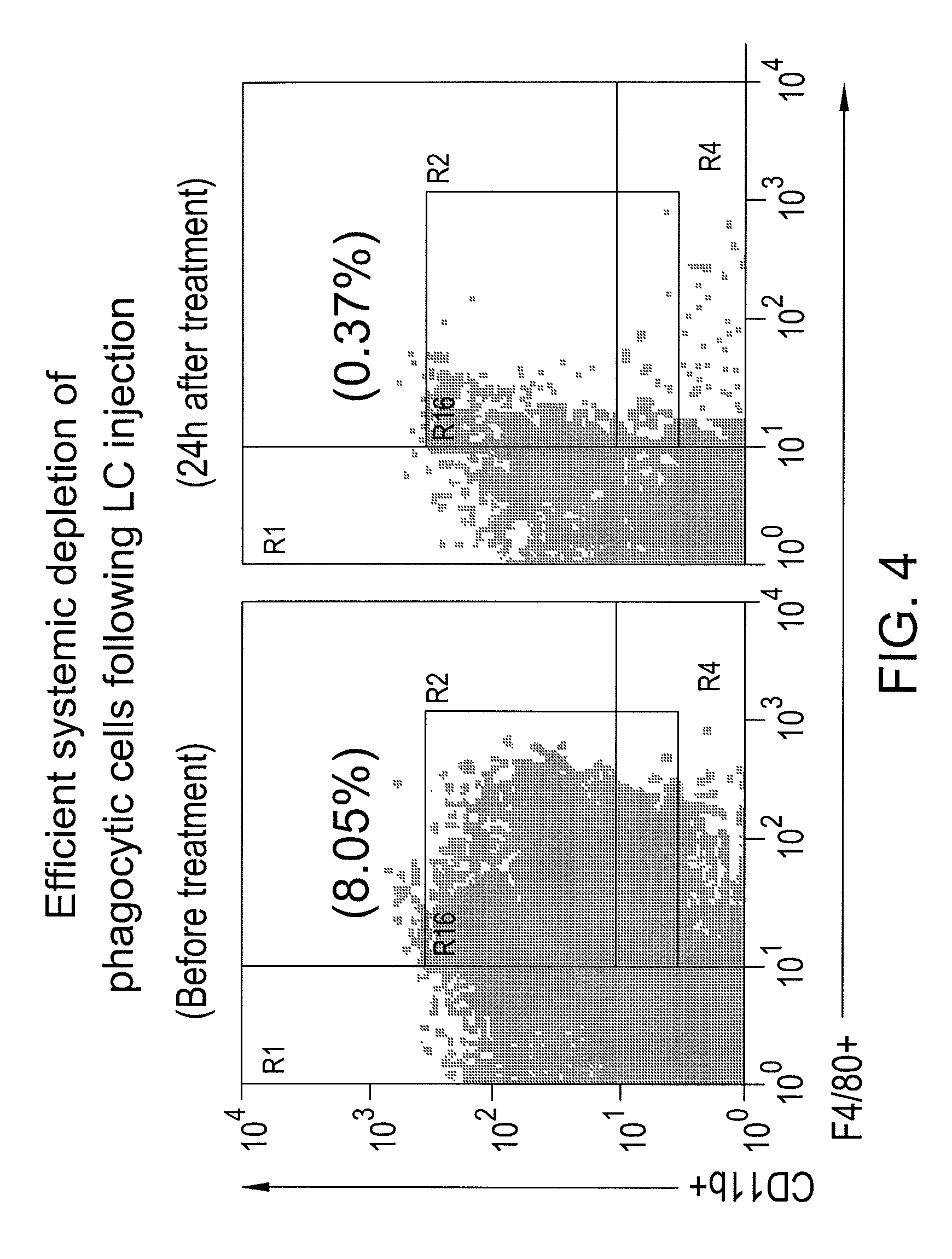
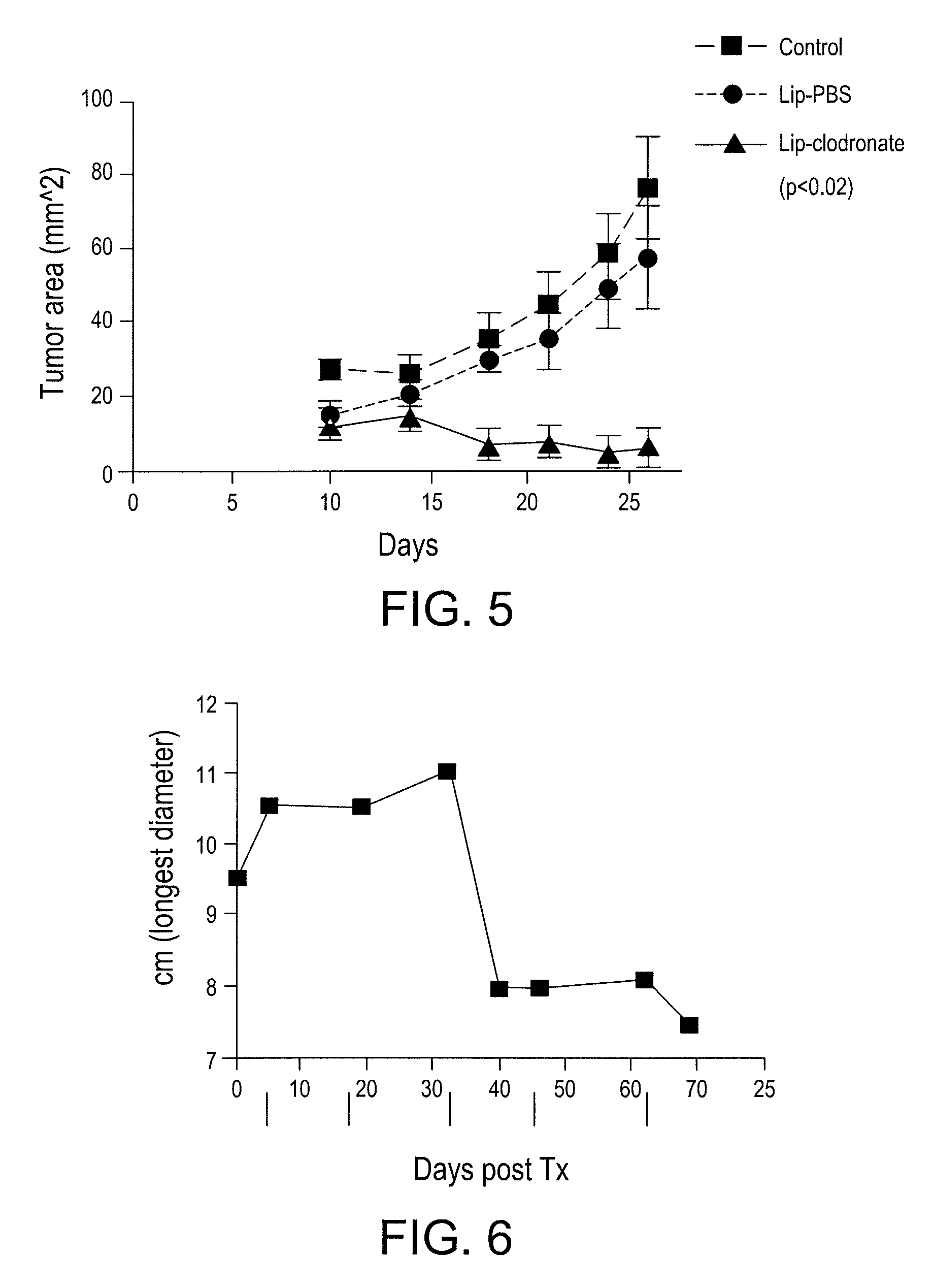
Myeloid derived suppressor cell (MDSC) inhibitory agents and vaccine and / or adjuvant enhancers are provided. Improved vaccine treatment regimens employing these agents are also provided. Cancer vaccines and methods for inhibiting tumor growth and cancer metastases are also presented. The myeloid derived suppressor cell (MDSC) inhibiting agents are described as bisphosphonates (such as liposomal clodronate) and CCR2 inhibitors and / or CCR2 antagonists. Methods for enhancing antibody titer levels in response to an antigen of interest are also provided.
