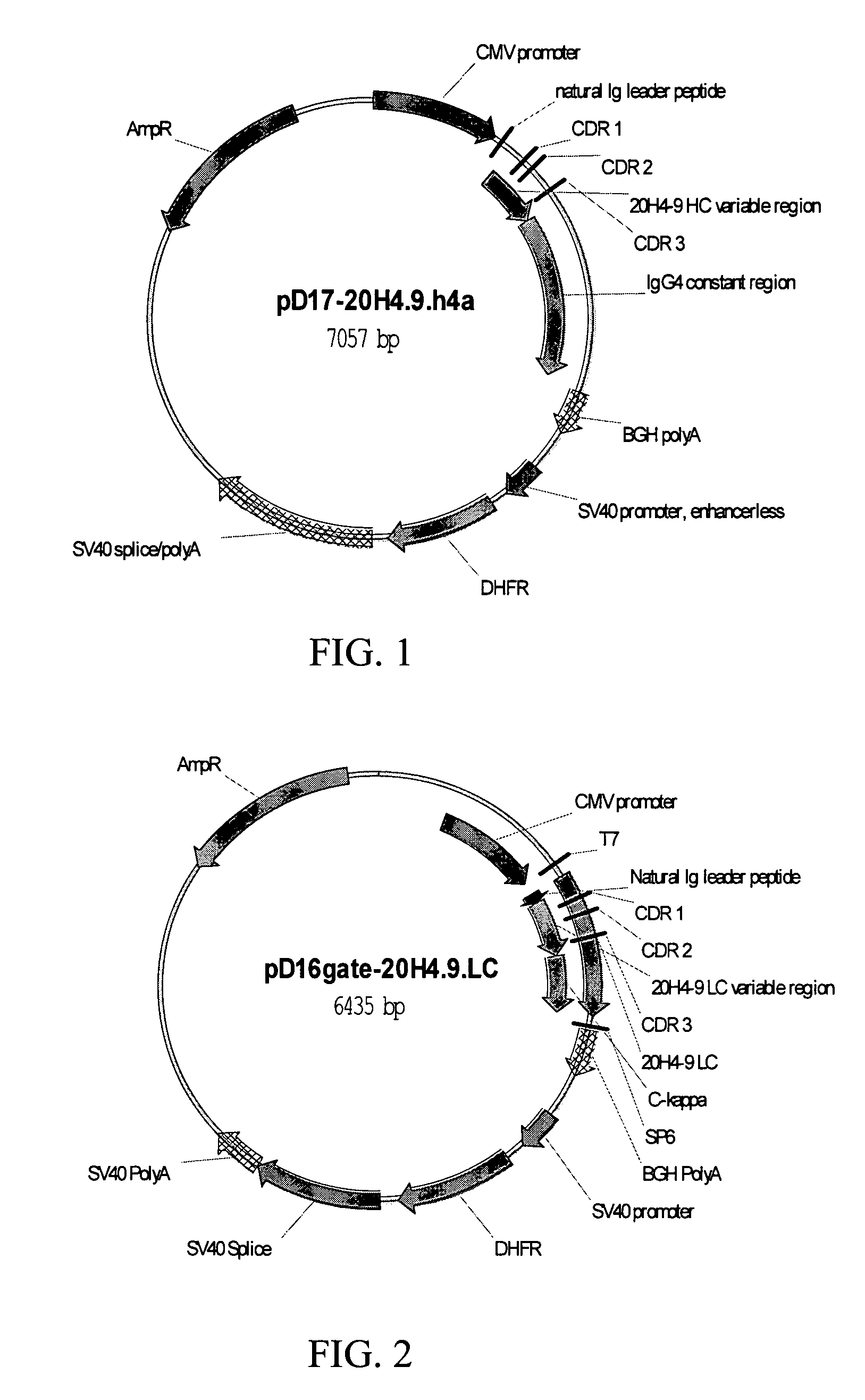
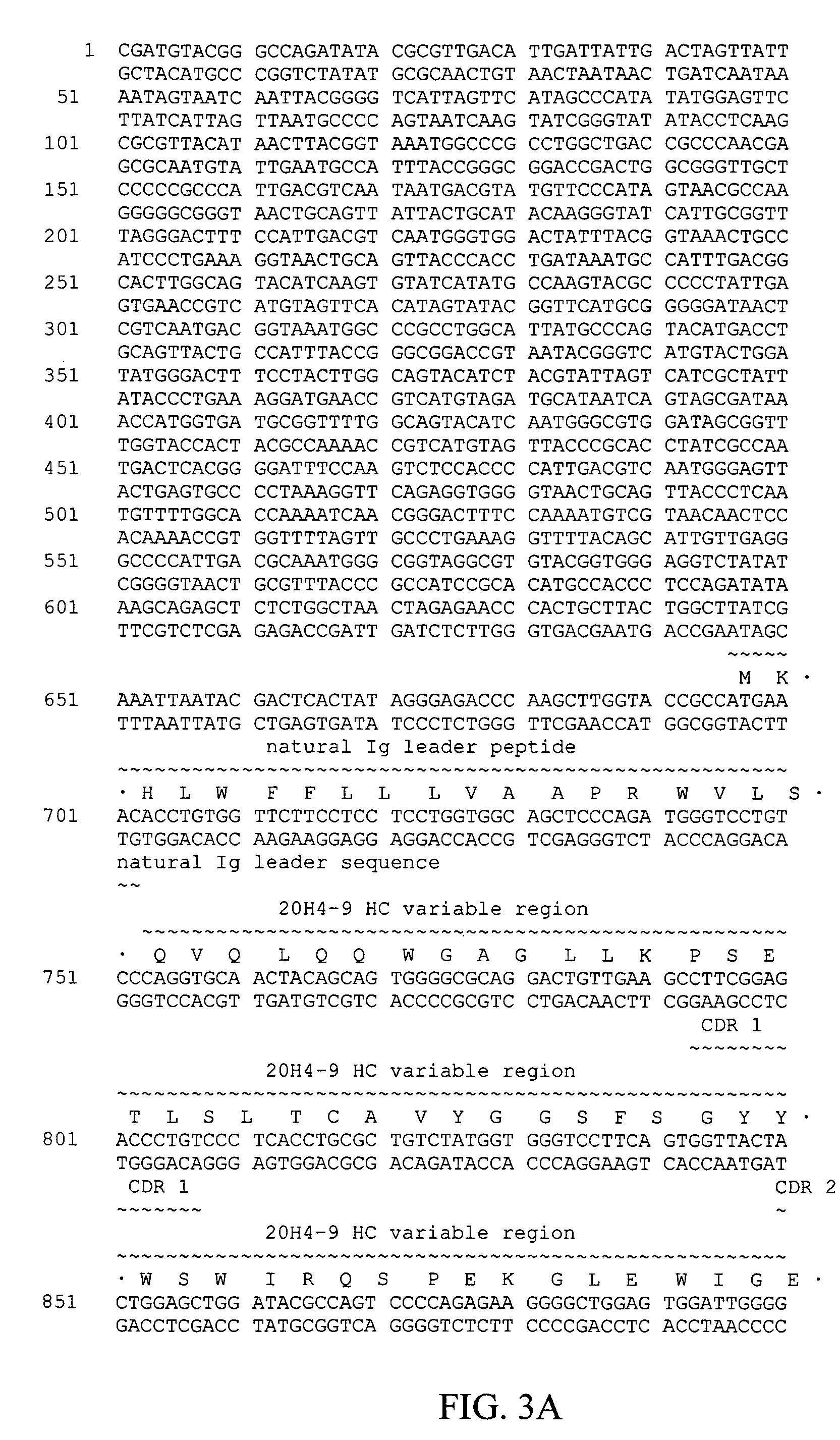
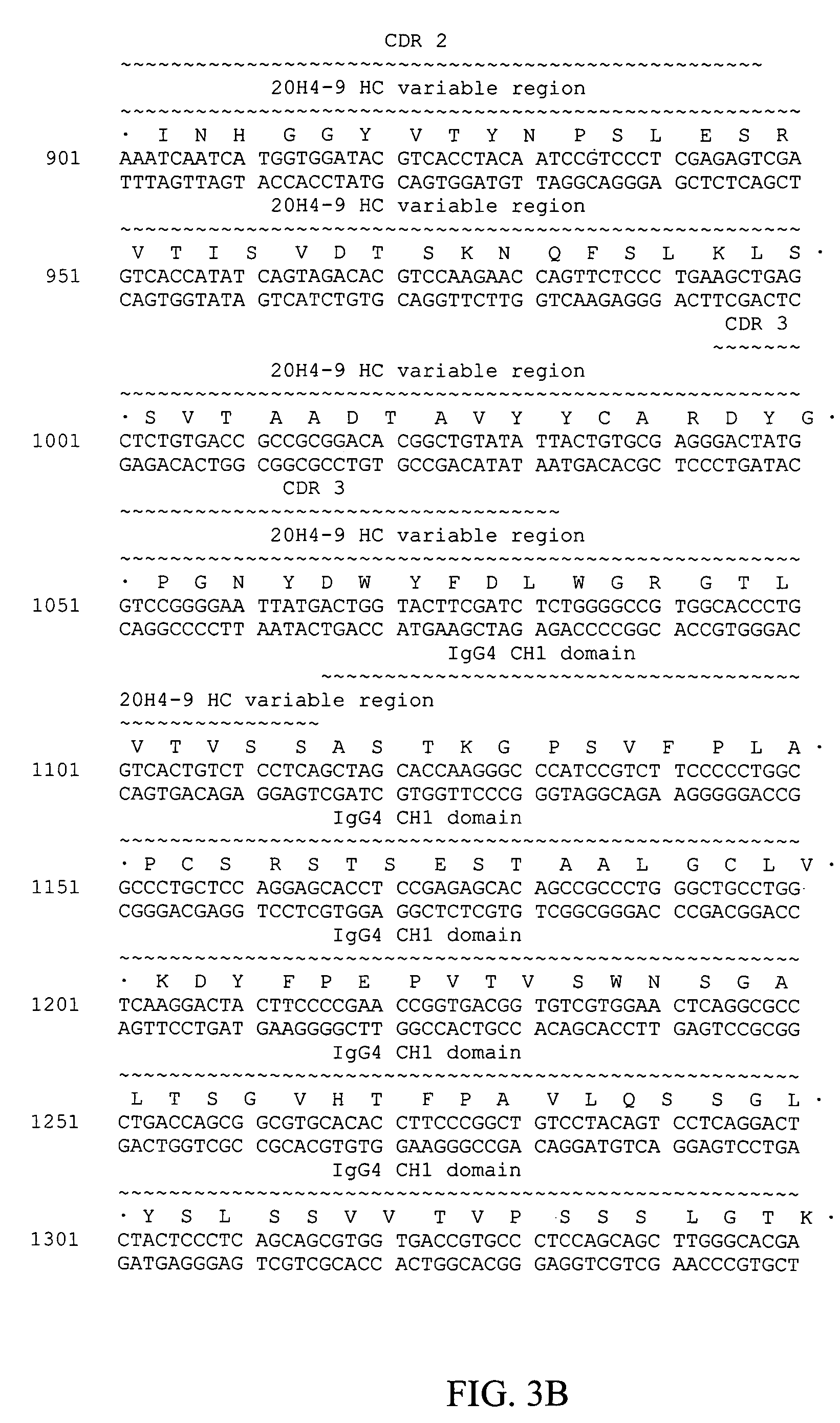
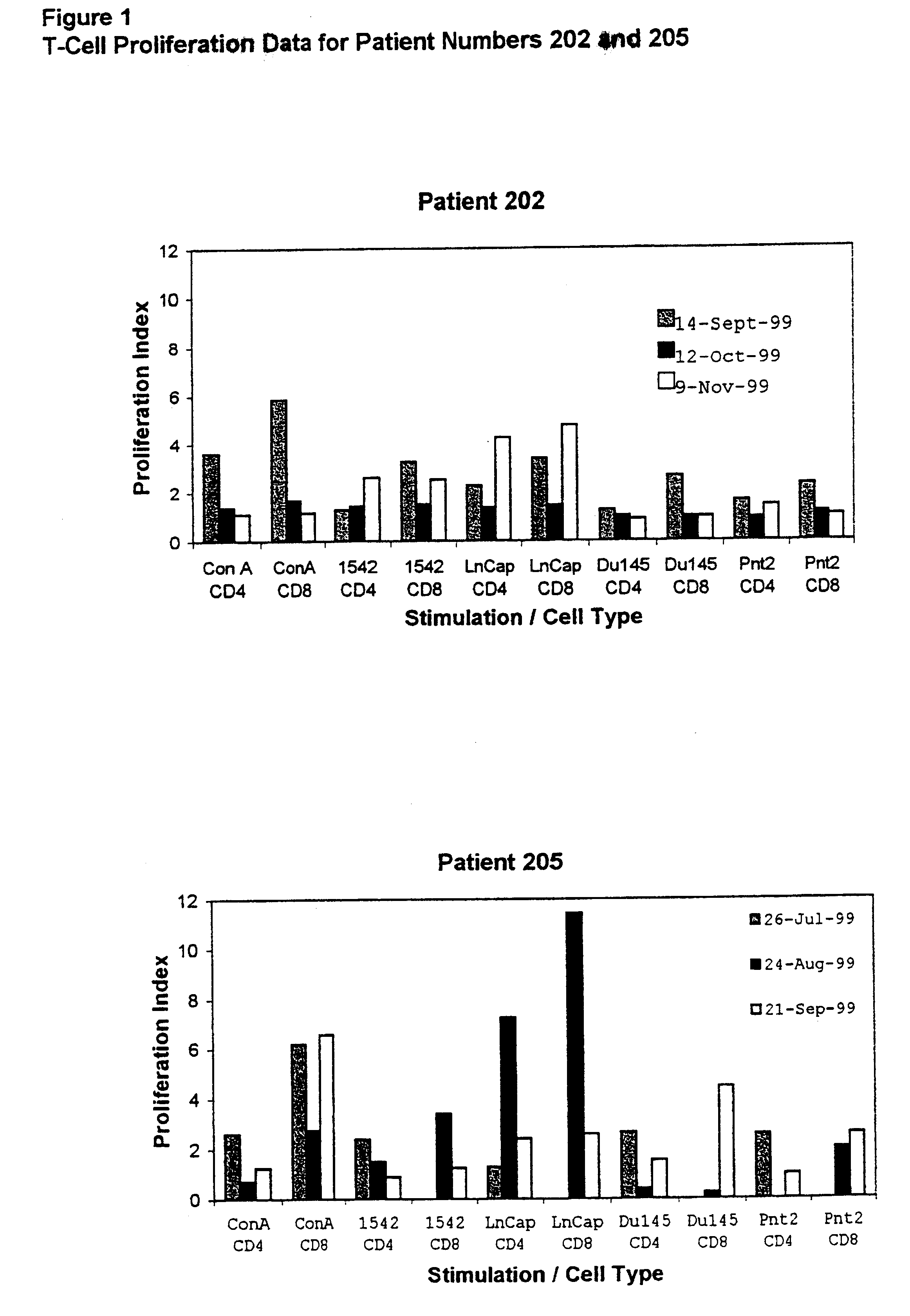
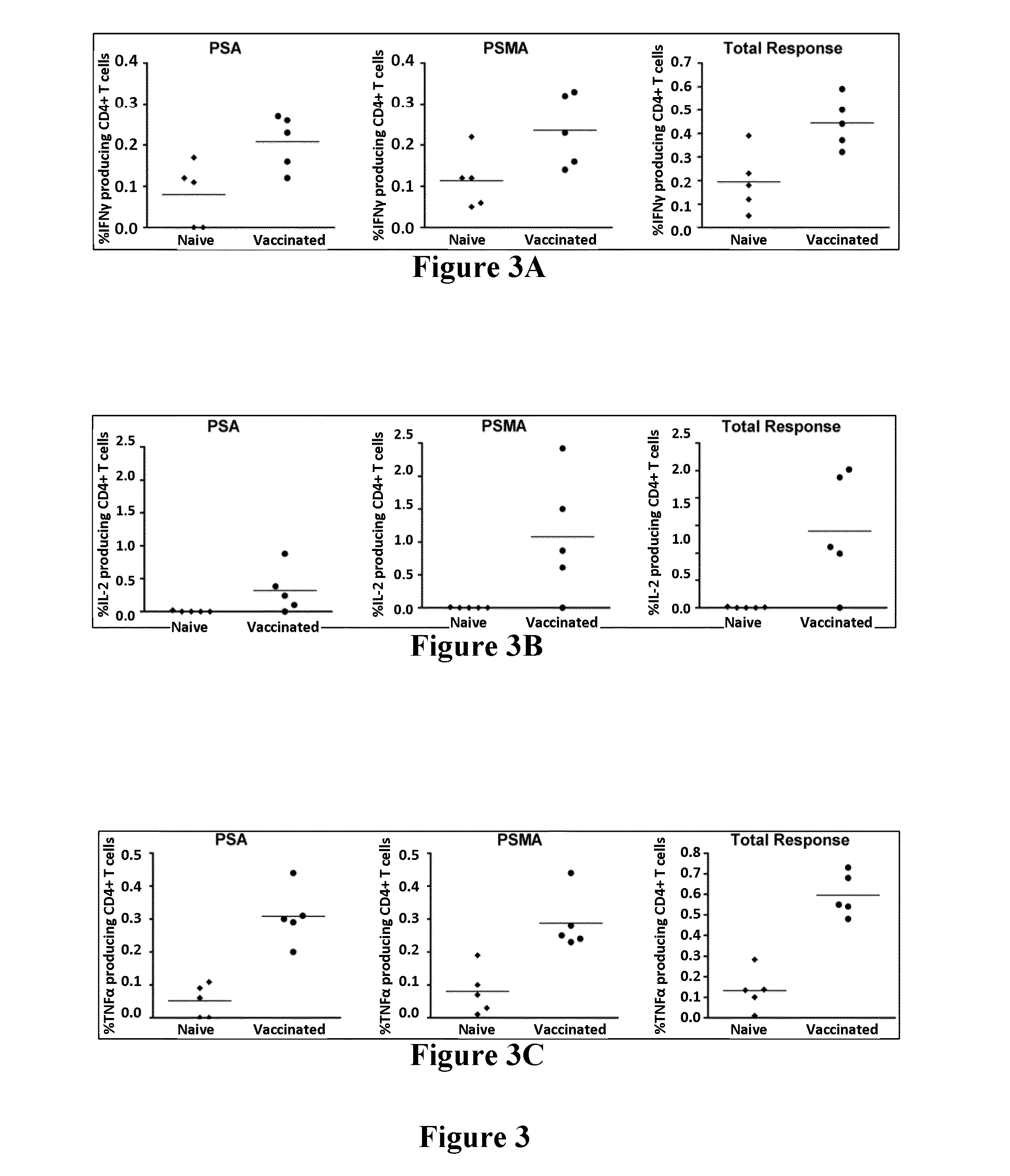
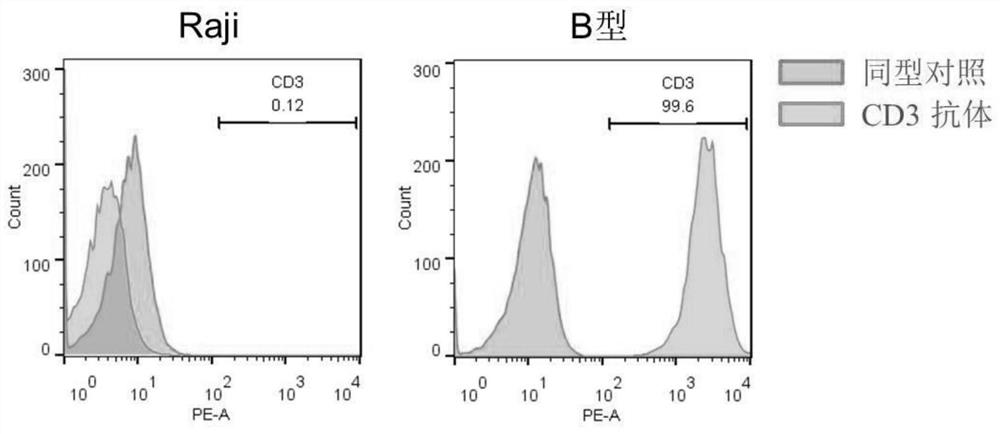
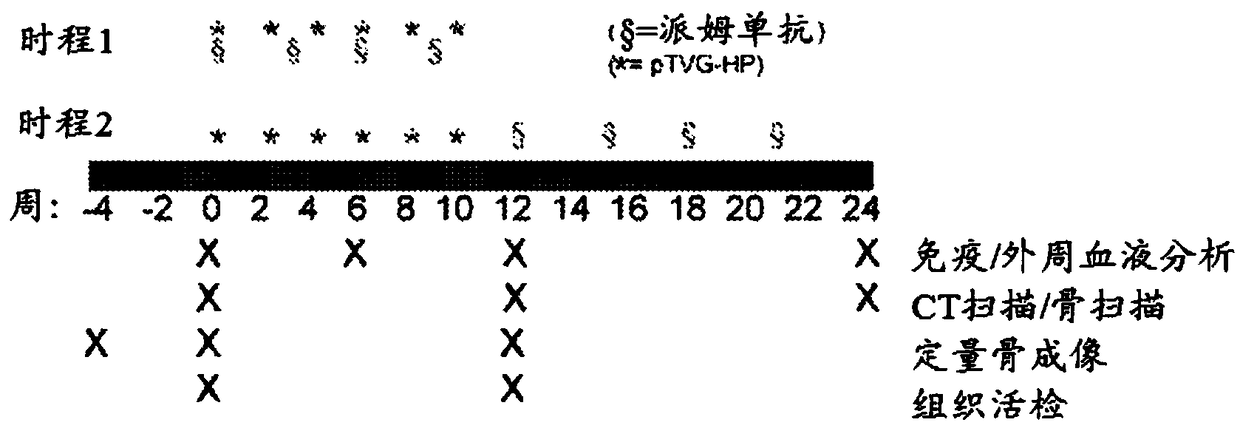
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89results about "Prostate cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fully human antibodies against human 4-1BB
Fully human antibodies and antigen-binding portions thereof that bind to human 4-1BB and that allow binding of human 4-1BB to a human 4-1BB ligand. In one aspect, the antibody is an IgG4 antibody. Also provided is a method for treating a disease in a subject comprising administering a therapeutically effective amount of the antibody to said subject.
Owner:BRISTOL MYERS SQUIBB CO
Cancer treatments
The invention relates to a product comprised of specific combinations of cell lines intended for use as an allogeneic immunotherapy agent for the treatment of prostate cancer in humans. The heterogeneity of the immunotherapeutic matches the heterogeneity of the antigenic profile in the target prostate cancer and immunises the recipients with many of the potential TAA and TSA which are expressed at various stages of the disease. The invention discloses a vaccine comprising a combination of three different cell lines prepared from primary or metastatic prostate cancer biopsy material. The cell lines are lethally irradiated utilising gamma irradiation at 50-300 Gy to ensure that they are replication incompetent.
