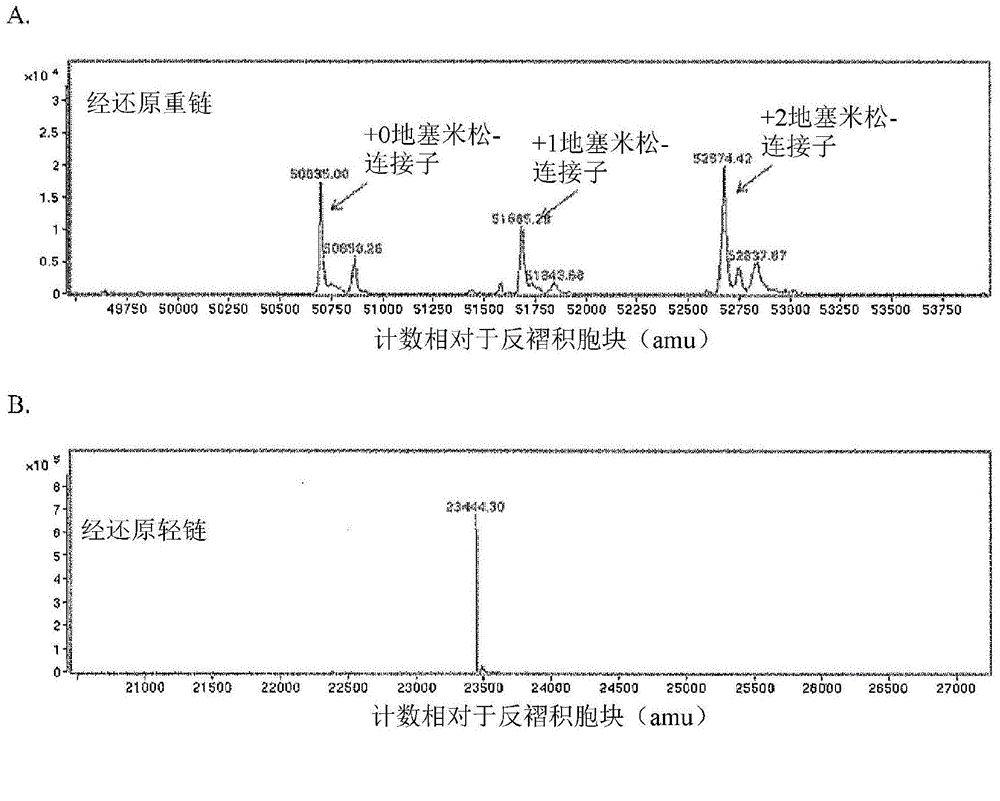
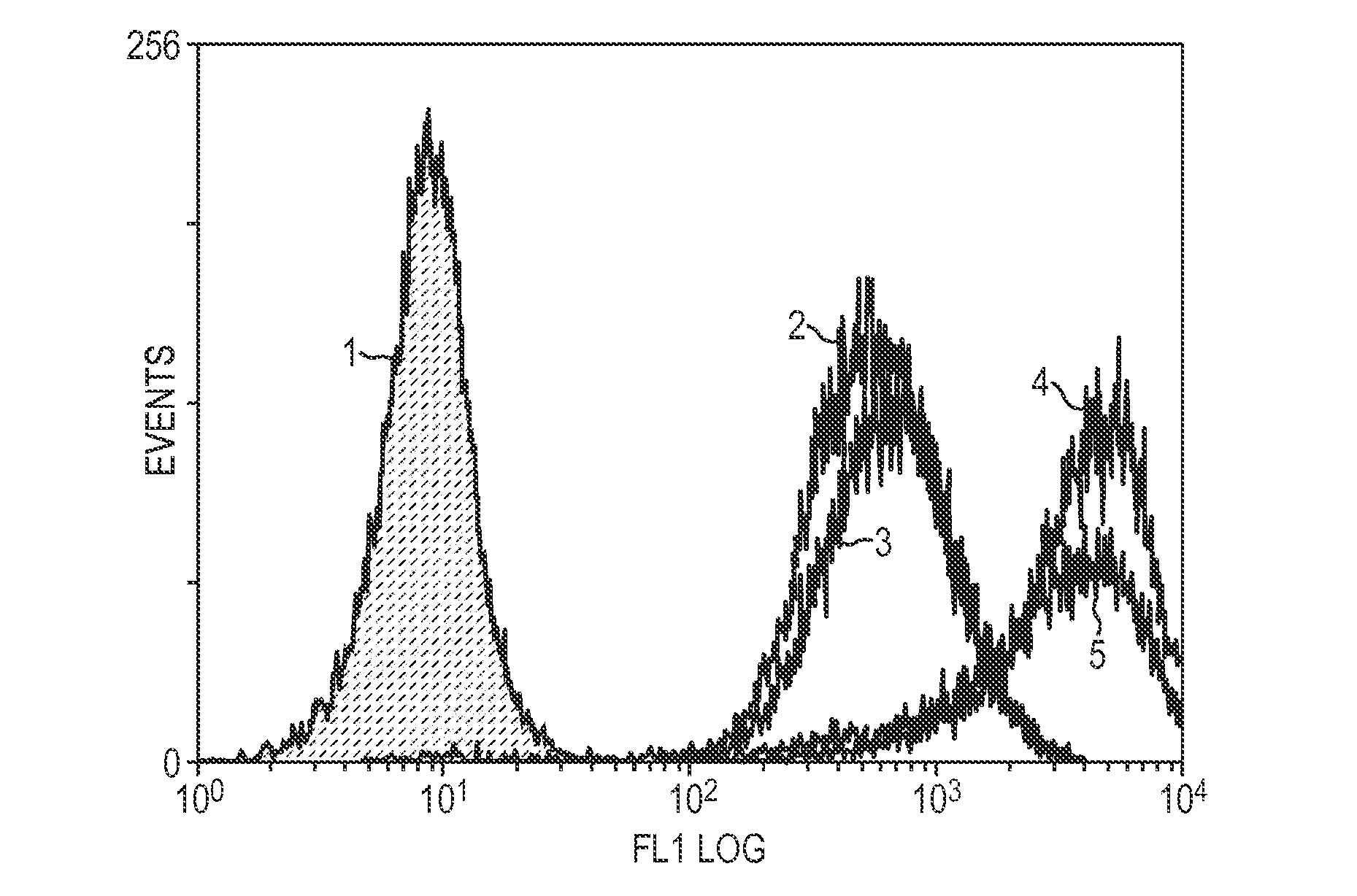