Owner:COLORADO STATE UNIVERSITY
Vector for anti-hpv vaccine and transformed microorganism by the vector
ActiveUS20050249752A1Effective preventionInduce responseOrganic active ingredientsBacteriaTumor-Related ProteinSurface display
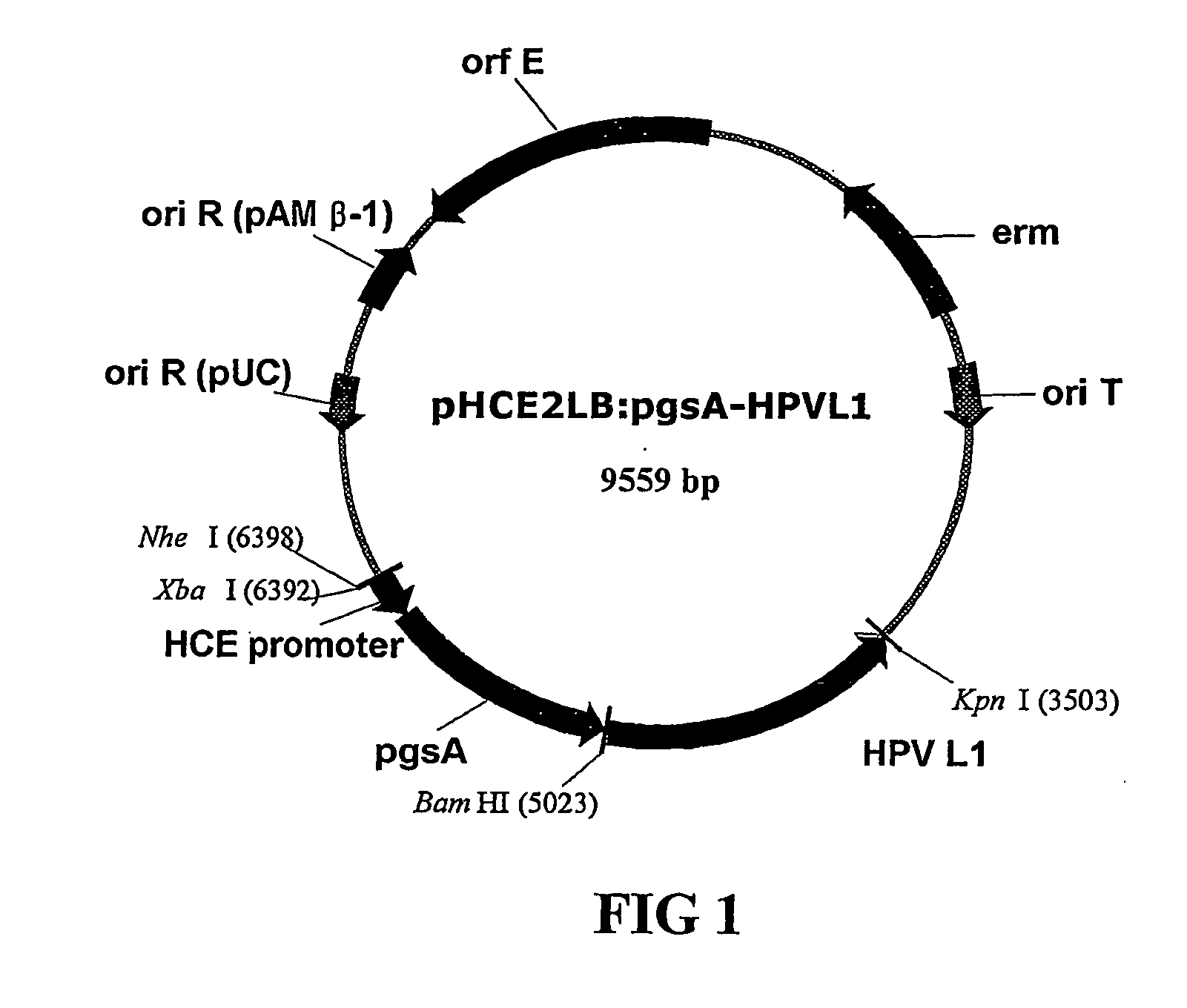
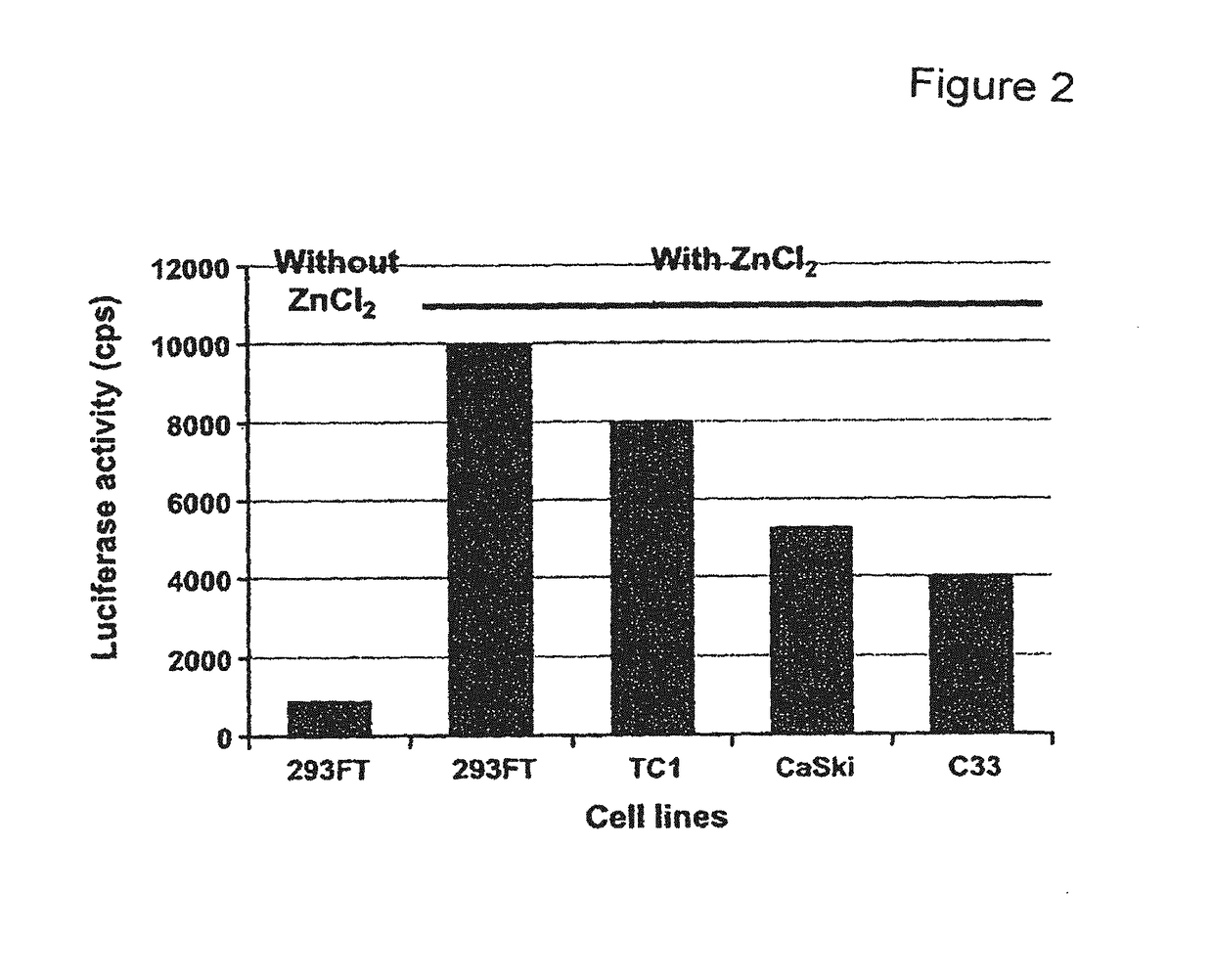
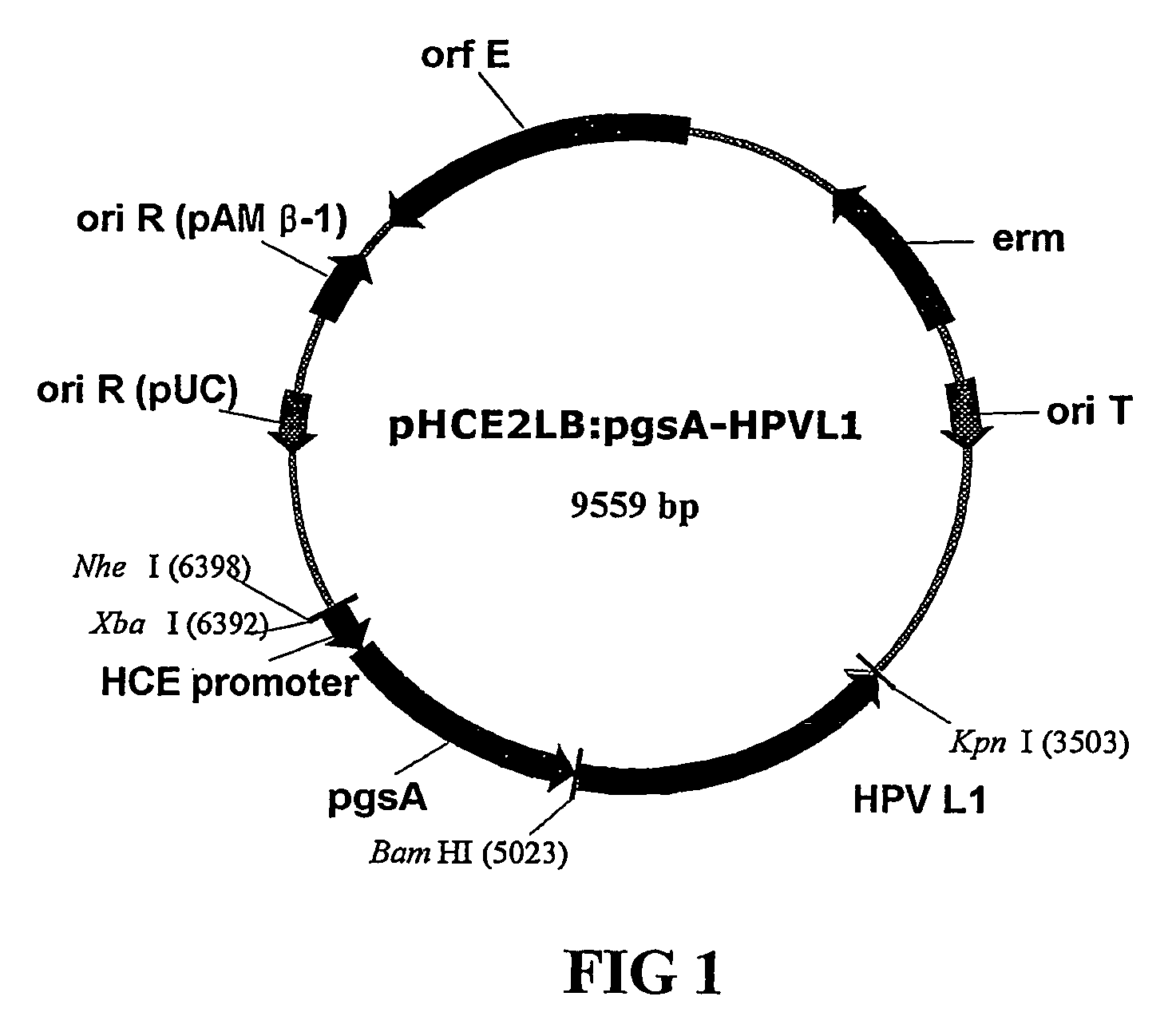
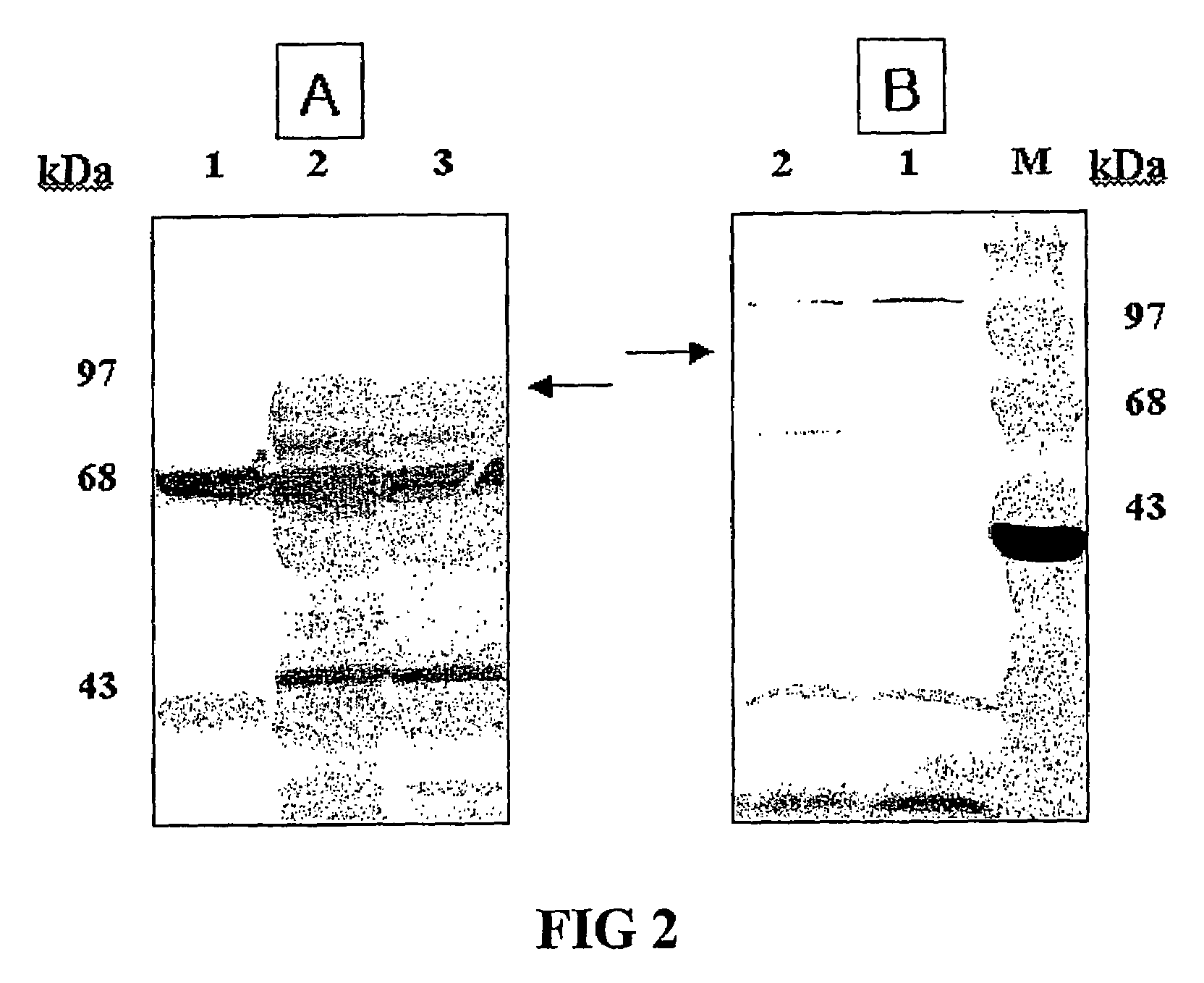
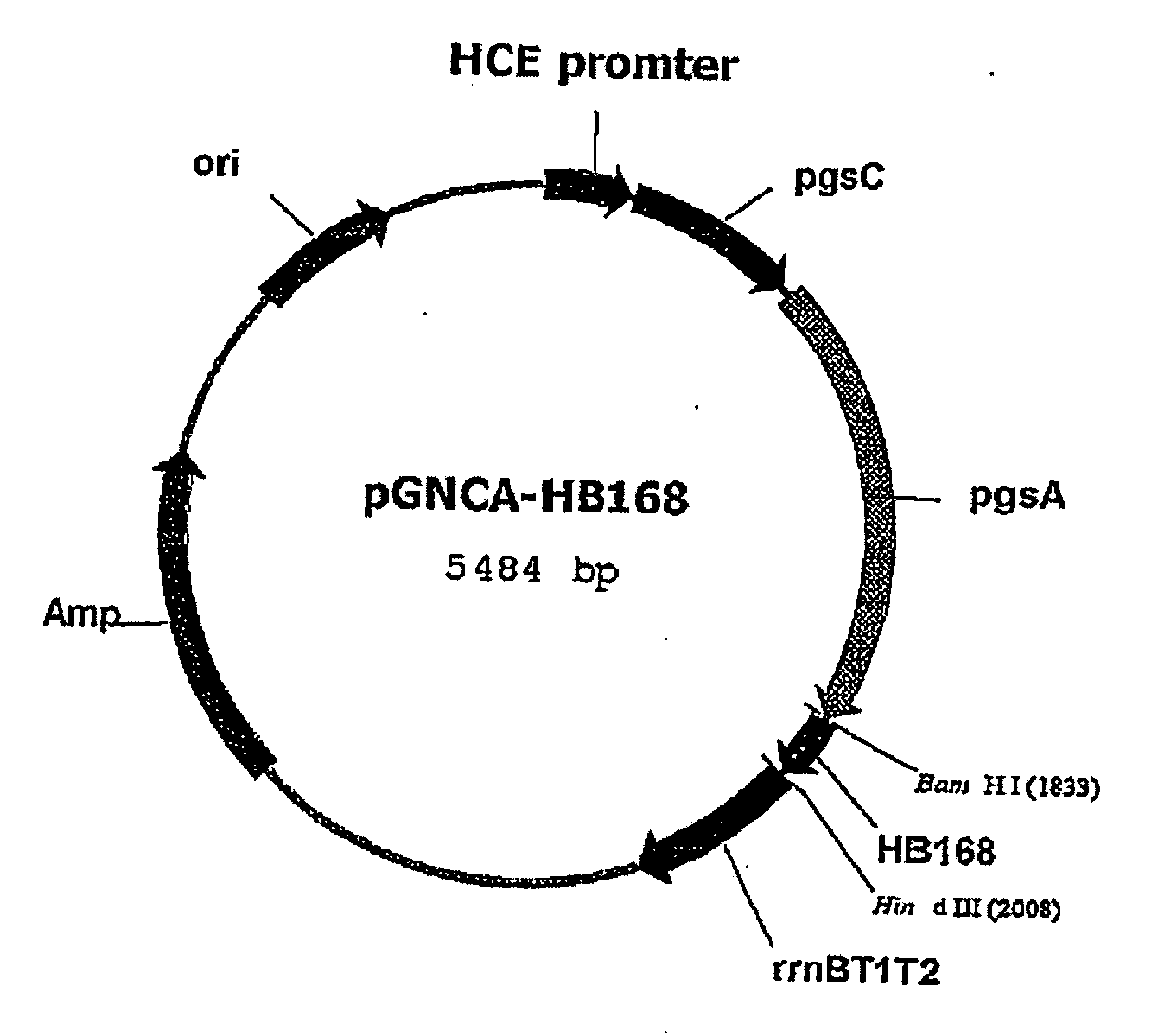
Expression vectors are described that can efficiently produce virion capsid protein, tumor-associated protein of human papillomavirus on a microbial surface. Bacterial strains harboring such surface display vectors, and the use of the bacterial strains or their extracts or purified products as complex vaccines, are also described. The surface display vectors contain one or more than two genes selected from among pgsB, pgsC and pgsA, encoding a poly-χ-glutamic acid synthetase complex (pgsBCA) of a Bacillus sp. strain, and genes that encode virion capsid proteins, tumor-associated proteins of human papillomavirus. Methodology for preparing the foregoing vectors, vaccines and transformed microorganisms are also described.