Owner:ONYVAX
Use of human prostrate cell lines in prostate cancer treatment
InactiveUS6972128B1Promote growthSufficient characteristicBiocidePeptide/protein ingredientsGamma irradiationProstate cancer
The invention here relates to a product comprised of a cell line or lines intended for use as an allogeneic immunotherapy agent for the treatment of cancer in mammals and humans. All of the studies of cell-based cancer vaccines to date have one feature in common, namely the intention to use cells that contain at least some TSAs and / or TAAs that are shared with the antigens present in patients' tumour. In each case, tumour cells are utilised as the starting point on the premise that only tumour cells will contain TSAs or TAAs of relevance, and the tissue origins of the cells are matched to the tumour site in patients. A primary aspect of the invention is the use of immortalised normal, non-malignant cells as the basis of an allogeneic cell cancer vaccine. Normal cells do not possess TSAs or relevant concentrations of TAAs and hence it is surprising that normal cells are effective as anti-cancer vaccines. For prostate cancer, for example, a vaccine may be based on one or a combination of different immortalised normal cell lines derived from the prostate. The cell lines are lethally irradiated utilising gamma irradiation at 50–300 Gy to ensure that they are replication incompetent prior to use in the mammal or human.
Owner:ONYVAX
Extracellular matrix cancer vaccine adjuvant
ActiveUS20100233214A1Cancer antigen ingredientsImmunological disordersCell-Extracellular MatrixAdjuvant
Compositions suitable for use as adjuvants in the preparation of vaccines, particularly those vaccines useful in the treatment of cancer, are provided. Methods for inhibiting tumor growth in an animal are also disclosed. Methods for immunizing an animal against cancer, such as prostate cancer, are also described. The adjuvants described are comprised of an extracellular matrix material, such as small intestinal submucosal (SIS) tissue. The preparations may take the form of sheets, gels, liquids (injectable), trocar, or other solid or semi-solid preparation. The invention provides for enhanced tumor inhibition of 2-fold or greater, compared to vaccine preparations without the extracellular matrix material, or from 4- to 5-fold, compared to preparations without the adjuvant promoting extracellular materials.
Owner:UNIV OF NOTRE DAME DU LAC
Prostate cancer vaccine
Androgen receptor-based vaccines for eliciting an immune reaction in vivo against cells expressing androgen receptor are disclosed. The vaccines are useful in the treatment of prostate cancer. Also disclosed are methods for inducing immune reaction to androgen receptor or treating prostate cancer in a mammal, using the vaccines and pharmaceutical compositions comprising the vaccines.