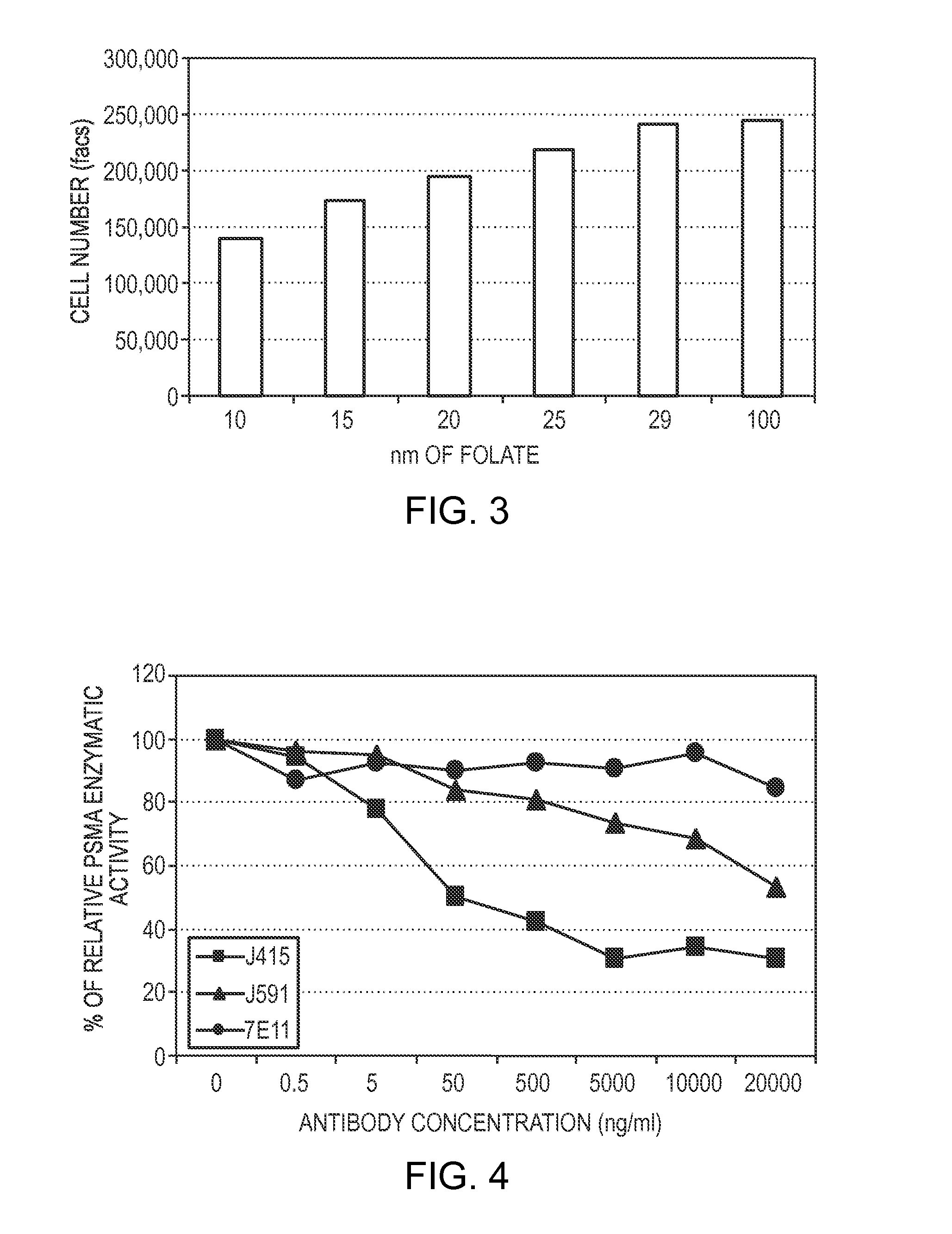