Owner:BIOLEADERS CORP
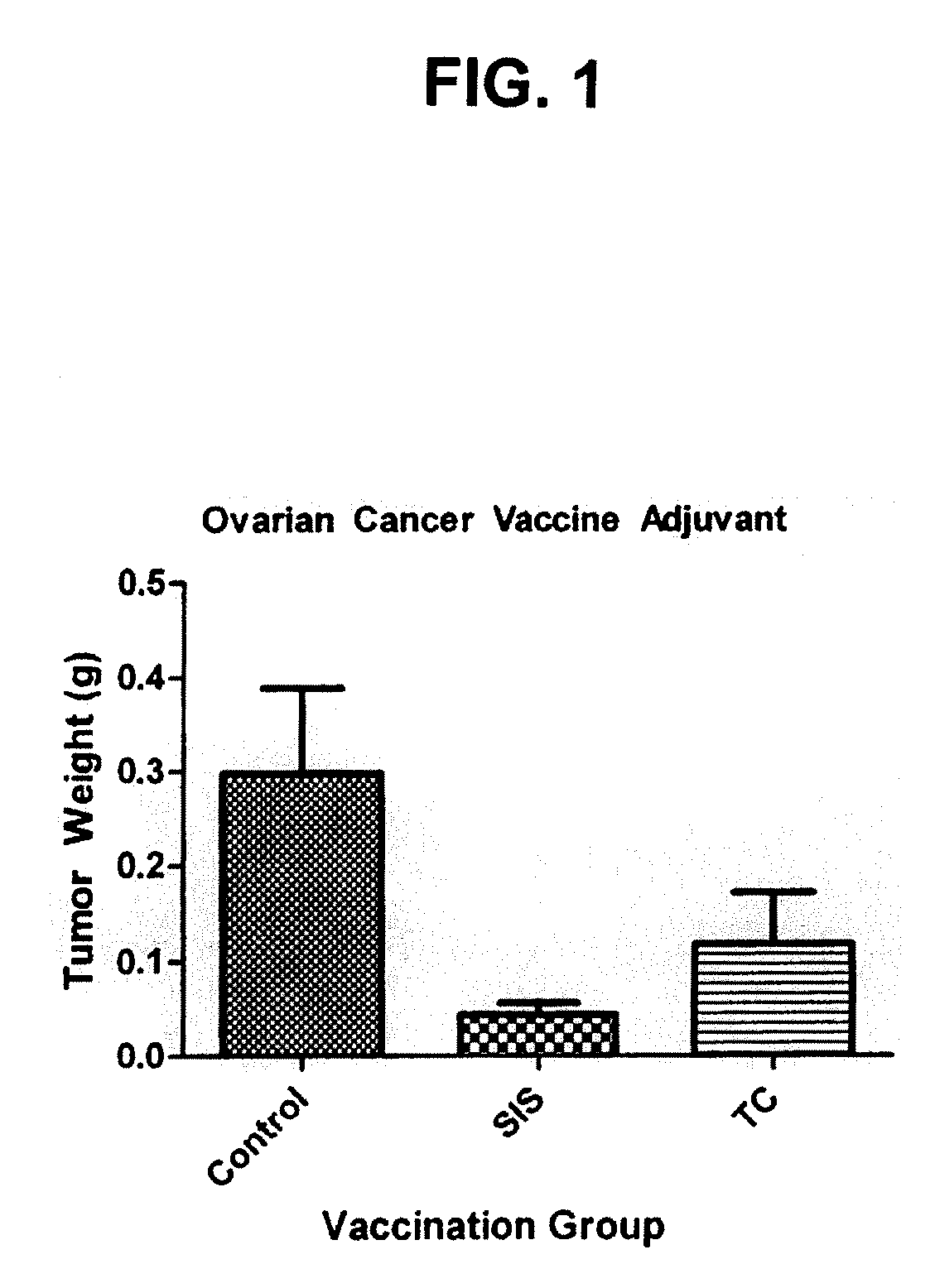
Extracellular matrix adjuvant & methods for prevention and/or inhibition of ovarian tumors and ovarian cancer
ActiveUS20110135690A1Improve anti-tumor activityEnhances immunizing and protective and anti-ovarian tumor physiological benefitCancer antigen ingredientsReproductive system cancer vaccineAdjuvantCell-Extracellular Matrix
Compositions suitable for use as ovarian cancer and / or tumor adjuvants in the preparation of ovarian cancer vaccines, particularly those vaccines useful in the treatment of human ovarian cancer, are provided. The ovarian cancer adjuvants described are comprised of an extracellular matrix material, such as small intestinal submucosal (SIS) tissue. The preparations may take the form of sheets, gels, liquids (injectable), tracer, or other solid or semi-solid preparation. Also disclosed are ovarian tumor inhibiting compositions that include extracellular matrix tissue adjuvants.
Owner:UNIV OF NOTRE DAME DU LAC
Human papillomavirus polypeptides and immunogenic compositions
InactiveUS7223408B2Reduced activityPotent activitySsRNA viruses positive-sensePeptide/protein ingredientsImmunogenicityFhit gene
Owner:WYETH HOLDINGS LLC
Human papillomavirus polypeptides and immunogenic compositions
ActiveUS20060014926A1Reduce biological activityPotent activitySsRNA viruses positive-sensePeptide/protein ingredientsImmunogenicityCancer research
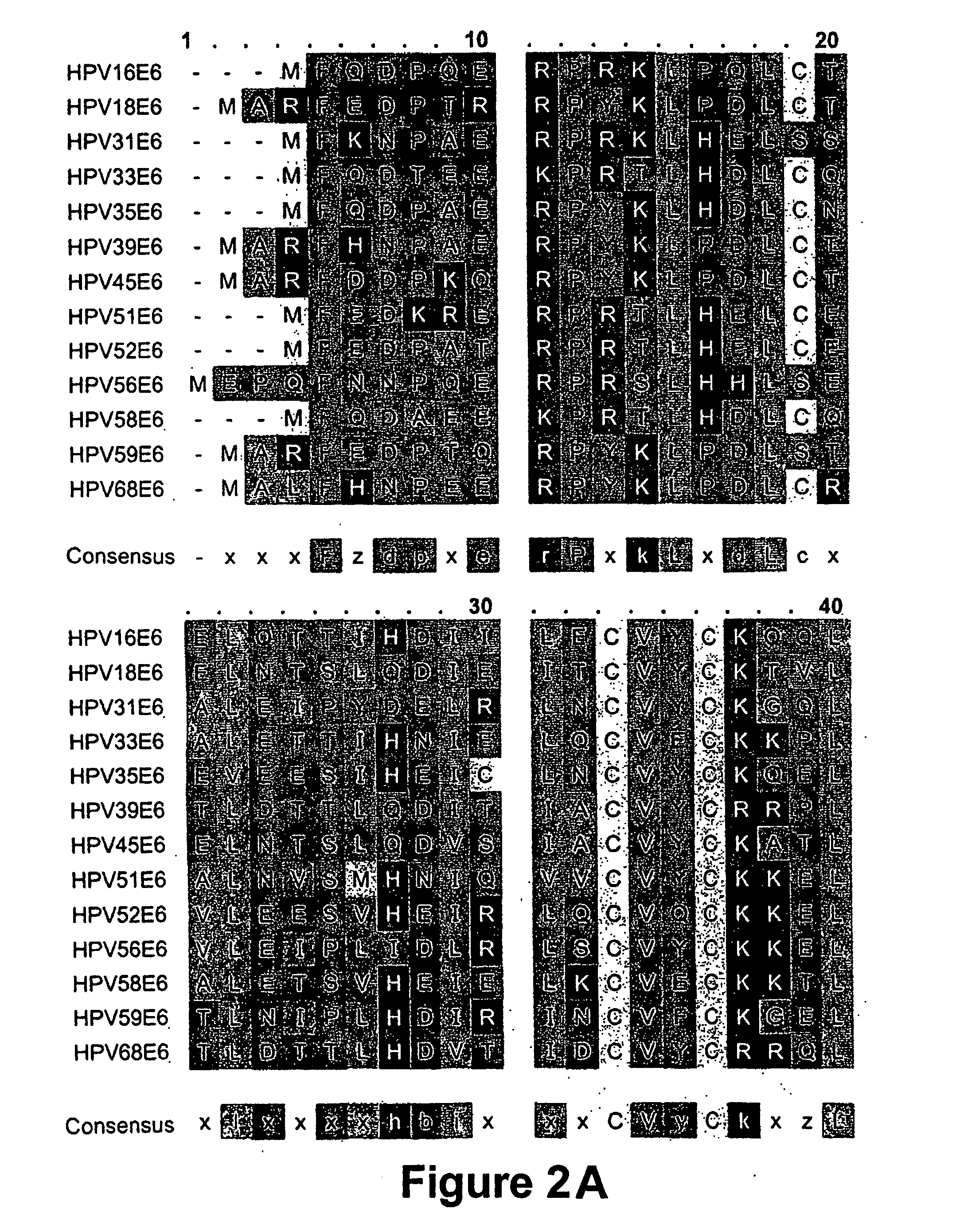
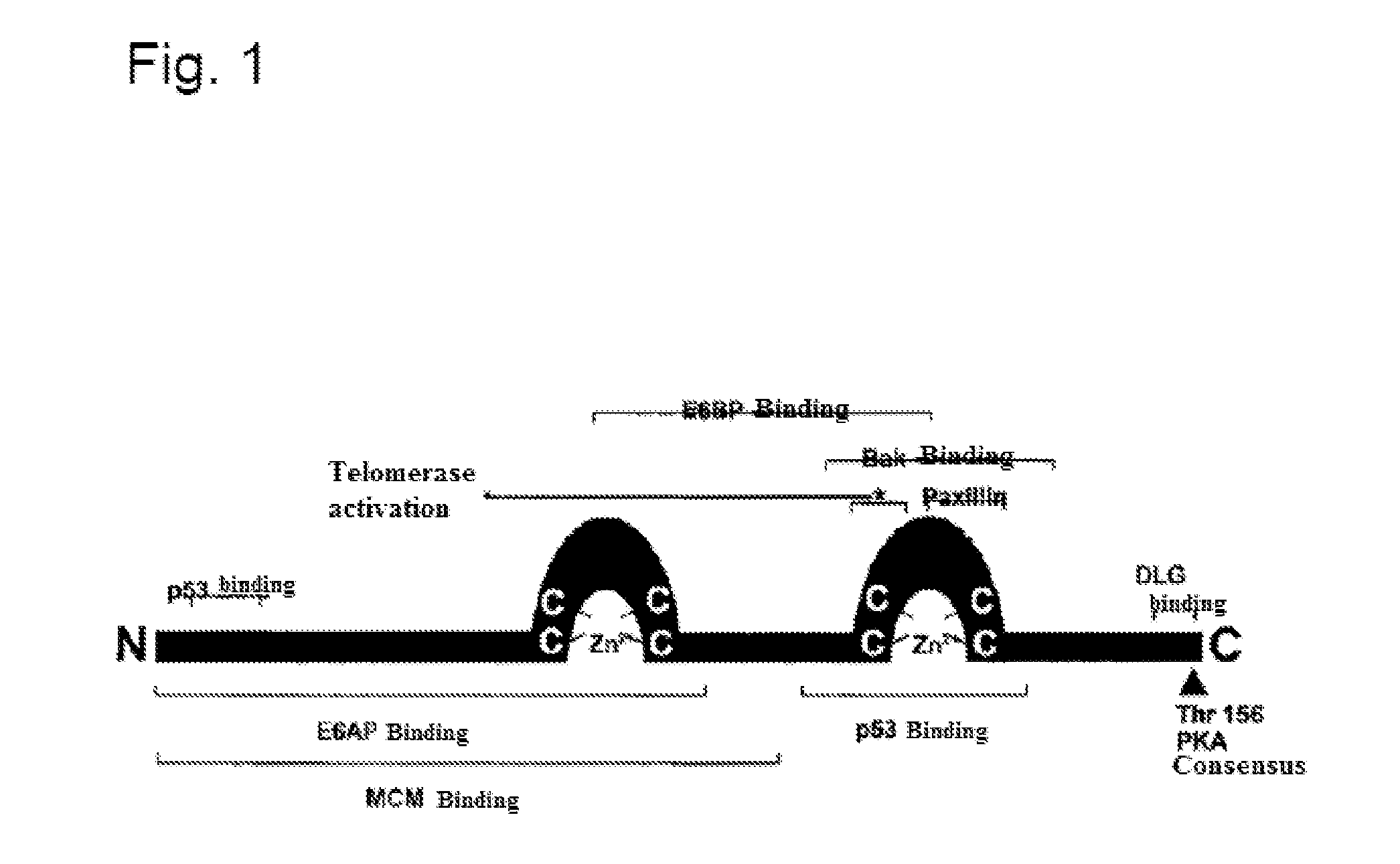
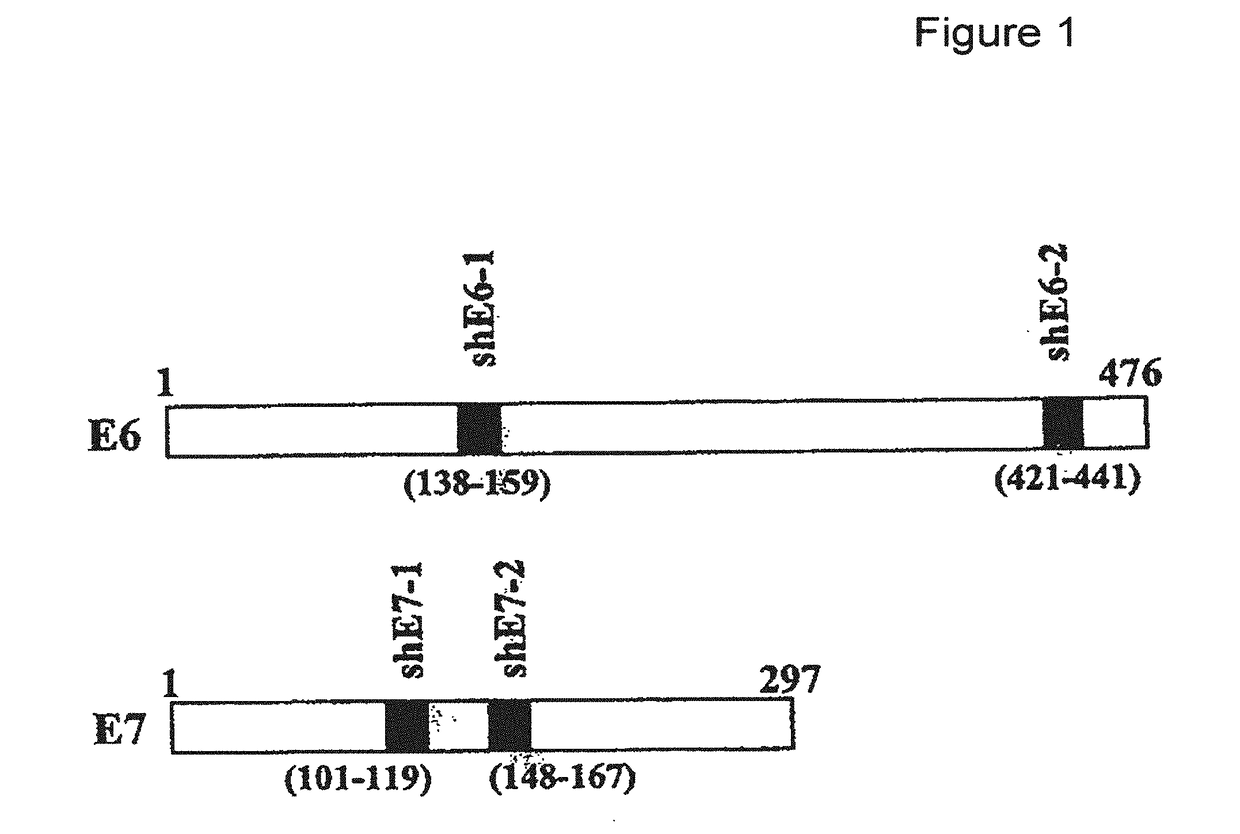
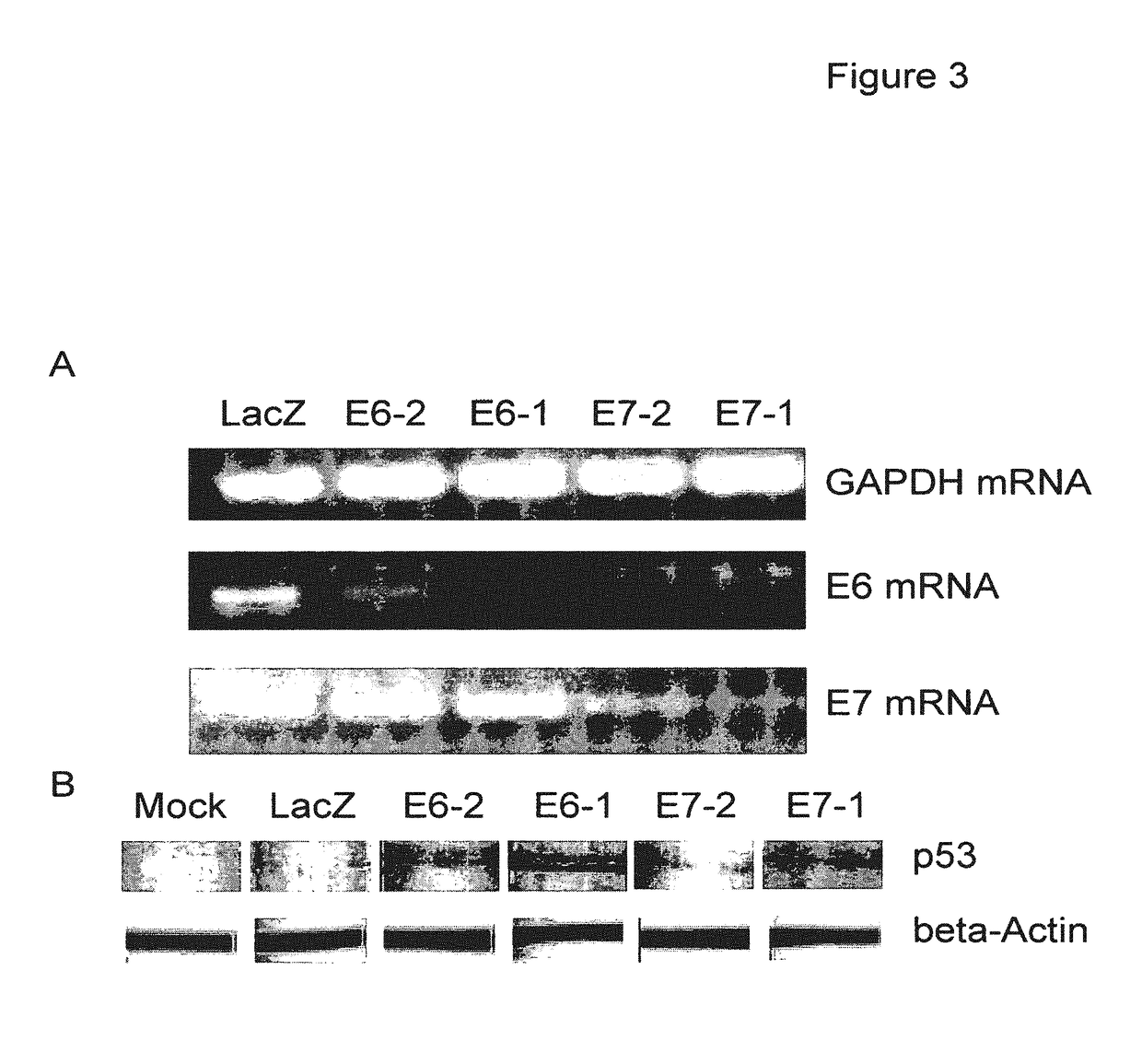