Owner:WISCONSIN ALUMNI RES FOUND
Methods and Compositions for Treating Prostate Cancer Using DNA Vaccines
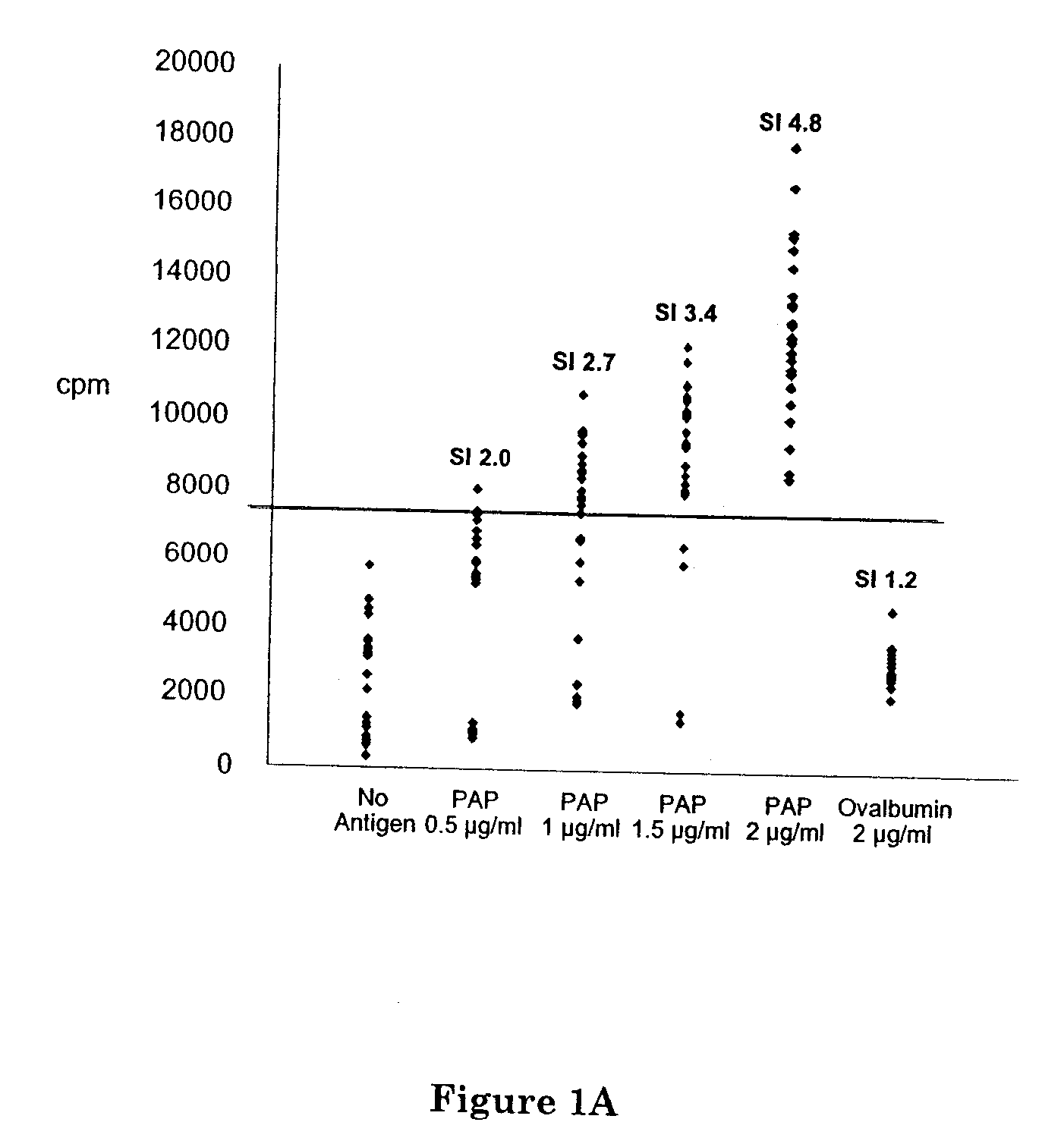
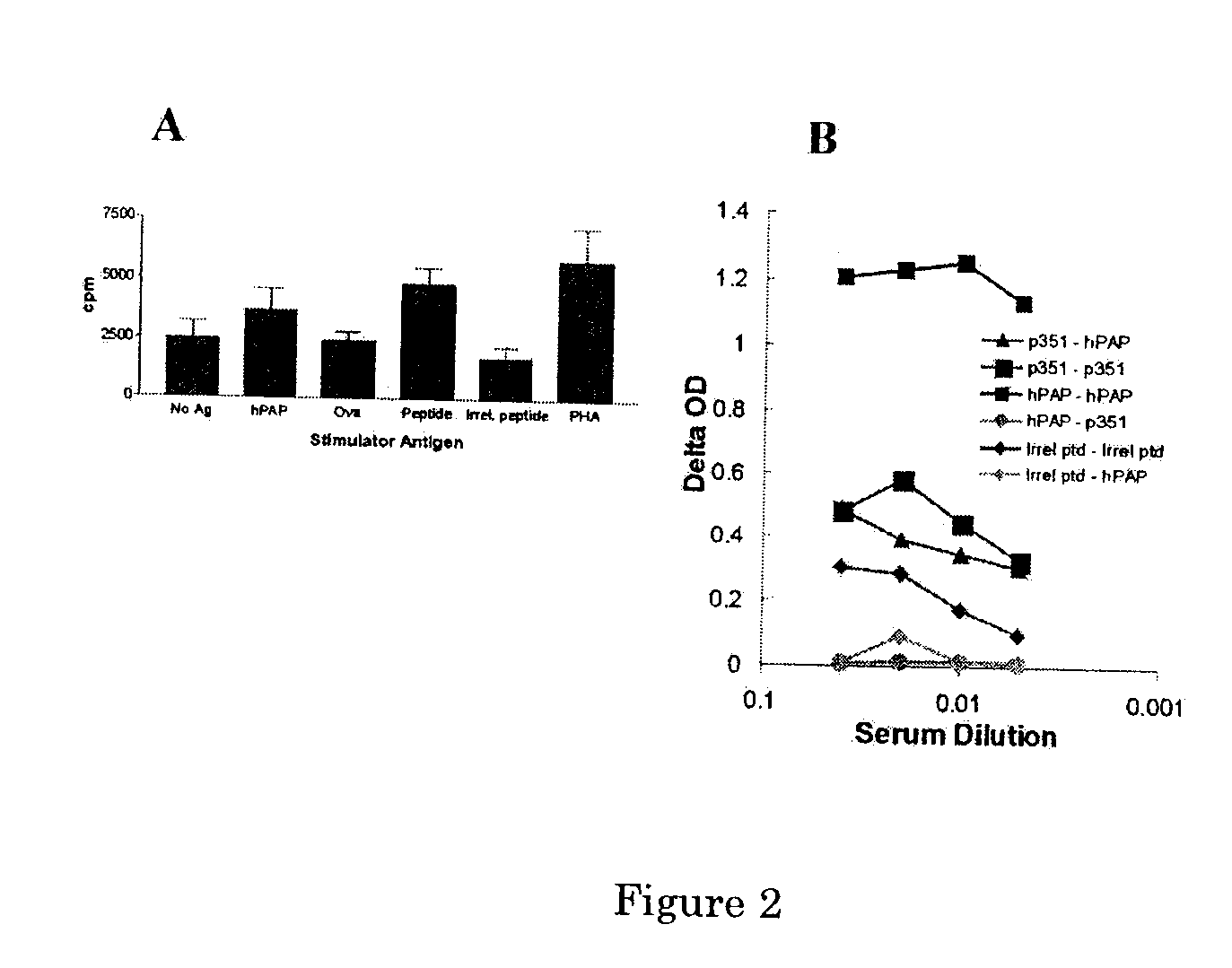
A DNA vaccine for the treatment of prostate cancer, comprising a plasmid vector comprising a nucleotide sequence encoding prostatic acid phosphatase (PAP) operably linked to a transcription regulatory element, wherein upon administration to a mammal a cytotoxic immune reaction against cells expressing PAP is induced. In preferred embodiment, the PAP encoded is a xenoantigen highly homologous to the autoantigen PAP of the mammal. Also disclosed are methods for inducing prostatitis, or inducing immune reaction to PAP, or treating prostate cancer in a mammal, using the DNA vaccine and pharmaceutical compositions comprising the vaccine. Preferably, xenoantigen vaccination is followed by boosting with autoantigen PAP from the same animal species as the mammal being treated.
Owner:WISCONSIN ALUMNI RES FOUND
Consensus prostate antigens, nucleic acid molecule encoding the same and vaccine and uses comprising the same
ActiveUS8927692B2Organic active ingredientsTumor rejection antigen precursorsProstate cancer cellAntigen
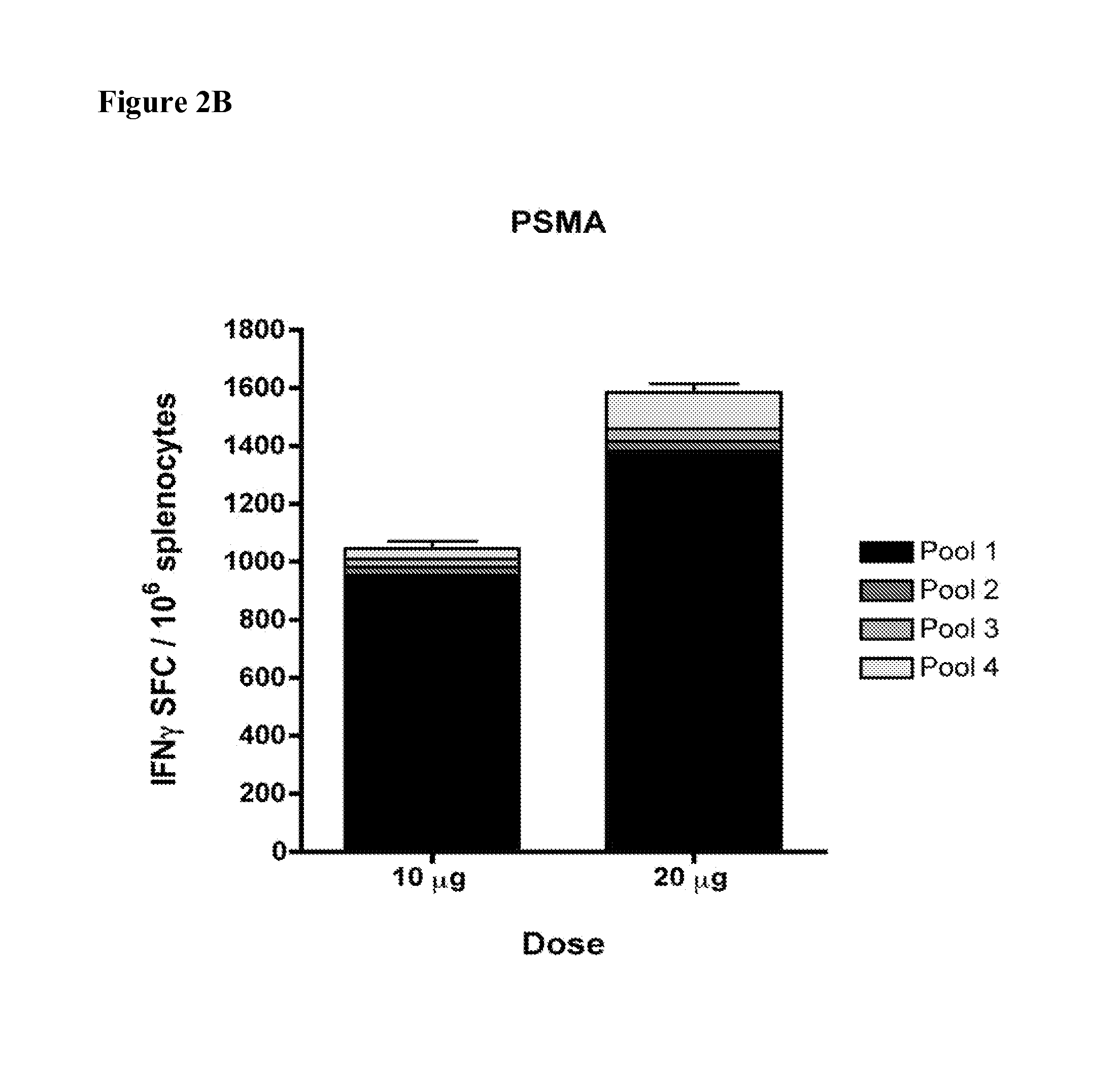
Provided herein are consensus amino acid sequences of prostate antigens that are capable of breaking tolerance in a targeted species, including PSA, PSMA, STEAP and PSCA antigens. Also provided are nucleic acid sequences that encode one or more consensus amino acid sequences of prostate antigens PSA, PSMA, STEAP and PSCA, as well genetic constructs / vectors and vaccines expressing the sequences. Also provided herein are methods for generating an autoimmune response against prostate cancer cells by administering one or more of the vaccines, proteins, and / or nucleic acid sequences that are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Biomarkers for prostate cancer and methods for their detection
InactiveUS20130156813A1Good curative effectExcessive amountSugar derivativesDepsipeptidesProstate cancerOncology
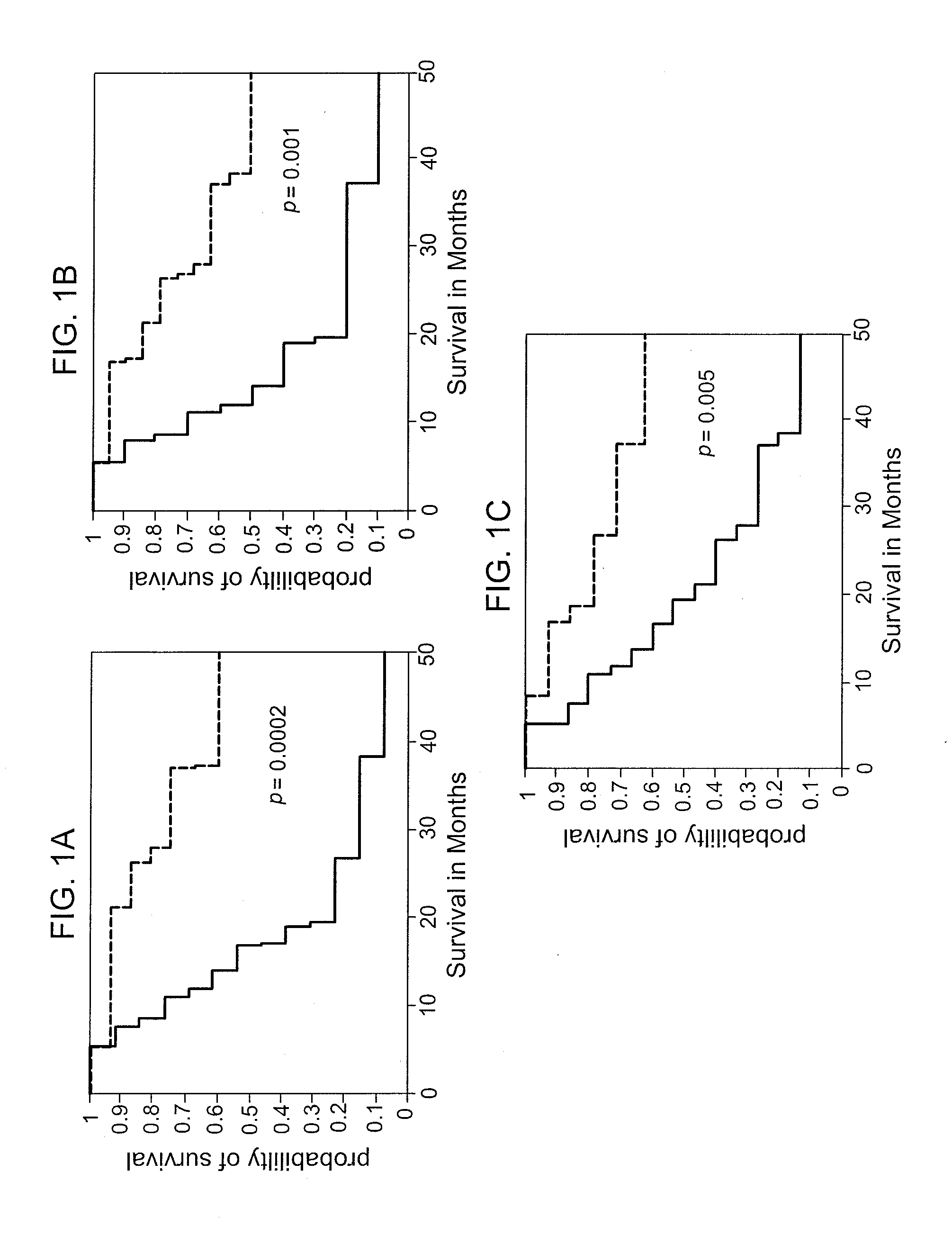
The invention provides a method for predicting the clinical response to a cancer vaccine in a patient having cancer, a method for determining the immune response to a cancer vaccine in a patient having cancer who has been administered a cancer vaccine, a method for determining the long-term survival in a patient having cancer, corresponding kits therefor, as well as methods of for improving the efficacy of a virus-based vaccine.