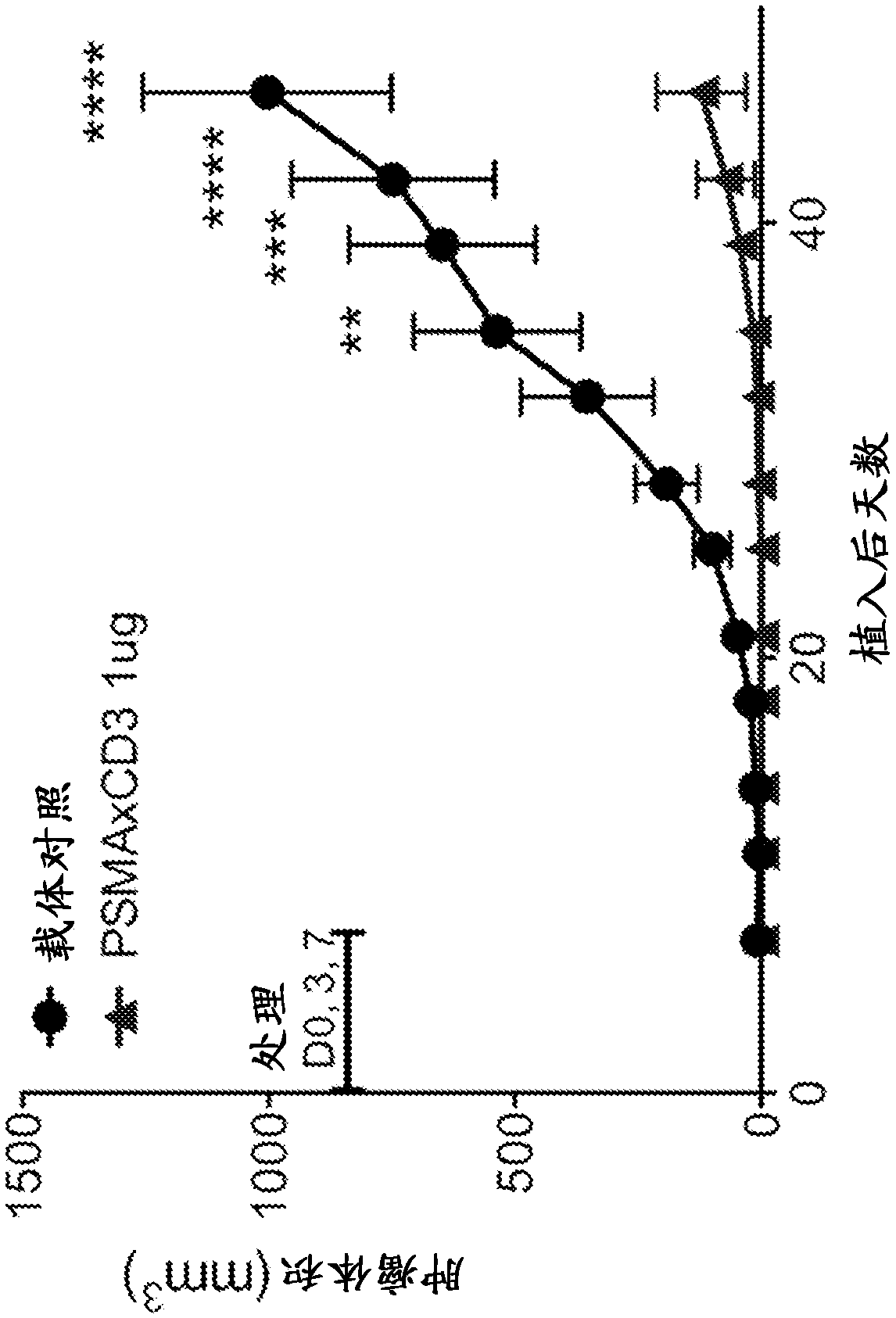
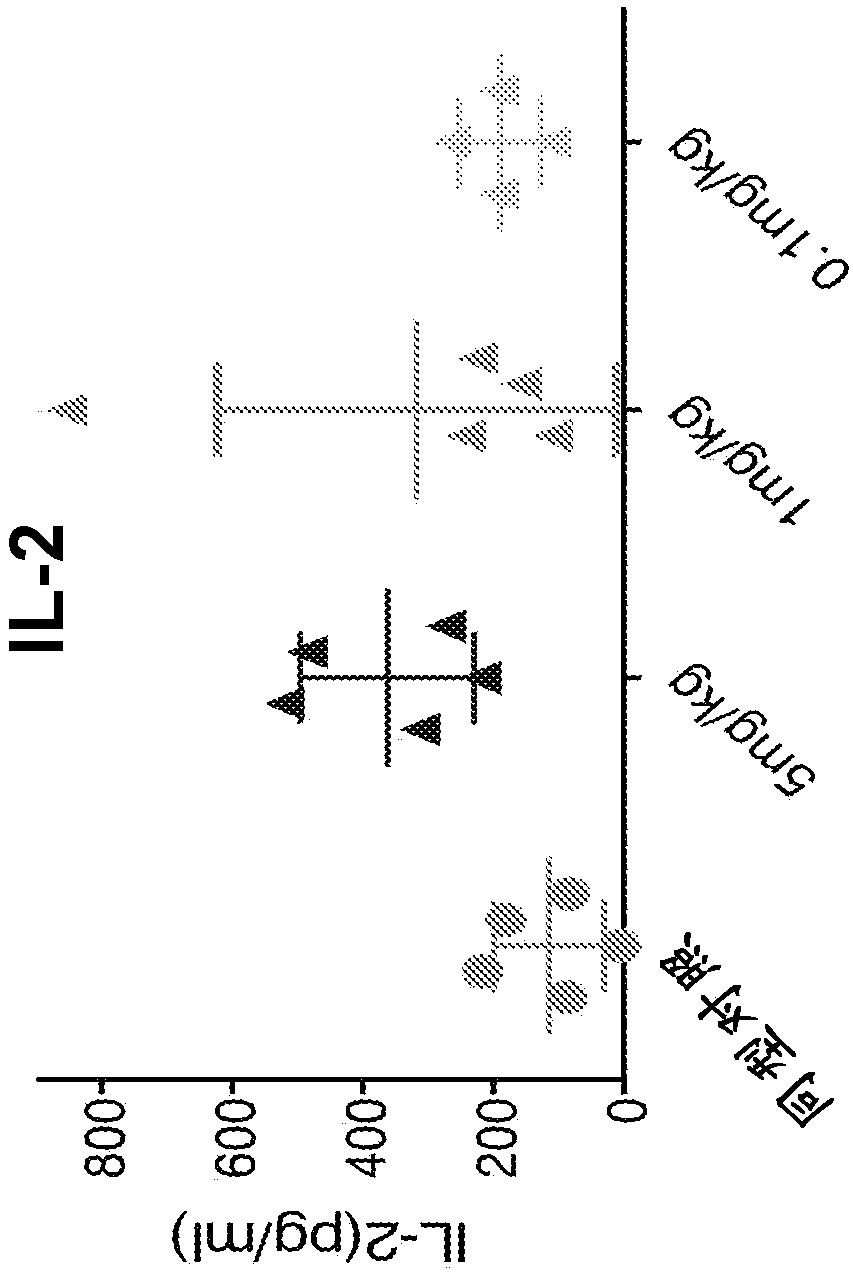