The present invention provides immunogenic and pharmaceutical compositions for the treatment and prevention of human papillomavirus (HPV)-associated cancers and in particular, cervical cancer. In particular, this invention relates to fusion proteins, and the nucleic acids encoding these fusion proteins, used to generate immune responses against HPV. Specifically, this invention provides for fusions of HPV E6 and E7 in which the E6 and / or E7 contains one or more mutations. These mutations abrogate the transformation activity of these oncogenic proteins and, thus, confer safety to the E6 / E7 fusions. In addition, these fusions maintain or increase the immunogenic efficacy of E6 and E7. Any gene or protein delivery method can be used to deliver or package the immunogenic compositions of the present invention.
Owner:WYETH HOLDINGS LLC
Agent for use in the topical or local treatment of cervical dysplasias
InactiveUS20130034584A1High expressionImprove responseViral antigen ingredientsAntiviralsInfected cellGynecology
The invention relates to an agent for treating cervical dysplasias, comprising a recombinant, genetically modified E1-deleted adenovirus replication defective in non-HPV infected cells, which is suitable for local external application in the region of the portio and the cervix uteri.
Owner:GENERAL VECTORS GMBH
Composite superimmunogen for bi-functional vaccine use for the treatment of illnesses associated with a stromal tissue disorder
The invention is relative to novel means of systemic or mucosal vaccinial therapy against some cancers, viral infections and allergy which are provided by the invention under the form of a family of composite superimmunogenic compounds for bifunctional vaccinial use able to induce an immune response raised towards two distinct targets, respectively, the causal pathogenic antigenic structure, on the one hand, and locally produced factors responsible for a subsequent immunotoxic or neoangiogenic stroma disorder, on the other hand.
