Combined pharmaceutical preparation for treating melanoma, lung cancers or colorectal cancers
A pharmaceutical preparation and technology for colorectal cancer, applied in the field of tumor treatment, can solve the problems of insufficient efficacy of PD-L1 inhibitor single drug, narrow application range of cilengitide, low objective tumor remission rate, etc., and achieve enhanced anti-tumor efficacy , low risk of action, reduced tumor growth effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
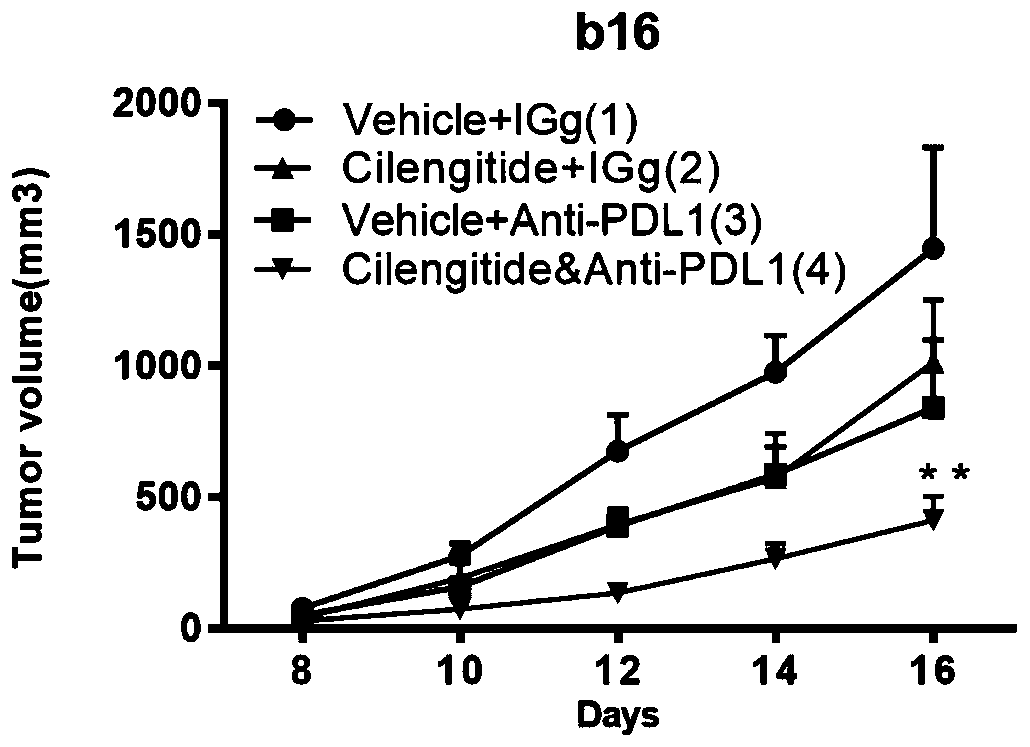
[0027] Forty-four 6-week-old female C57BL / 6 wild-type mice were selected, and each mouse was inoculated with 5×10 5 melanoma B16F10 cells, observed tumor formation 8 days later, and then divided them into control group (11), cilengitide group (11), PD-L1 antibody group (11) and cilengitide+ Anti-PDL-1 antibody combination group (11 rats). After grouping, the mice in the control group were given isotype control IgG, and the mice in the experimental group were given cilengitide (dissolved in PBS), 25 mg / Kg, once a day, intraperitoneally injected; or given anti-PDL-1 antibody, 200 μg each time (about 10mg / Kg), once every other day, a total of 4 administrations, intraperitoneal injection; or a combination of the two. After administration, the subcutaneous tumor size of each group of mice was measured every day, and the tumor volume was calculated (tumor volume=long diameter of tumor×short diameter of tumor) 2 / 2). On the ninth day after the administration, the mice in each grou...
Embodiment 2
[0031] At the end of the observation (the ninth day), one C57 was selected from the four groups in Example 1, and the peeled tumor tissue was fixed with 4% paraformaldehyde for 12 hours, and the fixed tissue was gradually treated with ethanol from low to high concentrations. Grade dehydration (50%, 75%, 85%, 95%, 100%), followed by three replacements of ethanol with xylene. Infiltrate the tissue quickly with paraffin wax with a melting point of 60 degrees. First trim the excess paraffin around the tissue block with a blade, and then place the sliced slices in a 42-degree oven for 2 hours. Next, the paraffin sections were dewaxed to water, soaked in environmental transparent agent (three cylinders 10min, 10min, 10min), gradient alcohol (anhydrous, 95%, 75%), 5min in each cylinder, then rinsed once with distilled water and soaked in in distilled water. After repairing with high temperature and high pressure, use 3% H2O2 to block endogenous peroxidase at room temperature for 2...