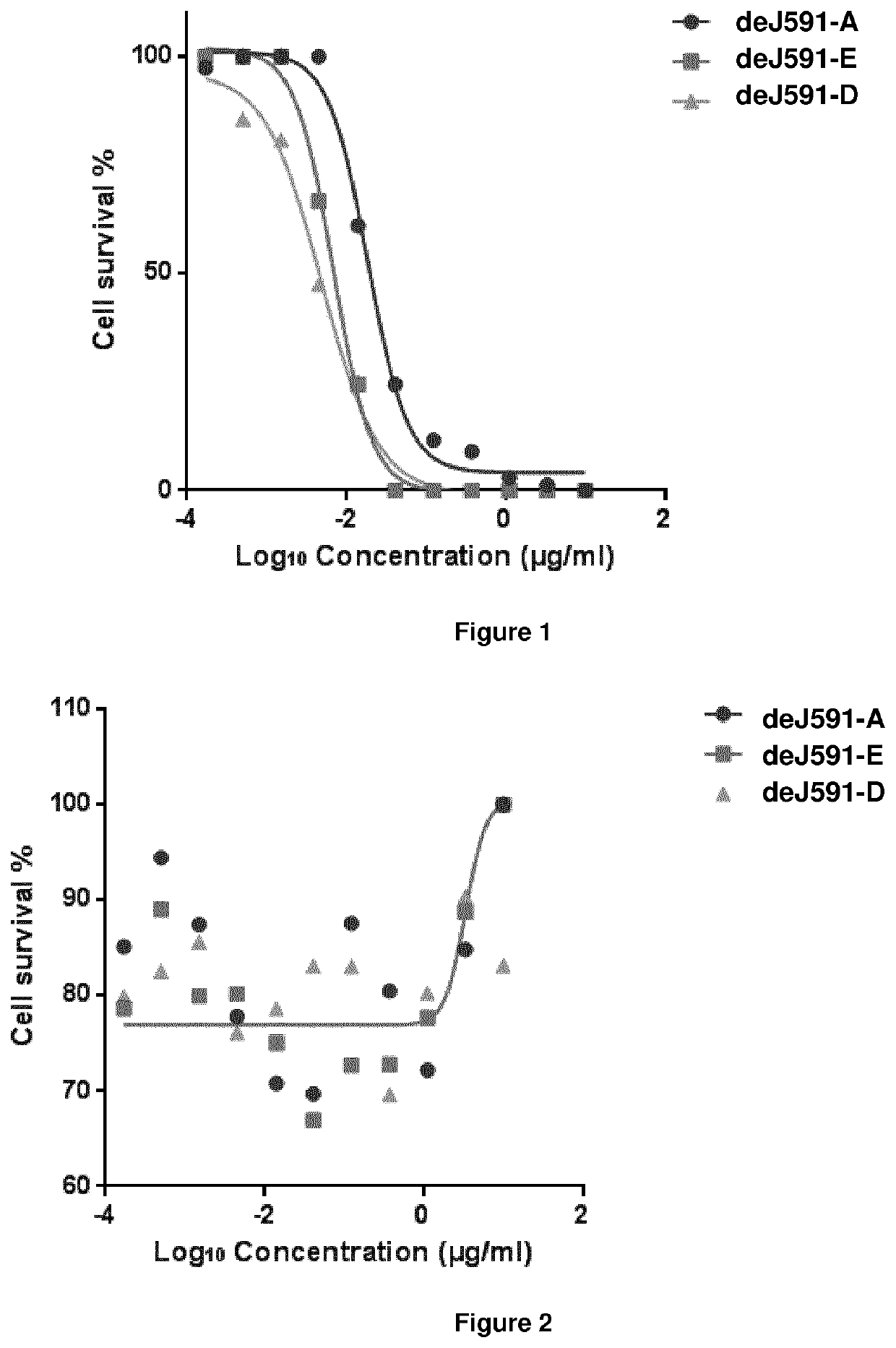