Owner:NEOVACS SA
HPV particles and uses thereof
ActiveUS20170368162A1Eliminate and reduce numberReduce and eliminate sizeSpecial deliveryMicroencapsulation basedDiseaseMucosal tissue
The invention relates to modified HPV particles that can be used therapeutically. Modified HPV particles may be used to deliver therapeutic agents, including siRNA molecules. Modified HPV particles may be used for the treatment of diseases or conditions of mucosal tissue, including HPV (human papilloma virus) infection and HPV-related tumors.
Owner:AURA BIOSCI +1
Vector for anti-HPV vaccine and transformed microorganism by the vector
ActiveUS7425438B2Effective preventionInduce responseOrganic active ingredientsBacteriaTumor-Related ProteinSurface display
Expression vectors are described that can efficiently produce virion capsid protein, tumor-associated protein of human papillomavirus on a microbial surface. Bacterial strains harboring such surface display vectors, and the use of the bacterial strains or their extracts or purified products as complex vaccines, are also described. The surface display vectors contain one or more than two genes selected from among pgsB, pgsC and pgsA, encoding a poly-χ-glutamic acid synthetase complex (pgsBCA) of a Bacillus sp. strain, and genes that encode virion capsid proteins, tumor-associated proteins of human papillomavirus, Methodology for preparing the foregoing vectors, vaccines and transformed microorganisms are also described.
Owner:BIOLEADERS CORP
Fusion protein as well as preparation method and application thereof
ActiveCN108484776ASingle ingredientClear structureAntibody mimetics/scaffoldsNucleic acid vectorImmunotherapyLinker peptide
The invention belongs to the immunotherapy and discloses a fusion protein as well as a preparation method and application thereof. The fusion protein disclosed by the invention comprises a protein HSP70 and a protein fused with an extracellular domain protein FPR1. The extracellular domain protein FPR1 comprises multiple amino acid sequences, and the amino acid sequences of different fragments ofthe extracellular domain protein FPR1 are connected by flexible linker peptides. The fusion protein disclosed by the invention is simple in preparation, high in specificity and long in duration time,and the immunotherapy effect can be obviously enhanced.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV
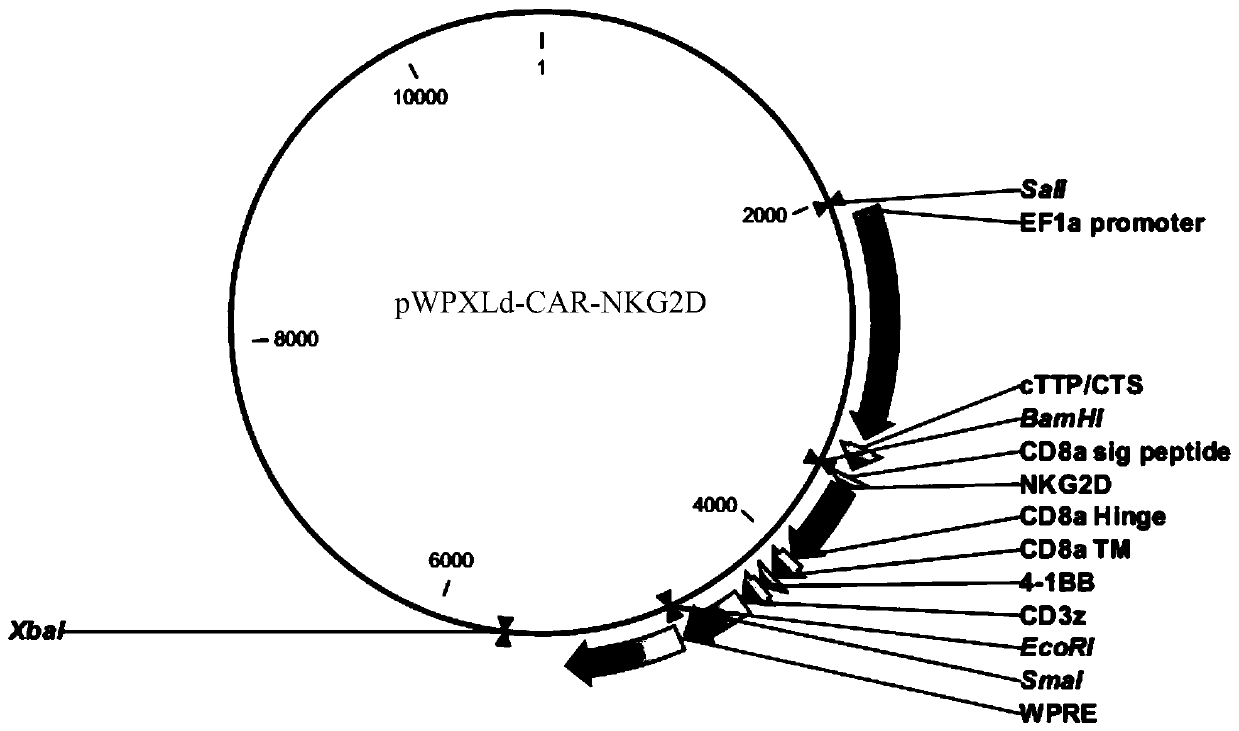
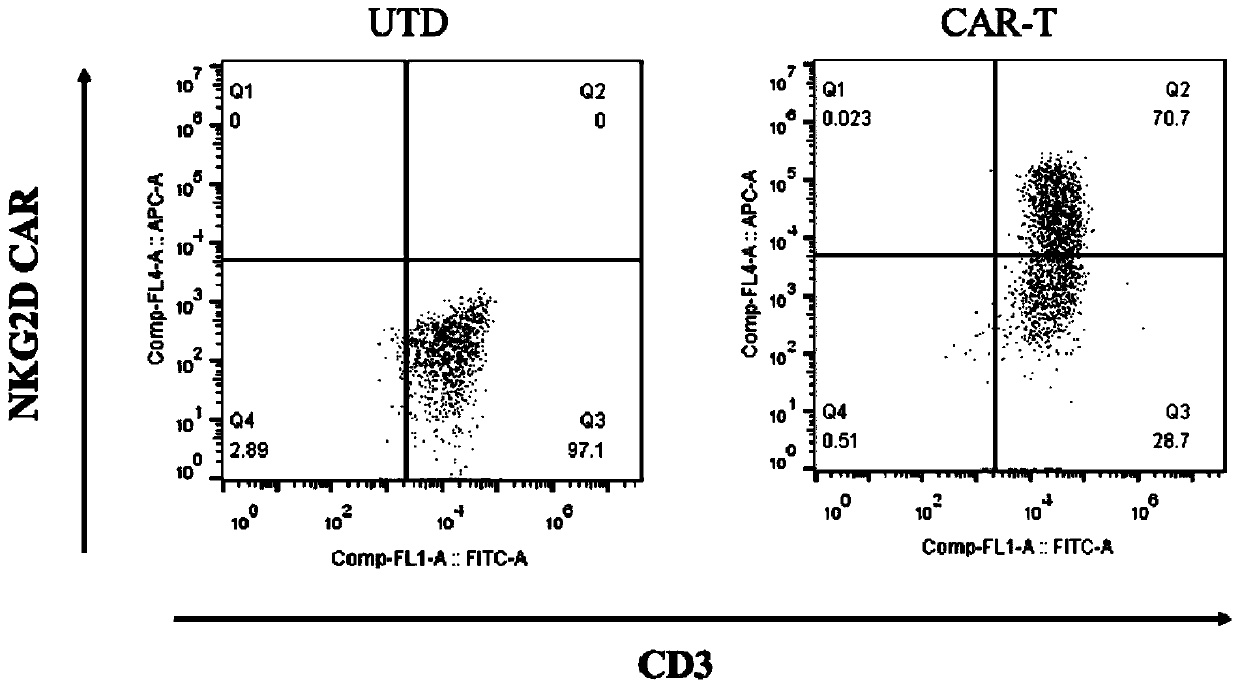
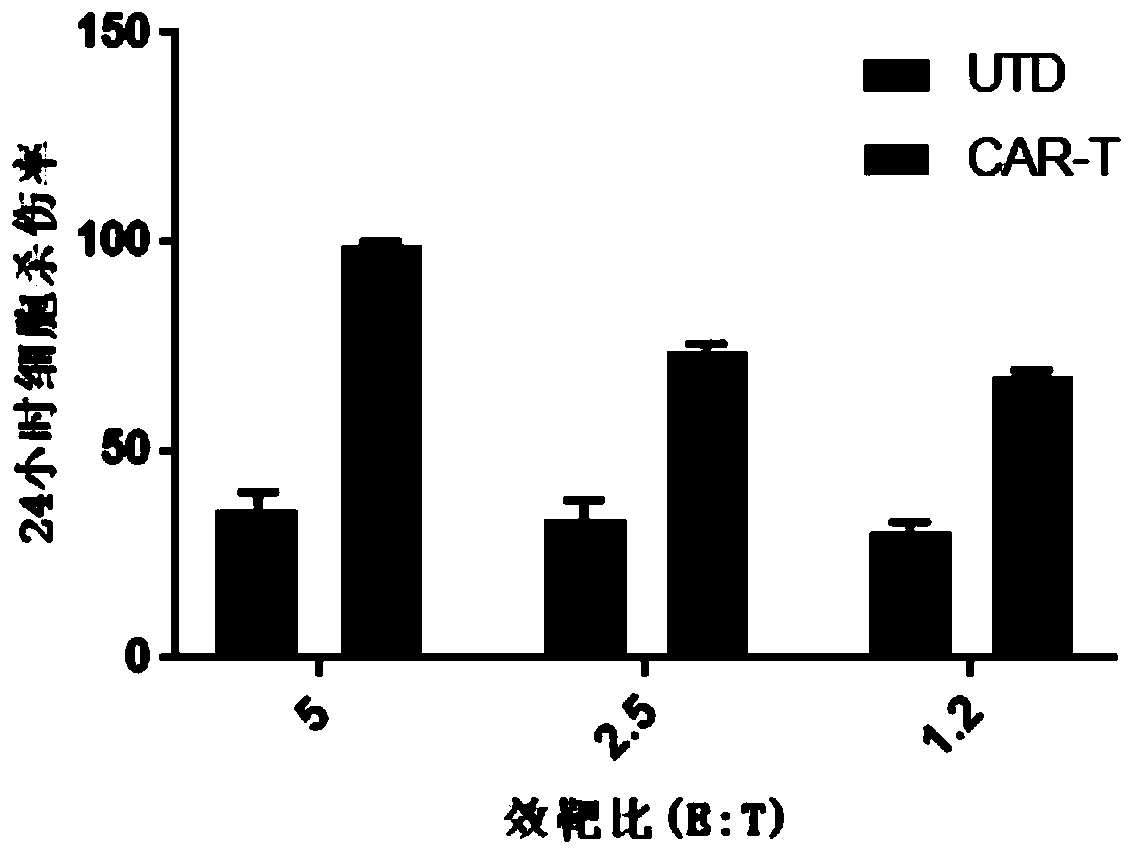
Chimeric antigen receptor targeting NKG2D, chimeric antigen receptor T-cell targeting NKG2D and preparation method thereof and application thereof
PendingCN111363046ALong-lasting strong killing effectNo damageBlood/immune system cellsImmunoglobulinsPancreas CancersSingle-Chain Antibodies
The invention provides a chimeric antigen receptor (CAR) targeting NKG2D. Amino acid sequences of the CAR targeting NKG2D comprises the amino acid sequences of a single-chain antibody targeting NKG2D,an extracellular hinge region, a transmembrane region and an intracellular signal region which are sequentially connected from an amino terminal to a carboxyl terminal, respectively. The single-chainantibody targeting NKG2D comprises the amino acid sequences shown in SEQ ID NO: 1. The invention also provides a chimeric antigen receptor T-cell (CAR-T) targeting NKG2D and a preparation method thereof, as well as application of the CAR targeting NKG2D and the CAR-T targeting NKG2D in the prevention, diagnosis and treatment of liver cancer, cervical cancer, pancreatic cancer and other malignanttumors.