Owner:UNITED STATES OF AMERICA
Chitosan-Derived Compositions
InactiveUS20190002594A1Organic active ingredientsPhotodynamic therapyMedical disorderKidney Neoplasm
The present invention relates generally to therapeutic compositions comprising chitosan-derived compositions used in connection with methods for treating neoplasms, such as for instance, malignant lung, thyroid and kidney neoplasms, and other types of malignant neoplasms, and other medical disorders.
Owner:IMMUNOPHOTONICS INC
Consensus Prostate Antigens, Nucleic Acid Molecule Encoding The Same And Vaccine And Uses Comprising The Same
ActiveUS20150150957A1Organic active ingredientsTumor rejection antigen precursorsAntigenProstate cancer cell
Provided herein are consensus amino acid sequences of prostate antigens that are capable of breaking tolerance in a targeted species, including PSA, PSMA, STEAP and PSCA antigens. Also provided are nucleic acid sequences that encode one or more consensus amino acid sequences of prostate antigens PSA, PSMA, STEAP and PSCA, as well as genetic constructs / vectors and vaccines expressing the sequences. Also provided herein are methods for generating an autoimmune response against prostate cancer cells by administering one or more of the vaccines, proteins, and / or nucleic acid sequences that are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Chitosan-Derived Compositions
The present invention relates generally to therapeutic compositions comprising chitosan-derived compositions used in connection with methods for treating neoplasms, such as for instance, malignant lung, thyroid and kidney neoplasms, and other types of malignant neoplasms, and other medical disorders.
Owner:IMMUNOPHOTONICS INC
Specific chimeric antigen receptor T cell targeting nkg2dl, preparation method and application thereof
ActiveCN109803983BEffective targeted attackHigh kill rateVirusesAntibody mimetics/scaffoldsAbnormal tissue growthAntigen receptors
A specific chimeric antigen receptor targeting NKG2DL, its coding sequence, and modified immune response cells, as well as their preparation method and application. The modified immune response cells can effectively target and attack various tumor cells, especially positive tumor cells expressing NKG2DL, and can be used to prepare preparations for treating tumors.
