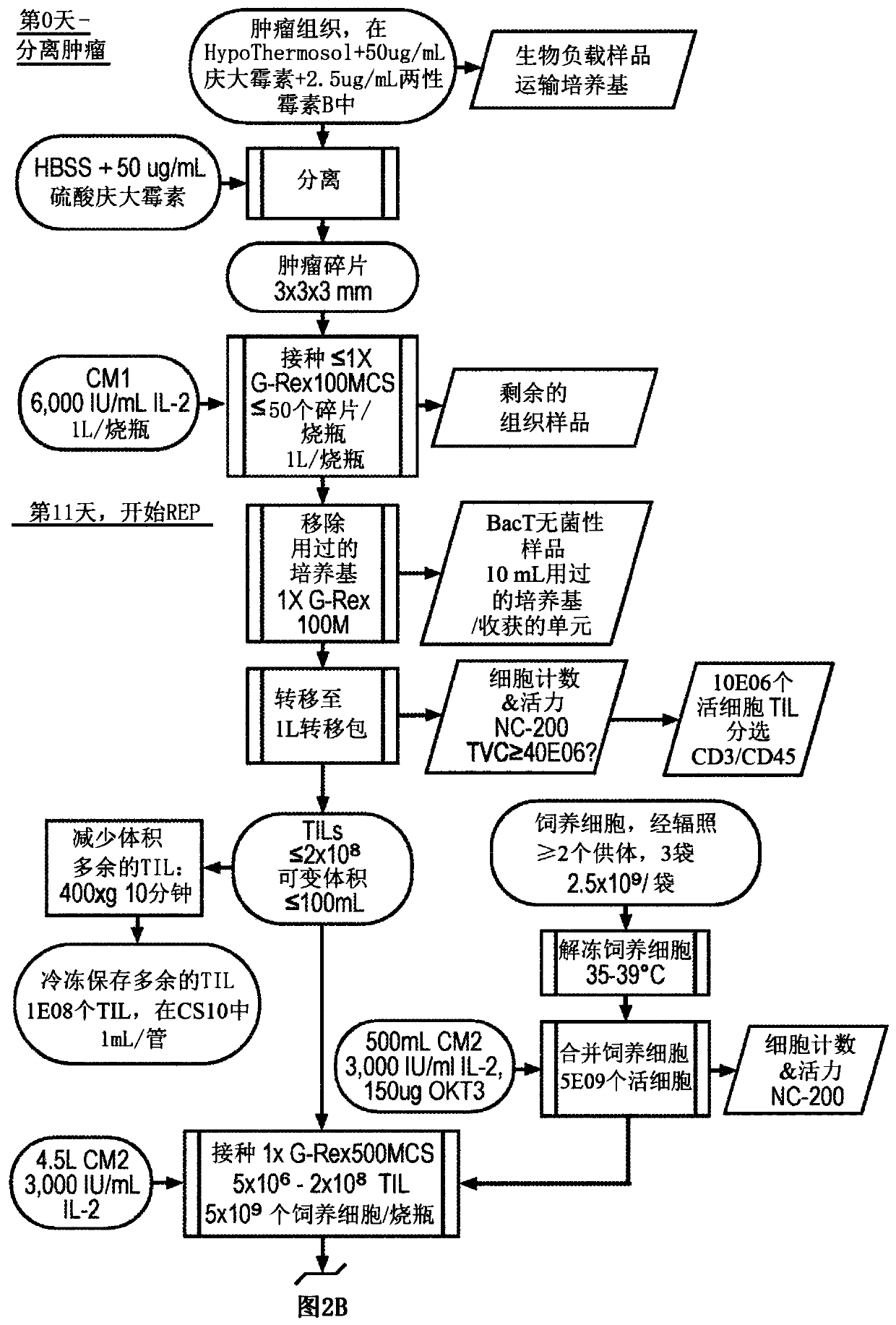
Til expansion from fine needle aspirates and small biopsies
A biopsy, IL-2 technology, applied in cell culture active agents, biochemical equipment and methods, microorganisms, etc., can solve problems such as the limitation of obtaining TIL
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1817] Example 1: Preparation of medium for PREP and REP processes
[1818] This example describes the procedure for preparing tissue culture medium for use in protocols involving the culture of tumor infiltrating lymphocytes (TILs) derived from a variety of tumor types including, but not limited to, metastatic melanoma, Head and neck squamous cell carcinoma (HNSCC), ovarian cancer, triple negative breast cancer and lung adenocarcinoma. This medium can be used to prepare any of the TILs described in this application and in the Examples.
[1819] Preparation of CM1
[1820] Remove the following reagents from refrigeration and warm them in a 37°C water bath: (RPMI1640, human AB serum, 200mML-glutamine). Prepare CM1 medium according to Table 32 below by adding each ingredient to the top of a 0.2 μm filter unit appropriate for the volume to be filtered. Store at 4°C.
[1821] Table 32: Preparation of CM1
[1822] Element final concentration Final volume 500mL ...
Embodiment 2
[1833] Example 2: Use of IL-2, IL-15 and IL-21 Cytokines Mixture
[1834] This example describes the use of IL-2, IL-15 and IL-21 cytokines as additional T cell growth in combination with the TIL method of the example factor.
[1835] Using the methods described herein, TILs were grown from colorectal, melanoma, cervical, triple-negative breast, lung, and kidney tumors in the presence of IL-2 in one experimental arm; and, in the other In one arm, IL-2 was replaced with a combination of IL-2, IL-15 and IL-21 at the beginning of the culture. At the completion of pre-REP, cultures were assessed for expansion, phenotype, function (CD107a+ and IFNγ) and TCR Vβ repertoire (TCR Vβ repertoire). IL-15 and IL-21 are described elsewhere herein and Gruijl et al., IL-21 promotes the expansion of CD27+CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells (IL-21 promotes CD27+CD28+ tumor infiltrating lymphocytes Expansion of ...
Embodiment 3
[1837] Example 3: Qualified individual batches of gamma-irradiated peripheral mononuclear cells
[1838] This example describes a novel, simplified procedure for qualifying individual batches of gamma-irradiated peripheral mononuclear cells (PBMC, also known as MNC) for use as isotypes in the exemplary methods described herein. Allogeneic feeder cells.
[1839] Each batch of irradiated MNC feeder cells was prepared from a single donor. Each lot or donor was screened individually for the ability to expand TILs in REPs in the presence of purified anti-CD3 (clone OKT3) antibodies and interleukin 2 (IL-2). Additionally, each batch of feeder cells was tested without the addition of TIL to verify that the dose of gamma radiation received was sufficient to render them replicatively incompetent.
[1840] background
[1841] REP of TILs requires gamma-irradiated growth-arrested MNC feeder cells. Membrane receptors on feeder MNCs bind to anti-CD3 (clone OKT3) antibodies and crosslin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com