Owner:SHENZHEN BINDEBIOTECH CO LTD
Modified monocytes/macrophage expressing chimeric antigen receptors and uses thereof
ActiveUS20200247870A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAdoptive cellular therapyDisease
The present invention includes methods and compositions for treating cancer, whether a solid tumor or a hematologic malignancy. By expressing a chimeric antigen receptor in a monocyte, macrophage or dendritic cell, the modified cell is recruited to the tumor microenvironment where it acts as a potent immune effector by infiltrating the tumor and killing the target cells. One aspect includes a modified cell and pharmaceutical compositions comprising the modified cell for adoptive cell therapy and treating a disease or condition associated with immunosuppression.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Specific chimeric antigen receptor T cell targeting nkg2dl, preparation method and application thereof
ActiveCN109803983BEffective targeted attackHigh kill rateVirusesAntibody mimetics/scaffoldsAbnormal tissue growthAntigen receptors
A specific chimeric antigen receptor targeting NKG2DL, its coding sequence, and modified immune response cells, as well as their preparation method and application. The modified immune response cells can effectively target and attack various tumor cells, especially positive tumor cells expressing NKG2DL, and can be used to prepare preparations for treating tumors.
Owner:NANJING KAEDI BIOTHERAPEUTICS LTD
Pharmaceutical composition for treating or preventing ovarian cancer
The invention relates to therapeutic and prophylactic treatment of ovarian cancer and metastases thereof. More specifically, the invention relates to immunogenic polypeptides comprising at least a portion of an ovarian tissue cell-associated protein or immunologically active variants thereof and to nucleic acids encoding such polypeptides and to the use thereof in immunotherapeutic methods of treatment. Said immunogenic polypeptides are provided by the zona pellucida (ZP) glycoproteins. ZP glycoproteins and fragments thereof that can induce a CD8+ and / or CD4+ T cell response as well as nucleic acid sequences encoding them can suitably be used in the present immunotherapeutic strategies.
Owner:PANTARHEI BIOSCI
Myeloid derived suppressor cell inhibiting agents
ActiveUS9320735B2Improve immune activityImprove efficiencySnake antigen ingredientsAntibody ingredientsAdjuvantCancer metastasis
Myeloid derived suppressor cell (MDSC) inhibitory agents and vaccine and / or adjuvant enhancers are provided. Improved vaccine treatment regimens employing these agents are also provided. Cancer vaccines and methods for inhibiting tumor growth and cancer metastases are also presented. The myeloid derived suppressor cell (MDSC) inhibiting agents are described as bisphosphonates (such as liposomal clodronate) and CCR2 inhibitors and / or CCR2 antagonists. Methods for enhancing antibody titer levels in response to an antigen of interest are also provided.
Owner:COLORADO STATE UNIVERSITY
Agent for use in the topical or local treatment of cervical dysplasias
InactiveUS8795684B2Improve responseIntensified release of papilloma viral antigensViral antigen ingredientsVirus peptidesInfected cellExternal application
The invention relates to an agent for treating cervical dysplasias, comprising a recombinant, genetically modified E1-deleted adenovirus replication defective in non-HPV infected cells, which is suitable for local external application in the region of the portio and the cervix uteri.
Owner:GENERAL VECTORS GMBH
Double-chimeric antigen receptor, T cell and construction method and application thereof
PendingCN110669138AToxicityImprove securityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsTumor antigenSialyl tn
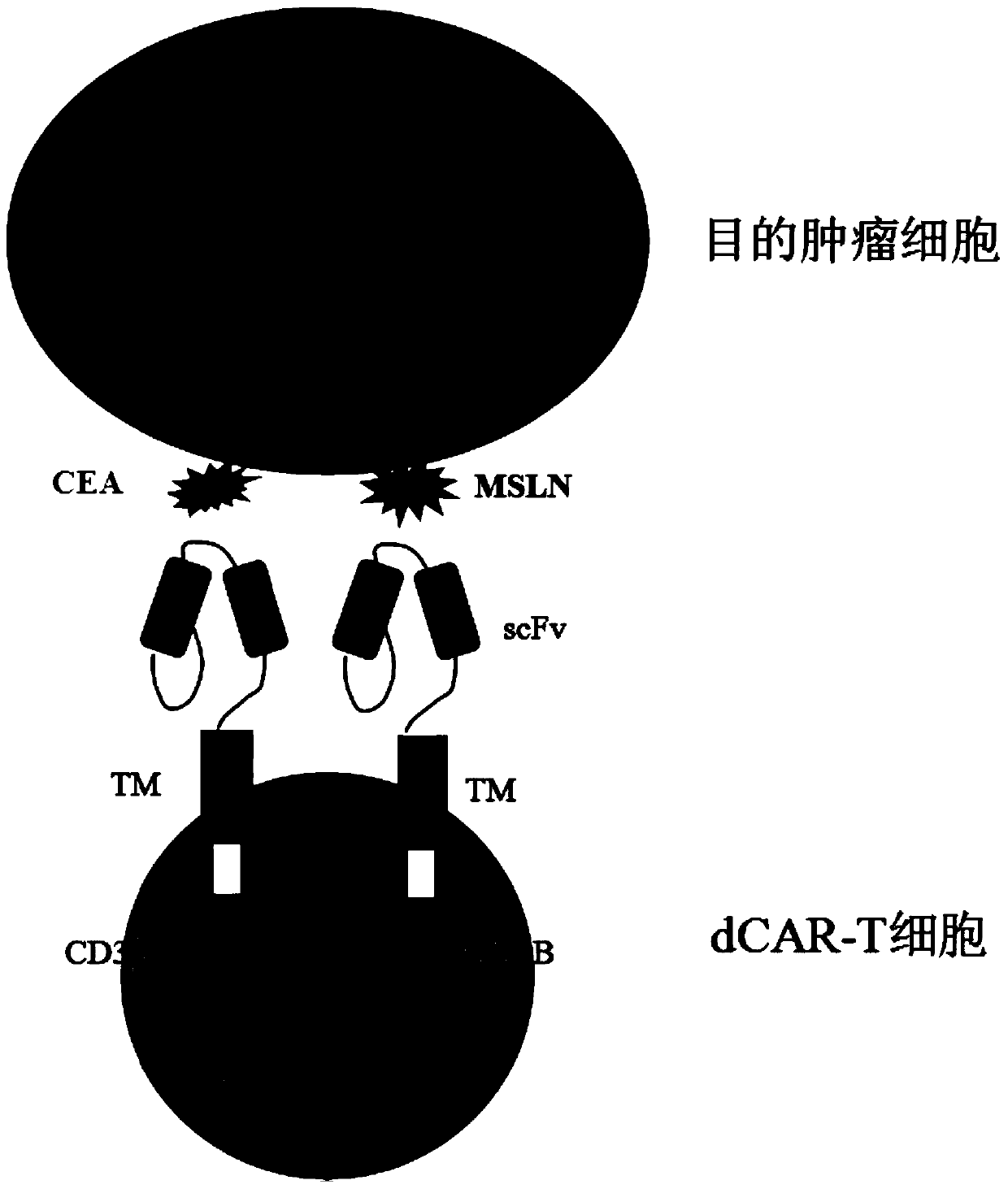
The invention discloses a double-chimeric antigen receptor, a T cell and a construction method and an application thereof, which belong to the field of cellular immunotherapy of tumors. The inventionspecifically relates to a specific structure and a construction method of the double chimeric antigen receptor T cell (dCAR-T cell), and preliminarily discusses the in-vivo and in-vitro activity of the dCAR-T cell. The selected tumor-associated antigens are mesothelin and carcino-embryonic antigens, and researches show that the two tumor antigens can be simultaneously expressed on the surface of asolid tumor, such as pancreatic cancer. The invention discloses an antigen receptor. The in-vitro and in-vivo tests prove that the constructed dCAR-T cell can be permanently and effectively activatedonly under the condition that two antigens are simultaneously recognized, and has efficient anti-tumor activity, so that a specific tumor killing function can be exerted, and the application of CAR-Tcell immunotherapy is improved.