Embodiment 3
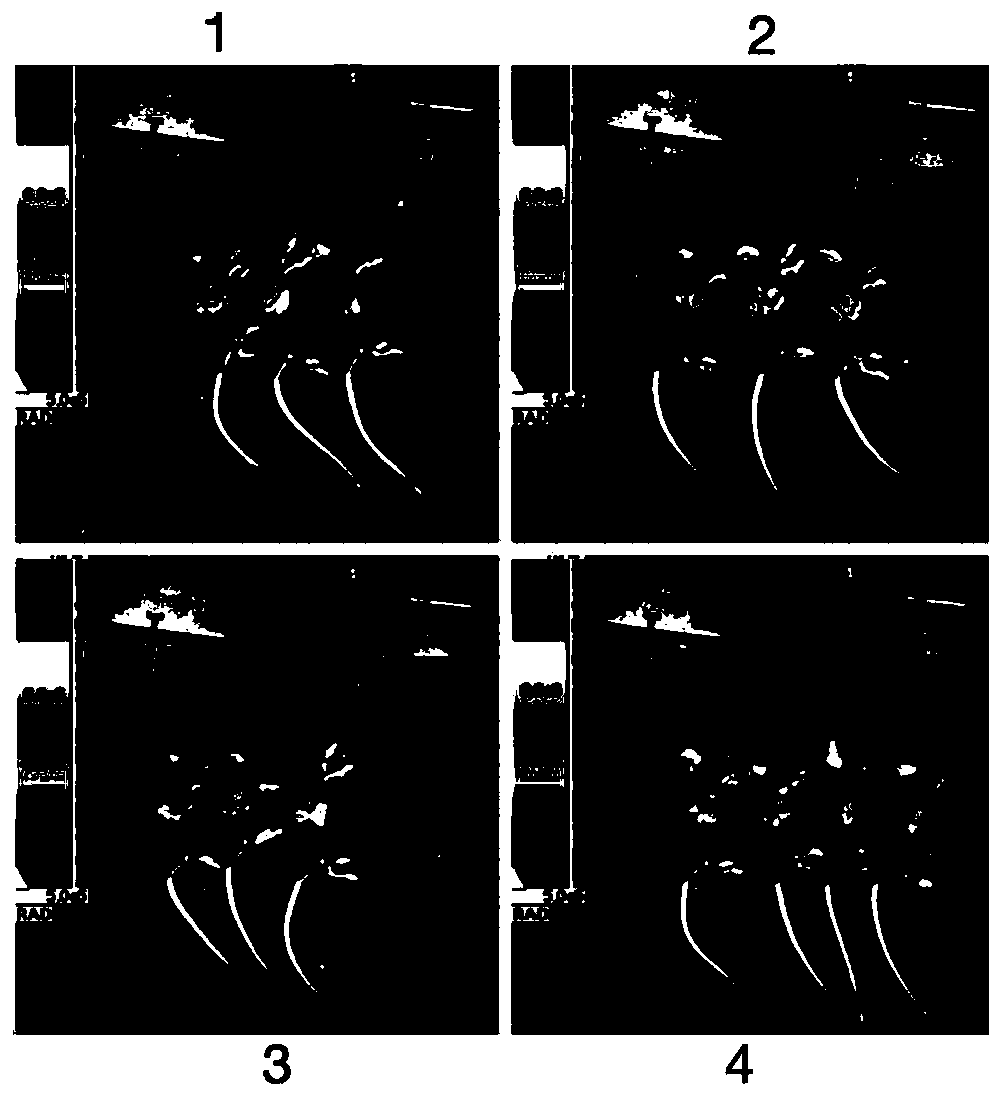
[0035] At the end point of observation (the ninth day), one C57 was selected from the four groups in Example 1, and the tumor tissue and spleen were peeled off to make a single cell suspension, and the lymphocytes in the tumor tissue were extracted with percoll, and the recommended dosage was used according to the instructions. Add fluorescently labeled antibodies, mix well, place at 4°C, and incubate in the dark for 30 minutes, add PBS containing 0.1% BSA to resuspend cells, and use flow cytometry for detection and analysis.
[0036] Such as Figure 8 As shown, the results of flow cytometry of the tumor tissues of the four groups of mice showed that CD3 in the tumor tissues of the mice in the combination group + CD8 + T cell infiltration was significantly increased. Such as Figure 9 As shown, CD3 in spleen tissue of four groups of mice + CD8 + There was no significant difference in T cell infiltration.
[0037] The above experiments confirmed that simultaneous administ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com