Owner:NANJING KAEDI BIOTHERAPEUTICS LTD
Cancer therapy
PendingCN108883203APersistent PAP-specific immunityGenetic material ingredientsAntibody ingredientsProstate cancerCancer diet therapy
Owner:MADISON VACCINES INC
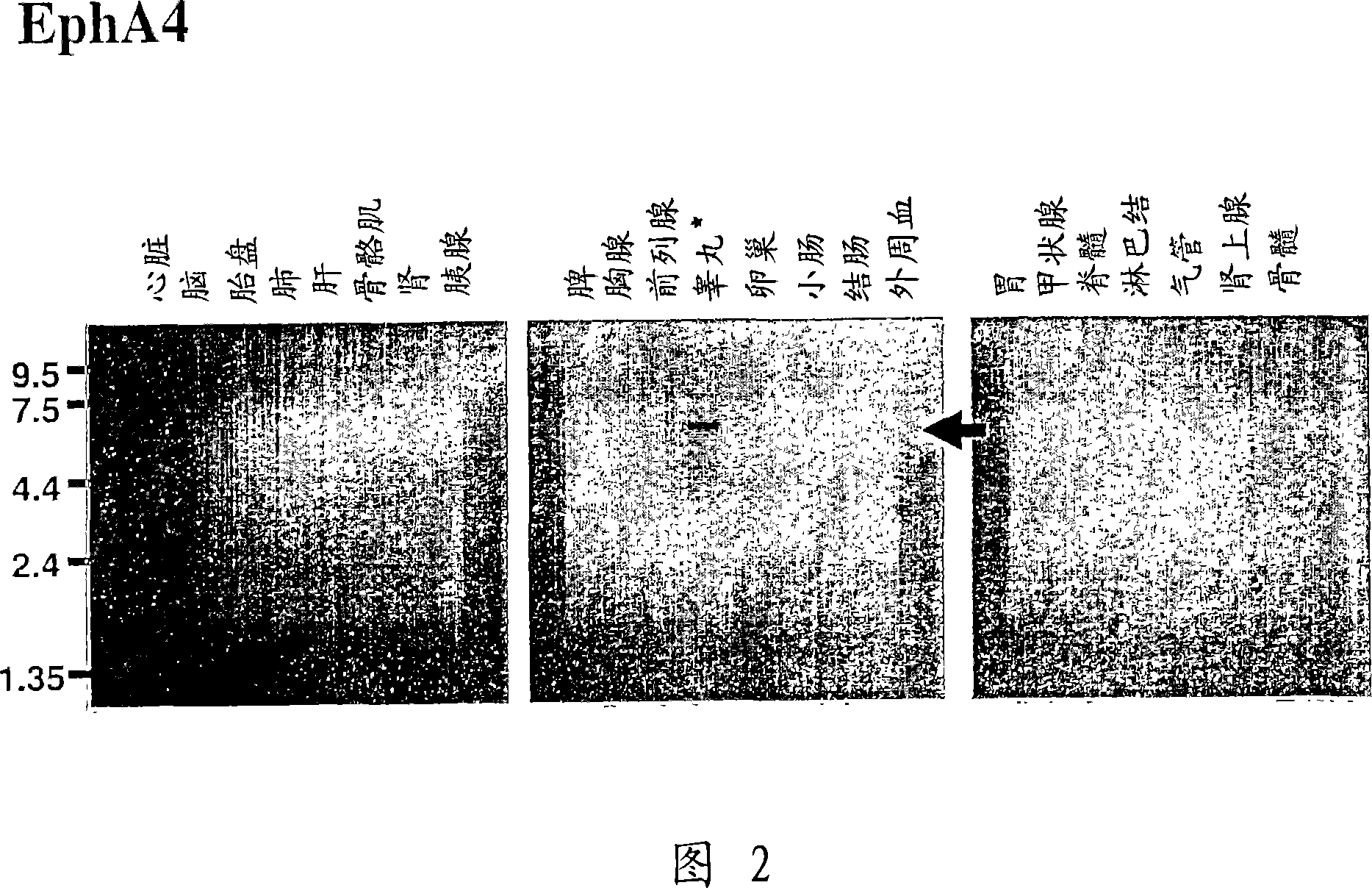
EphA4 as therapeutic target of PRC and PDACa
Objective methods for diagnosing a predisposition to developing prostate cancer (PRC) are described herein. In one embodiment, the diagnostic method involves the determining a expression level of EphA4. The present invention further provides methods of screening for therapeutic agents useful in the treatment of PRC, methods of treating PRC. The invention also features a method for inhibiting growth of a cancer cell by contacting the cell with a composition of a siRNA of EPHA4. Methods of treating cancer are also within the invention. The invention also features products, including nucleic acid sequences and vectors as well as to compositions comprising them, useful in the provided methods. The invention also provides a method for inhibiting of tumor cell, for example pancreatic cancer cell, particularly pancreatic ductal adenocarcinoma (PDACa).
Owner:ONCOTHERAPY SCI INC
Peripheral blood TCR marker for prostate cancer, and detection kit and application thereof
PendingCN111679074AImprove featuresImprove accuracyBiological material analysisAntibody medical ingredientsProstate cancerOncology
The invention discloses a peripheral blood TCR marker for prostate cancer, and a detection kit and application of the peripheral blood TCR marker. The peripheral blood TCR marker comprises at least one of proteins of which the sequences are shown as SEQ ID NO. 1-100. The peripheral blood TCR marker, the detection kit and the application thereof are based on a high-throughput sequencing method, only a small amount of peripheral blood needs to be collected for extracting RNA, an immune map library is established by treating samples, a characteristic TCR sequence in prostate cancer peripheral blood is firstly determined through high-throughput sequencing and TCR data analysis, and then a test result of a sample to be tested is compared with the characteristic TCR sequence so as to determine whether a patient has prostate cancer or not. The peripheral blood TCR marker can simultaneously compare a huge number of prostate cancer specific TCR sequences, has higher specificity and accuracy compared with single detection of one or more markers, and improves the diagnosis efficiency.
Owner:CHENGDU EXAB BIOTECH CO LTD
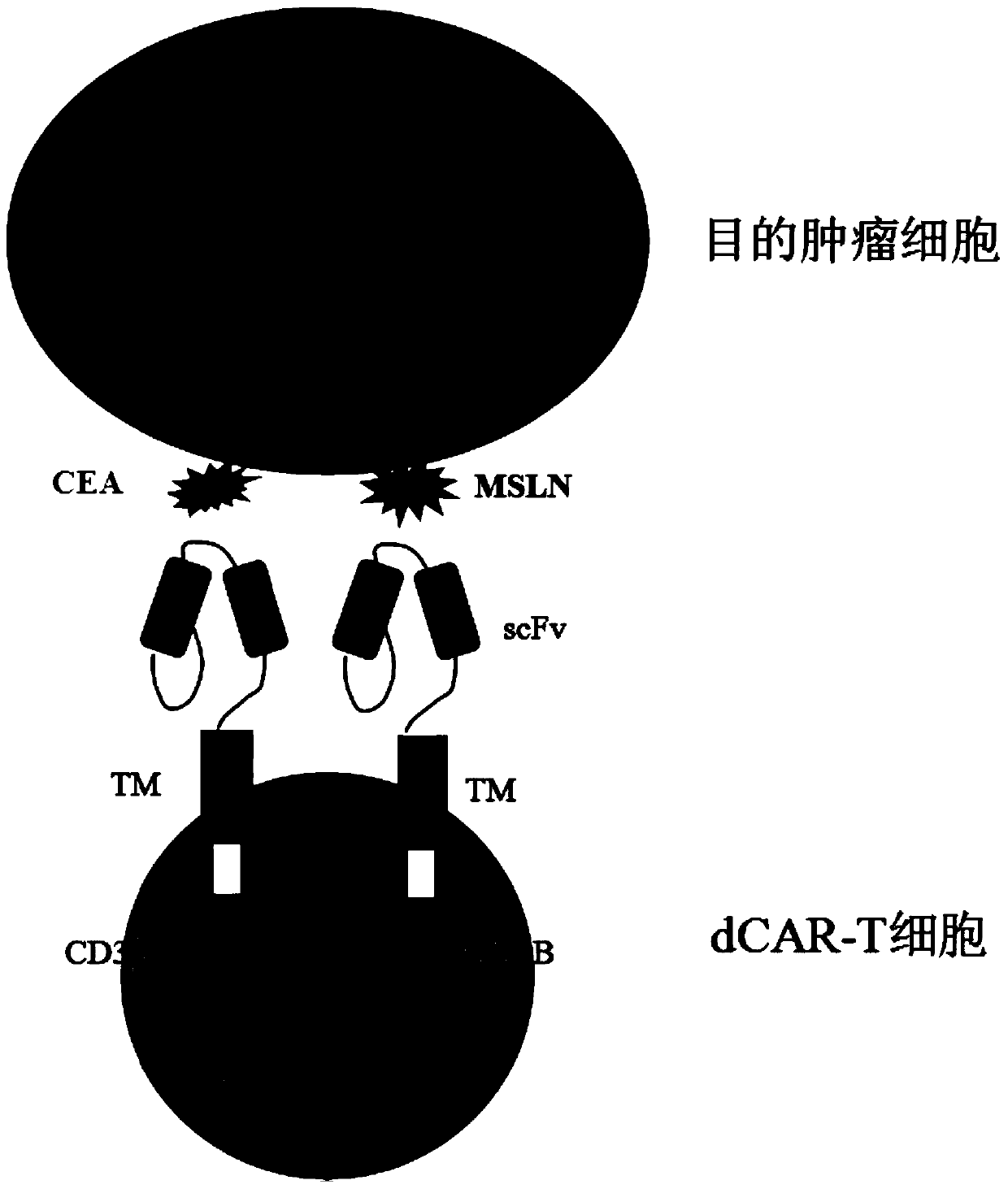
Double-chimeric antigen receptor, T cell and construction method and application thereof
PendingCN110669138AToxicityImprove securityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsTumor antigenSialyl tn
The invention discloses a double-chimeric antigen receptor, a T cell and a construction method and an application thereof, which belong to the field of cellular immunotherapy of tumors. The inventionspecifically relates to a specific structure and a construction method of the double chimeric antigen receptor T cell (dCAR-T cell), and preliminarily discusses the in-vivo and in-vitro activity of the dCAR-T cell. The selected tumor-associated antigens are mesothelin and carcino-embryonic antigens, and researches show that the two tumor antigens can be simultaneously expressed on the surface of asolid tumor, such as pancreatic cancer. The invention discloses an antigen receptor. The in-vitro and in-vivo tests prove that the constructed dCAR-T cell can be permanently and effectively activatedonly under the condition that two antigens are simultaneously recognized, and has efficient anti-tumor activity, so that a specific tumor killing function can be exerted, and the application of CAR-Tcell immunotherapy is improved.