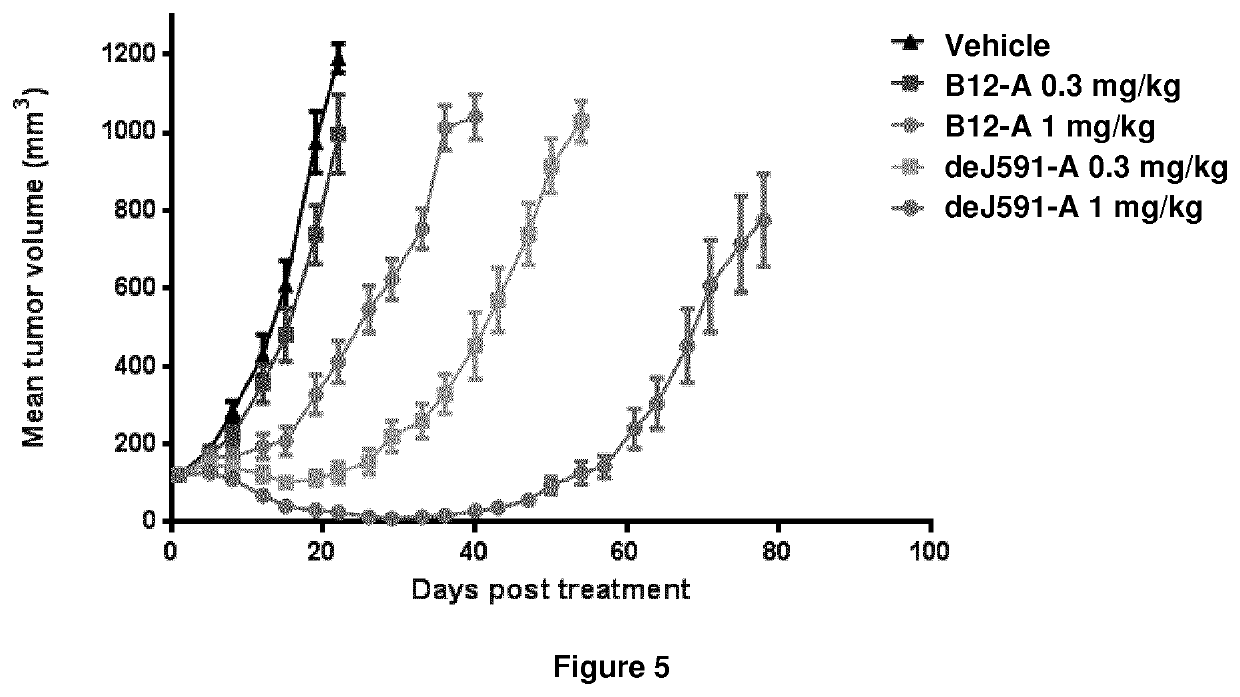
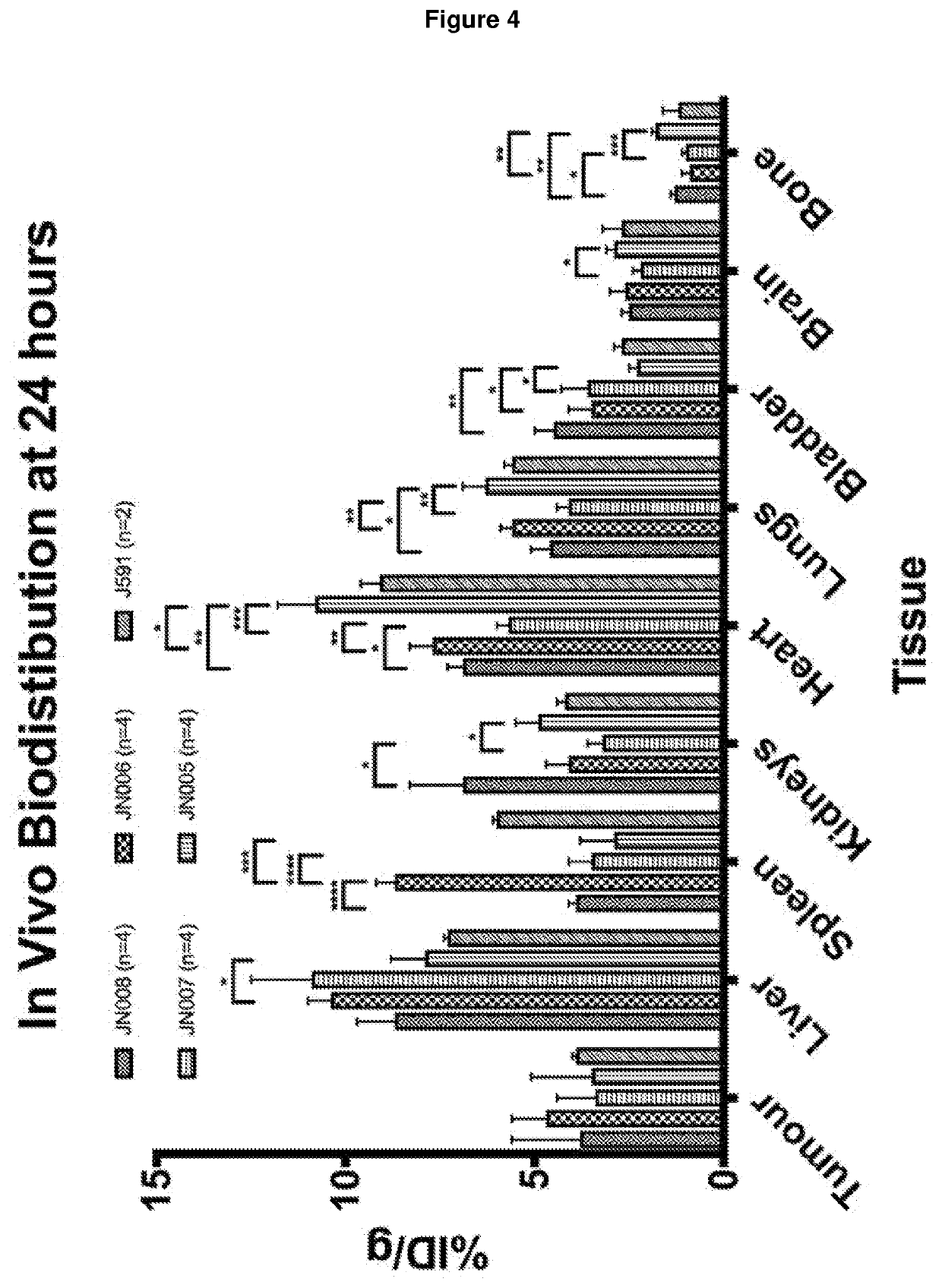