Owner:CHINA PHARM UNIV
Peripheral blood TCR marker for cervical cancer as well as detection kit and application of peripheral blood TCR marker
PendingCN111650373AImprove featuresImprove accuracyBiological material analysisAntibody medical ingredientsCervical caOncology
The invention discloses a peripheral blood TCR marker for cervical cancer as well as a detection kit and application of the peripheral blood TCR marker. The marker comprises at least one of proteins of which the sequences are shown as SEQ ID NO. 1-100. The method is based on a high-throughput sequencing method; only a small amount of peripheral blood needs to be collected; the method comprises thefollowing steps: extracting RNA, treating samples to establish an immune map library, carrying out high-throughput sequencing and TCR data analysis, firstly determining a characteristic TCR sequencein the peripheral blood of cervical cancer, and then comparing a test result of a sample to be tested with the characteristic TCR sequence so as to determine whether a patient suffers from the cervical cancer or not. According to the present invention, the huge number of the cervical cancer specific TCR sequences can be simultaneously compared, and compared to the single detection of one or more markers, the high specificity and the high accuracy are provided, and the diagnosis efficiency is improved.
Owner:CHENGDU EXAB BIOTECH CO LTD
Vaccine
The disclosure relates to polypeptides and pharmaceutical compositions comprising polypeptides that find use in the prevention or treatment of cancer, in particular breast cancer, ovarian cancer and colorectal cancer. The disclosure also relates to methods of inducing a cytotoxic T cell response in a subject or treating cancer by administering pharmaceutical compositions comprising the peptides, and companion diagnostic methods of identifying subjects for treatment. The peptides comprise T cell epitopes that are immunogenic in a high percentage of patients.
Owner:TREOS BIO LTD
Recombinant protein and medicine composition and application thereof
ActiveCN106632694AFix security issuesEffective treatmentPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman bodyAdjuvant
The invention relates to the field of biological medicine, in particular to recombinant protein, a medicine composition and application thereof. The recombinant protein comprises human papilloma virus E6 and E7 plasmodium fusion polypeptides, has specific amino acid sequences, and special space structures, so that strong immunogenicity, particularly cell-mediated immunity can be realized; the human body safety problem is solved through point mutation. The medicine composition provided by the invention comprises the recombinant protein and auxiliary agents, and can stimulate and reinforce the specific T-cell immune response aiming at the human papilloma virus E6 and E7 protein, can be used for effectively treating cervical cancer, and has good application prospects.
Owner:南京益康生物医药有限公司
Vector for Anti-hpv vaccine and transformed microorganism by the vector
ActiveUS20090117151A1Effective preventionInduce responseOrganic active ingredientsVirusesTumor-Related ProteinHuman papillomavirus
Owner:BIOLEADERS CORP
Canine tumor cell and allogeneic dendritic cell fused vaccine and method for preparing the same
The present invention provides a dendritic cell-based vaccine by fusing a canine tumor cell and an allogeneic dendritic cell, and a method for preparing the same. The fusion cells expressing canine tumor antigens are generated by fusing canine bone marrow-derived dendritic cells and canine tumor cells. The canine immune system can be induced to produce tumor specific T lymphocytes and natural killer cells when the fusion cells used as a vaccine is injected into a canine body.
Owner:NAT TAIWAN UNIV
Til expansion from fine needle aspirates and small biopsies
The present disclosure provides methods for expanding TIL populations from fine needle aspirates (FN As) or small biopsies which contain low numbers of TILs, using the methods disclosed herein including in a closed system that leads to improved phenotype and increased metabolic health of the TILs in a shorter time period.
Owner:IOVANCE BIOTHERAPEUTICS INC
Compositions and methods for treating human papillomavirus-mediated disease
InactiveUS20110158952A1Permit treatmentEffective treatmentBiocideOrganic active ingredientsDiseaseEpitope
The invention includes methods of treating an HPV-mediated disease by administration to an individual of a pharmaceutical composition comprising a nucleic acid that encodes a polypeptide comprising an epitope of a naturally occurring HPV protein. The methods include the selection of individuals for treatment with the composition according to a the age of the recipient, as well as the use of the composition to elicit a cross-reactive anti-HPV immune response.
Owner:EISAI INC
Chimeric antigen receptor of cell for targeted expression of Claudin 18.2 and application of chimeric antigen receptor
ActiveCN113354739AEfficient killingStrong killing and cytokine release functionVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
The invention discloses a chimeric antigen receptor of a cell for targeted expression of Claudin 18.2 (CLDN 18.2), and particularly discloses a chimeric antigen receptor with an amino acid sequence as shown in SEQ ID NO.14. The chimeric antigen receptor comprises a Claudin 18.2-targeted single-chain antibody, a hinge region, a transmembrane structural domain and an intracellular signal structural domain. The Claudin 18.2-targeted chimeric antigen receptor disclosed by the invention can achieve effective and specific targeted expression of malignant cells (such as tumor cells) of the Claudin 18.2 surface antigen, so that a more efficient method with fewer side effects and adverse reactions is provided for treating some tumors expressing the Claudin 18.2 surface antigen.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Chimeric antigen receptor T lymphocyte and application thereof to preparation of product for treating solid tumors
ActiveCN111875708ALift specific immune responseEnhance anti-tumor immune responseVirusesAntineoplastic agentsSingle-Chain AntibodiesAntiendomysial antibodies
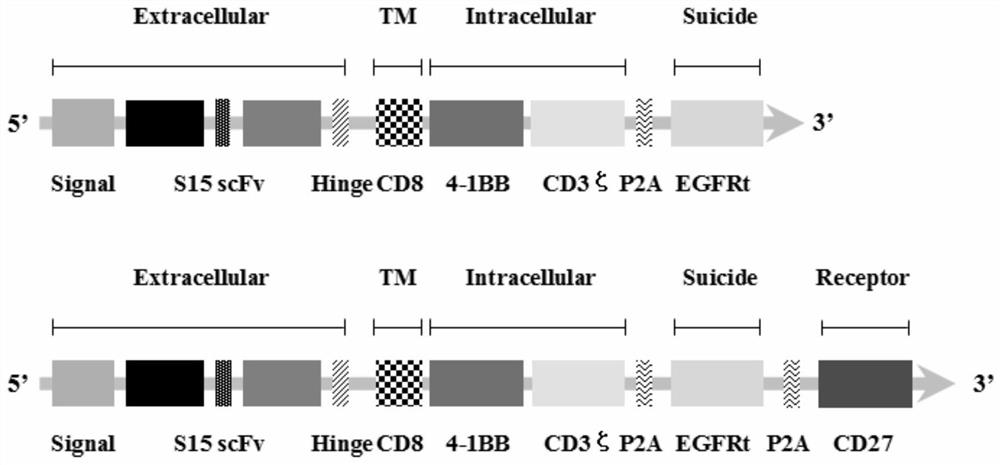
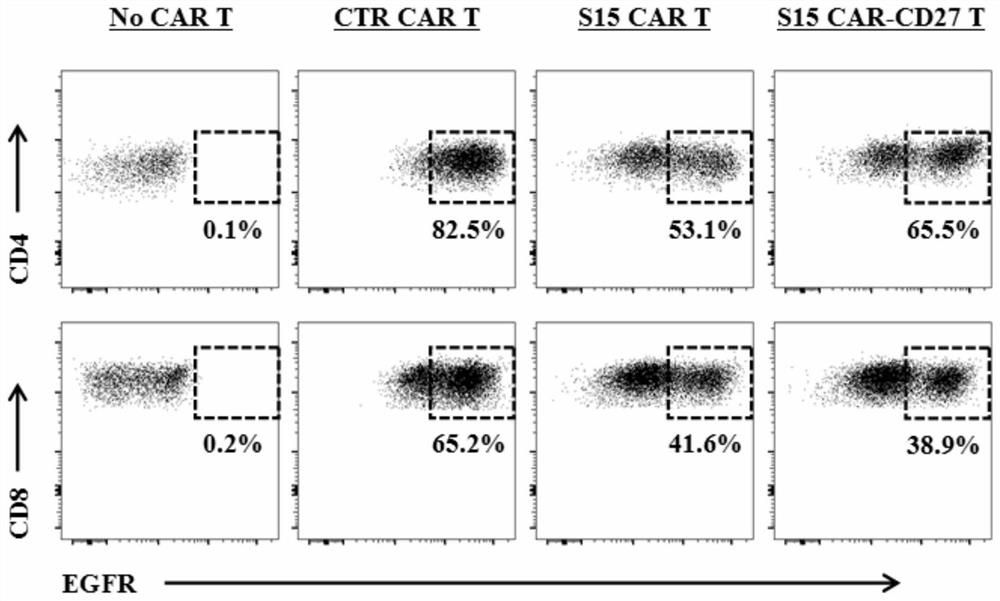
The invention discloses a chimeric antigen receptor T lymphocyte and an application thereof to preparation of a product for treating solid tumors. A chimeric antigen receptor in the chimeric antigen receptor T lymphocyte sequentially comprises a human CD8 lead peptide, an anti-Siglec-15 single-chain antibody, a human CD8 hinge transmembrane region, a human 4-1BB intracellular region, a human CD3 zeta intracellular region, a self-cleavage peptide, a CSF2Ra signal peptide, an EGFRt protein, a self-cleavage peptide and a human CD27. Experiments prove that the chimeric antigen receptor T lymphocyte provided by the invention highly expresses IFN gamma and CD107a, has a strong killing function on Siglec-15 positive tumor cells, and has killing efficiency of more than 80% under the condition thatthe effect-target ratio is 1: 1. A tumor transplantation model experiment shows that the chimeric antigen receptor T lymphocyte also has a strong killing function on Siglec-15 positive tumor cells inan animal body.
Owner:CARBIOGENE THERAPEUTICS CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com