Owner:CHINA PHARM UNIV
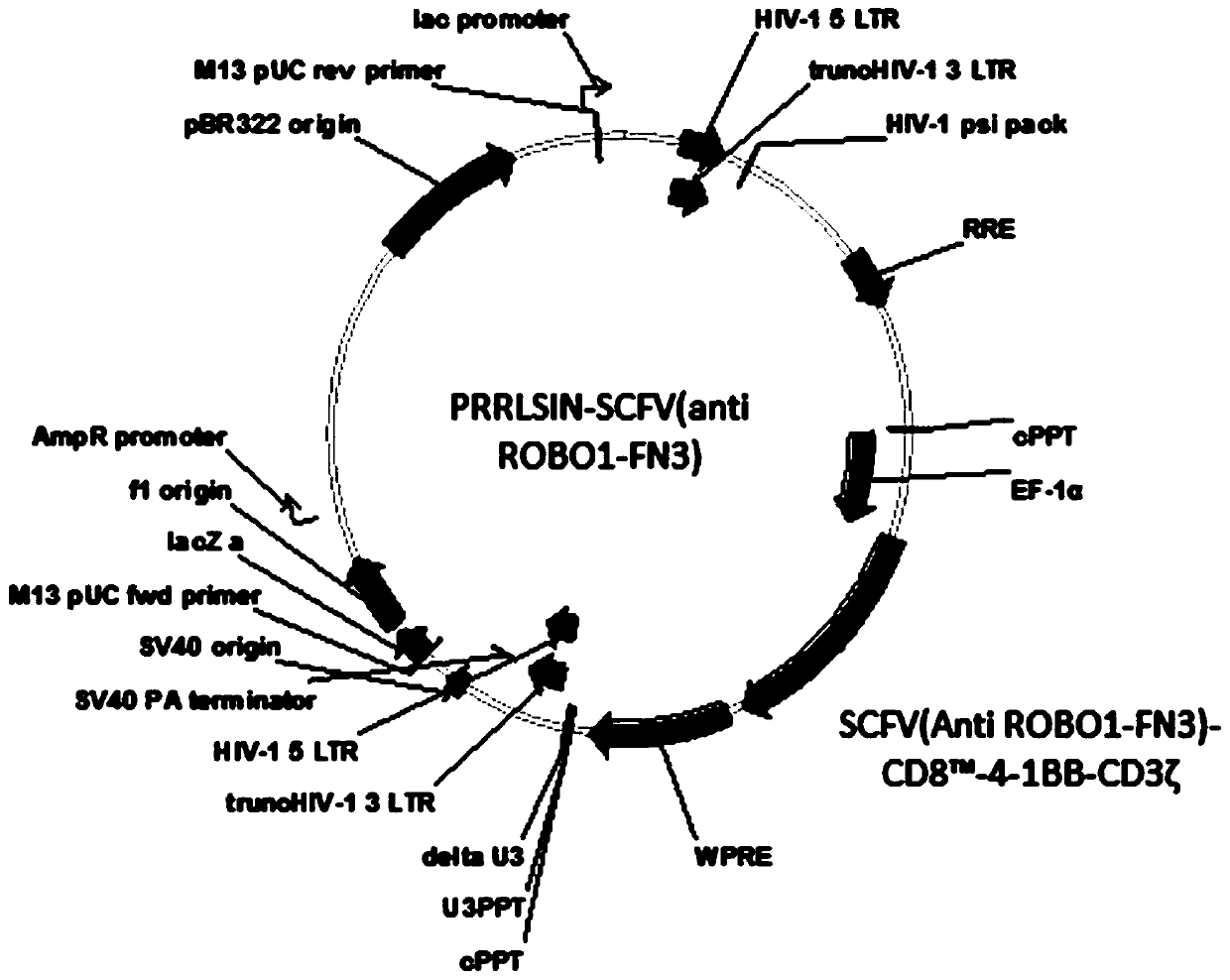
ROBO1 CAR-NK cell carrying suicide gene as well as preparation method and application of ROBO1 CAR-NK cell
PendingCN111269925ASmall side effectsGood killing effectVirusesHydrolasesNatural Killer Cell Inhibitory ReceptorsROBO1
The invention discloses an ROBO1 CAR-NK cell carrying a suicide gene as well as a preparation method and application of the ROBO1 CAR-NK cell. In order to improve the safety and controllability of theCAR-NK therapy, a suicide gene switch element is integrated into a genome through a lentiviral transfection technology on the basis of the current ROBO1 CAR-NK cell to form the CAR-NK with the suicide gene. By adding the suicide gene, the CAR-NK cells can be better controlled, and the clinical safety is further improved.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
Use of human prostate cell lines in cancer treatment
InactiveUS20050249756A1Promote growthSufficient characteristicBacterial antigen ingredientsPeptide/protein ingredientsGamma irradiationHuman prostate
The invention here relates to a product comprised of a cell line or lines intended for use as an allogeneic immunotherapy agent for the treatment of cancer in mammals and humans. All of the studies of cell-based cancer vaccines to date have one feature in common, namely the intention to use cells that contain at least some TSAs and / or TAAs that are shared with the antigens present in patients' tumour. In each case, tumour cells are utilised as the starting point on the premise that only rumour cells will contain TSAs or TAAs of relevance, and the tissue origins of the cells are matched to the tumour site in patients. A primary aspect of the invention is the use of immortalised normal, non-malignant cells as the basis of an allogeneic cell cancer vaccine. Normal cells do not possess TSAs or relevant concentrations of TAAs and hence it is surprising that normal cells are effective as anti-cancer vaccines. For prostate cancer, for example, a vaccine may be based on one or a combination of different immortalised normal cell lines derived from the prostate. The cell lines are lethally irradiated utilising gamma irradiation at 50-300 Gy to ensure that they are replication incompetent prior to use in the mammal or human.
Owner:ONYVAX
Pin-Prc Transition Genes
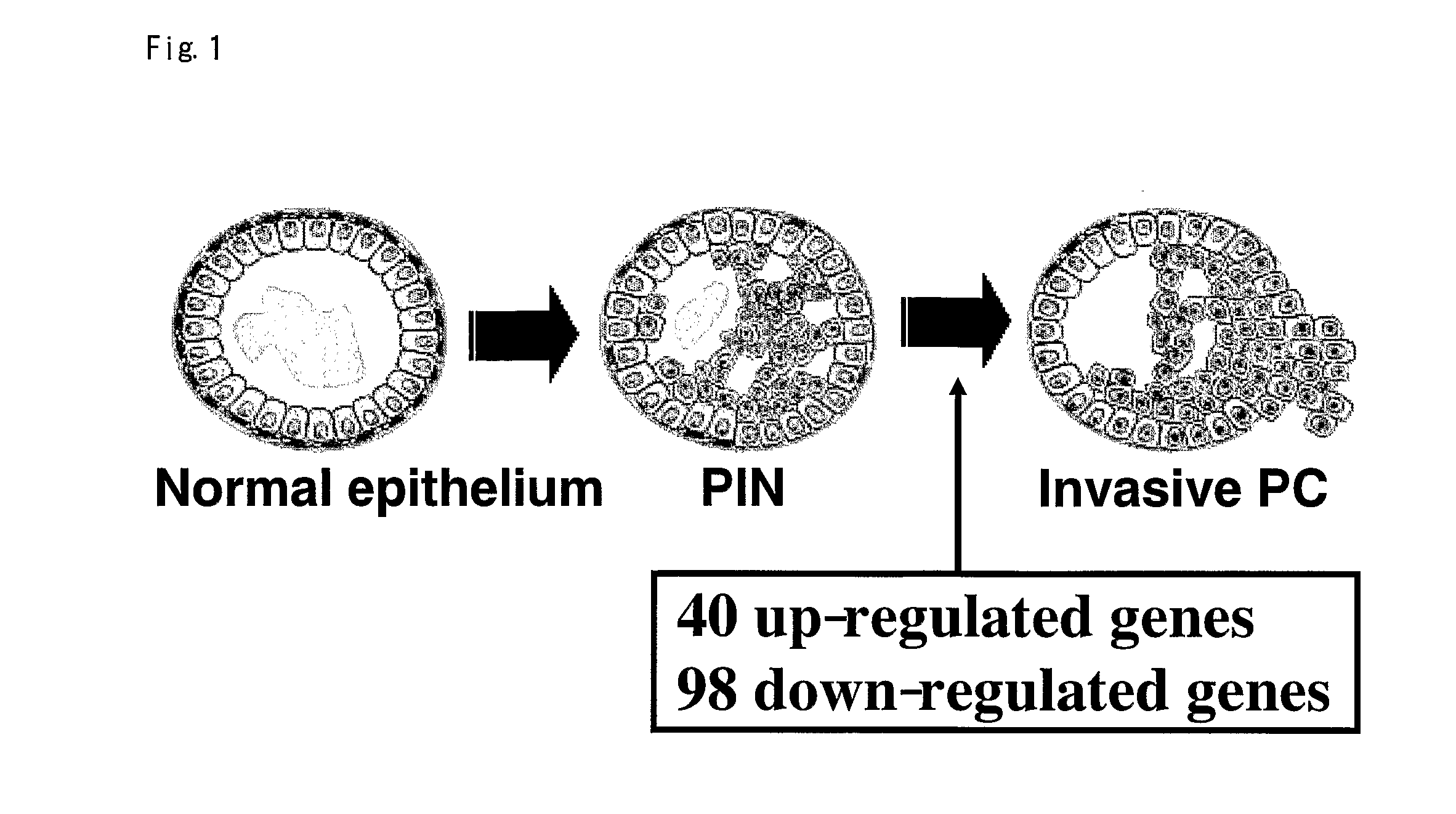
InactiveUS20080063640A1Increase intervalReduce morbidityCompound screeningApoptosis detectionProstate cancerCancer research
Objective methods for diagnosing a predisposition to developing prostate cancer (PRC) are described herein. In one embodiment, the diagnostic method involves the determining a expression level of PRC-associated gene that discriminate between PRC and PIN. The present invention further provides methods of screening for therapeutic agents useful in the treatment of PRC, methods of treating PRC.