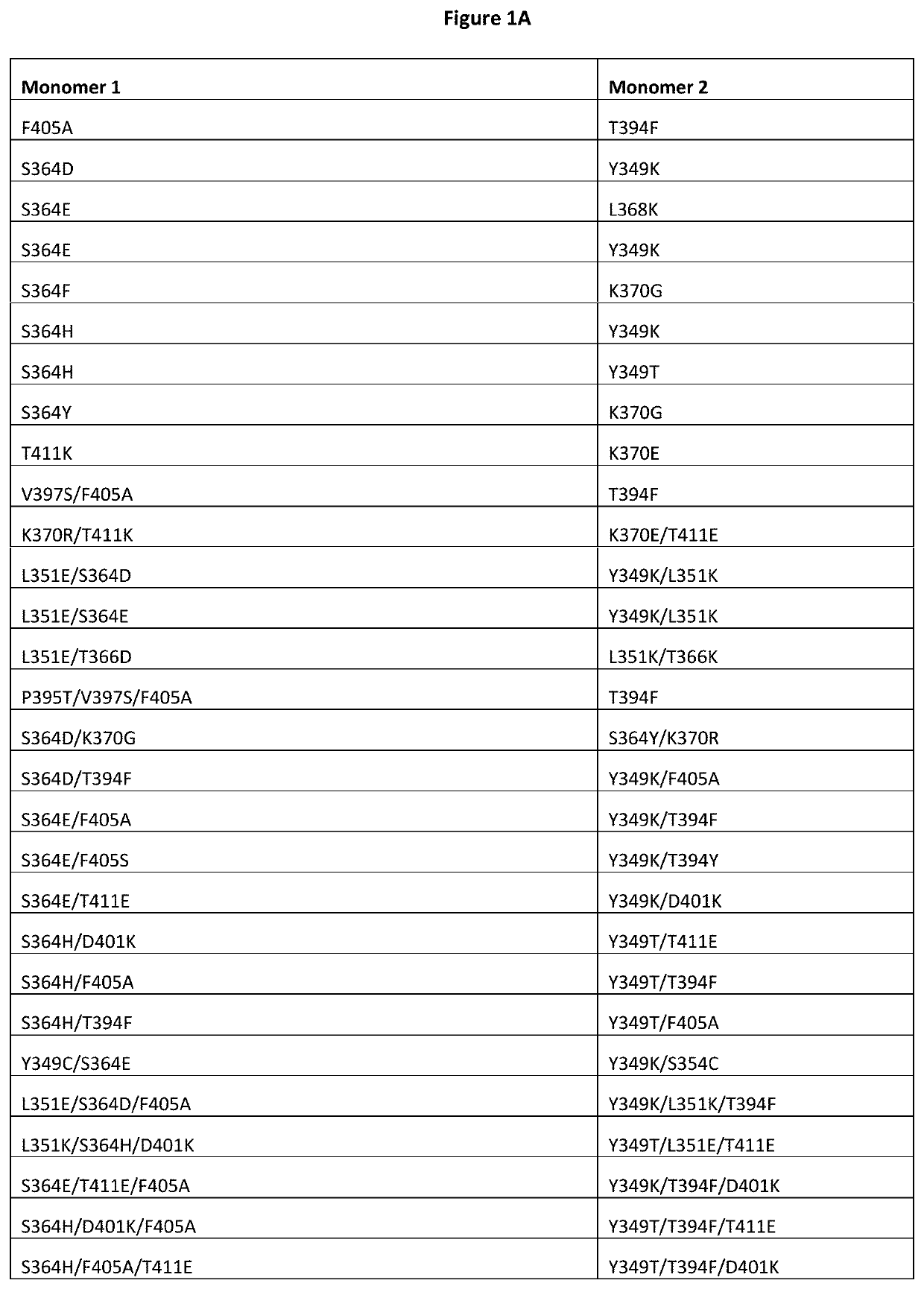