Owner:ONCOTHERAPY SCI INC
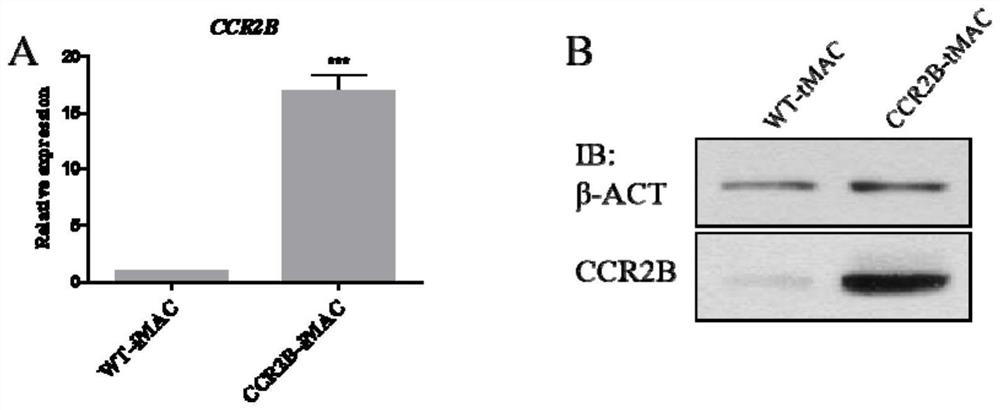
Monocyte and macrophage for expressing chemokine receptor with solid tumor directional chemotactic ability, and preparation and application thereof
ActiveCN113322238ADownregulation of expression levelReduce non-specific immune attackPeptidesBlood/immune system cellsPluripotential stem cellLentivirus
The invention provides a monocyte and a macrophage for expressing a chemokine receptor with solid tumor directional chemotactic ability, and preparation and application thereof, and relates to the field of biotechnology. The invention provides a macrophage, the macrophage overexpresses the chemokine receptor, the macrophage has the ability of chemotactic migration to a solid tumor and remarkable effects of chemotactic migration to the tumor and infiltration, the specific killing efficiency and utilization rate of adoptively transplanted macrophage can be improved, and non-specific immune attack to normal tissues is reduced. The invention provides a preparation method of macrophages, which comprises the following steps: constructing a lentivirus expression system containing a chemokine receptor gene, integrating the chemokine receptor gene into pluripotent stem cells or mononuclear macrophages by using the lentivirus expression system, performing induced differentiation to obtain the macrophages, and the macrophages can stably overexpress the chemokine receptor for a long time.
Owner:ZHEJIANG UNIV
Immunogenic compositions comprising a xenogenic prostate protein p501s
InactiveUS20060140965A1Enhancing induction of immunityBreaking the tolerance against the autologous antigenGenetic material ingredientsCancer antigen ingredientsHuman prostateImmunogenicity
The present invention relates to pharmaceutical / immunogenic compositions and methods for inducing an immune response against tumour-related antigens. More specifically, the invention relates to non-human prostate-specific antigens, more precisely to the non-human prostate-specific P501S, which can be used as xenogeneic antigen in prostate cancer vaccine therapy and as diagnostic agents for prostate tumours in humans, to immunogenic compositions containing them, to methods of manufacture of such compositions and to their use in medicine. Methods for formulating vaccines for immunotherapeutically treating P501S-expressing prostate tumors, prostatic hyperplasia, and prostate intraepithelilial neoplasia (PIN) are also provided.
Owner:GLAXO GROUP LTD +1
Psma binding antibody and use thereof
ActiveCN108699157AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody medical ingredientsAntiendomysial antibodiesSquamous Carcinomas
The present invention provides a novel PSMA binding antibody termed 10B3 and pharmaceutical and diagnostic uses of the antibody 10B3. The PSMA antibody 10B3 does not cross-compete with the state of the art PMSA binding antibody J591 and has a reduced induction of antigen shift compared to J591 and a unique reactivity with squamous cell carcinoma (SCC) cells of different origin.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS +1
Chimeric antigen receptor of cell for targeted expression of Claudin 18.2 and application of chimeric antigen receptor
ActiveCN113354739AEfficient killingStrong killing and cytokine release functionVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
The invention discloses a chimeric antigen receptor of a cell for targeted expression of Claudin 18.2 (CLDN 18.2), and particularly discloses a chimeric antigen receptor with an amino acid sequence as shown in SEQ ID NO.14. The chimeric antigen receptor comprises a Claudin 18.2-targeted single-chain antibody, a hinge region, a transmembrane structural domain and an intracellular signal structural domain. The Claudin 18.2-targeted chimeric antigen receptor disclosed by the invention can achieve effective and specific targeted expression of malignant cells (such as tumor cells) of the Claudin 18.2 surface antigen, so that a more efficient method with fewer side effects and adverse reactions is provided for treating some tumors expressing the Claudin 18.2 surface antigen.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Peptides, combination of peptides, and cell based medicaments for use in immunotherapy against urinary bladder cancer and other cancers
InactiveUS20190330274A1Enhance stability and solubilityAid in diagnosisPeptide/protein ingredientsAntibody mimetics/scaffoldsWilms' tumorEx vivo
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Ii-Key hybrid polypeptide and preparation method and application of tumor vaccine of Ii-Key hybrid polypeptide
InactiveCN111040038AImprove anti-cancer effectAntibody mimetics/scaffoldsPeptide preparation methodsMolecular bindingTGE VACCINE
The invention discloses an Ii-Key hybrid polypeptide. LRMK tetrapeptide in the amino acid sequence of a stable polypeptide chain (Ii) of MHC class II molecules can be linked by polypeptide recognizedby TH cells, shows MHC class II molecule binding efficacy greater than 250 times in vitro, and is easier to recognize and present by antigen presenting cells. The invention provides a preparation method of a hybrid polypeptide tumor vaccine and application of a combination of the hybrid polypeptide tumor vaccine and an immunologic adjuvant, namely granulocyte-macrophage colony stimulating factor (GM-CSF) to treatment of cancers. The hybrid polypeptide tumor vaccine disclosed by the invention is an efficient cancer immunotherapy vaccine for activating an autoimmune system with a latest mechanism. The Ii-Key hybrid polypeptide tumor vaccine provided by the invention has the advantage of improved anti-cancer effect.
Owner:深圳市博圣康生物科技有限公司
PSMA Binding Antibody and Uses Therof
ActiveUS20190022205A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody medical ingredientsAntiendomysial antibodiesSquamous Carcinomas
The present invention provides a novel PSMA binding antibody termed 10B3 and pharmaceutical and diagnostic uses of the antibody 10B3. The PSMA antibody 10B3 does not cross-compete with the state of the art PMSA binding antibody J591 and has a reduced induction of antigen shift compared to J591 and a unique reactivity with squamous cell carcinoma (SCC) cells of different origin.
Owner:UNIV TUBINGEN +1
Combination of a PD-1 antagonist and a listeria-based vaccine for treating prostate cancer
InactiveCN106999561ABacterial antigen ingredientsViral antigen ingredientsTissue specific antigenProstate cancer
The present disclosure describes combination therapies comprising an antagonist of Programmed Death 1 receptor (PD-1) and a Listeria based strain that expresses prostate-tissue specific antigen (PSA), and the use of the combination therapies for the treatment of prostate cancer.
Owner:ADVAXIS +1
Immunoenhancer, immunotherapy medicine composition, preparation method of composition and application of immunoenhancer and composition
ActiveCN108567977AStrong immune responseImprove immunityVirus peptidesAntiviralsDiseaseGranular cell
The invention discloses an immunoenhancer, an immunotherapy medicine composition, a preparation method of the immunotherapy medicine composition and an application of the immunoenhancer and the immunotherapy medicine composition. The immunoenhancer at least comprises interferon and granular cell-macrophage colony stimulating factors, and the immunotherapy medicine composition at least comprises antigen and the immunoenhancer. The immunoenhancer can remarkably enhance the immunity of a body and improve the antigen presenting efficiency of the body, so that the body can establish effective immune activation and response. Strong antibody and cellular immune protection reaction and pathogen removing capacity can be generated, and the immunoenhancer can be applied to therapy of diseases and tumors caused by microorganisms such as viruses and bacteria.
Owner:FUDAN UNIV
Application of chimeric antigen receptor T cell targeting NKG2D in treatment of prostate cancer, and medicine for treating prostate cancer
PendingCN111632135AGood treatment effectNo damageAntibody medical ingredientsProstate cancer vaccineProstate cancer cellAntigen receptors
Owner:SHENZHEN BINDEBIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com