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
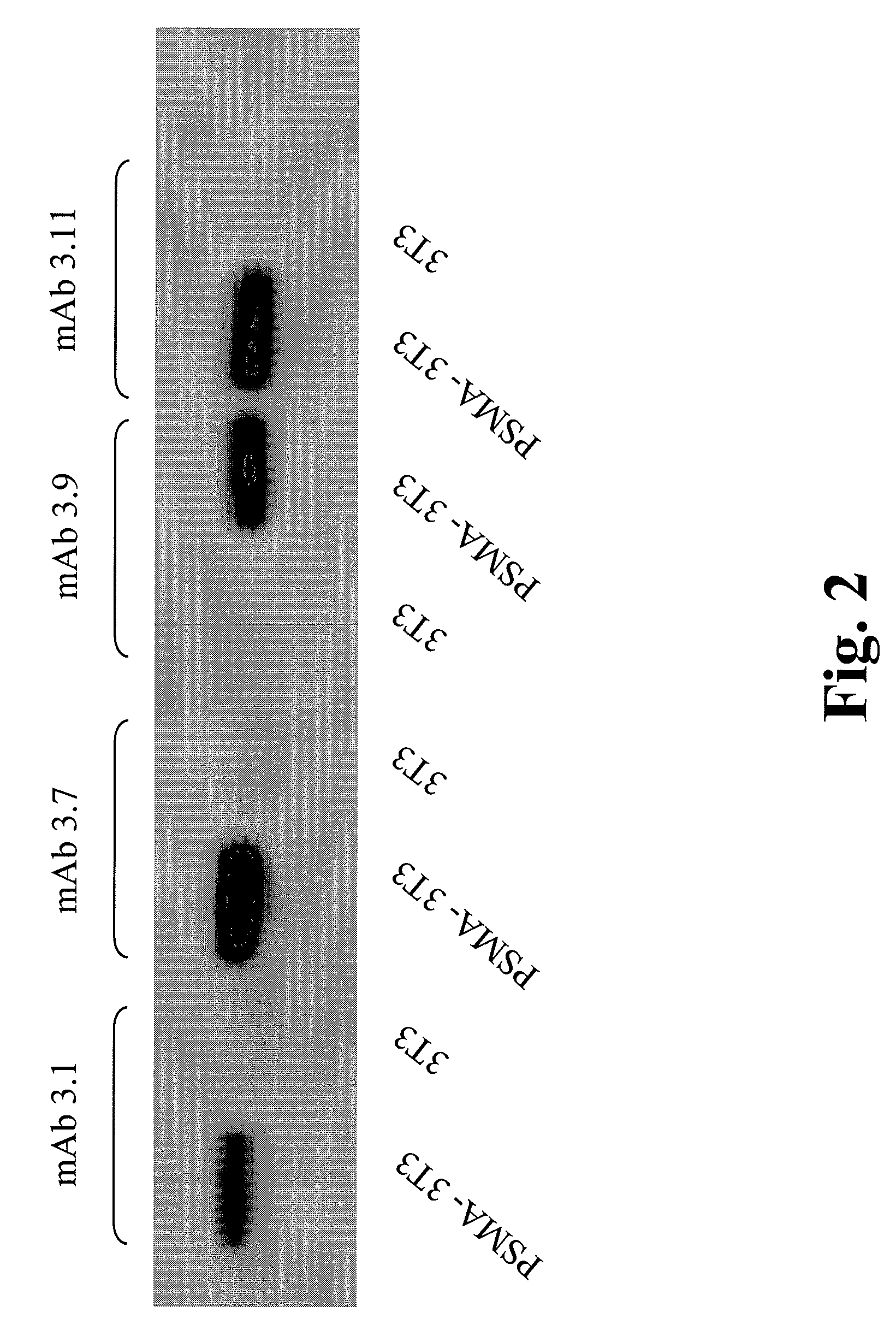
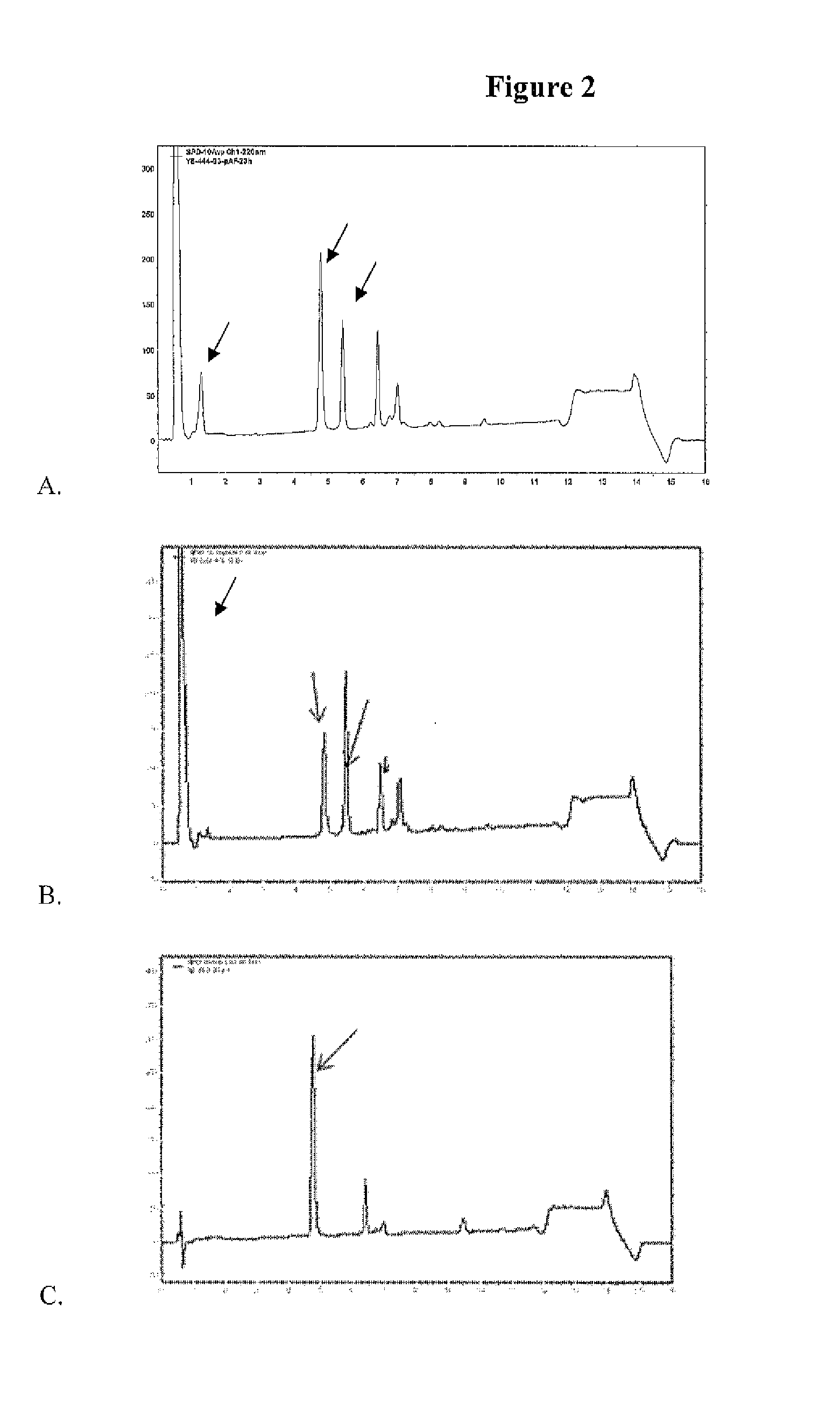
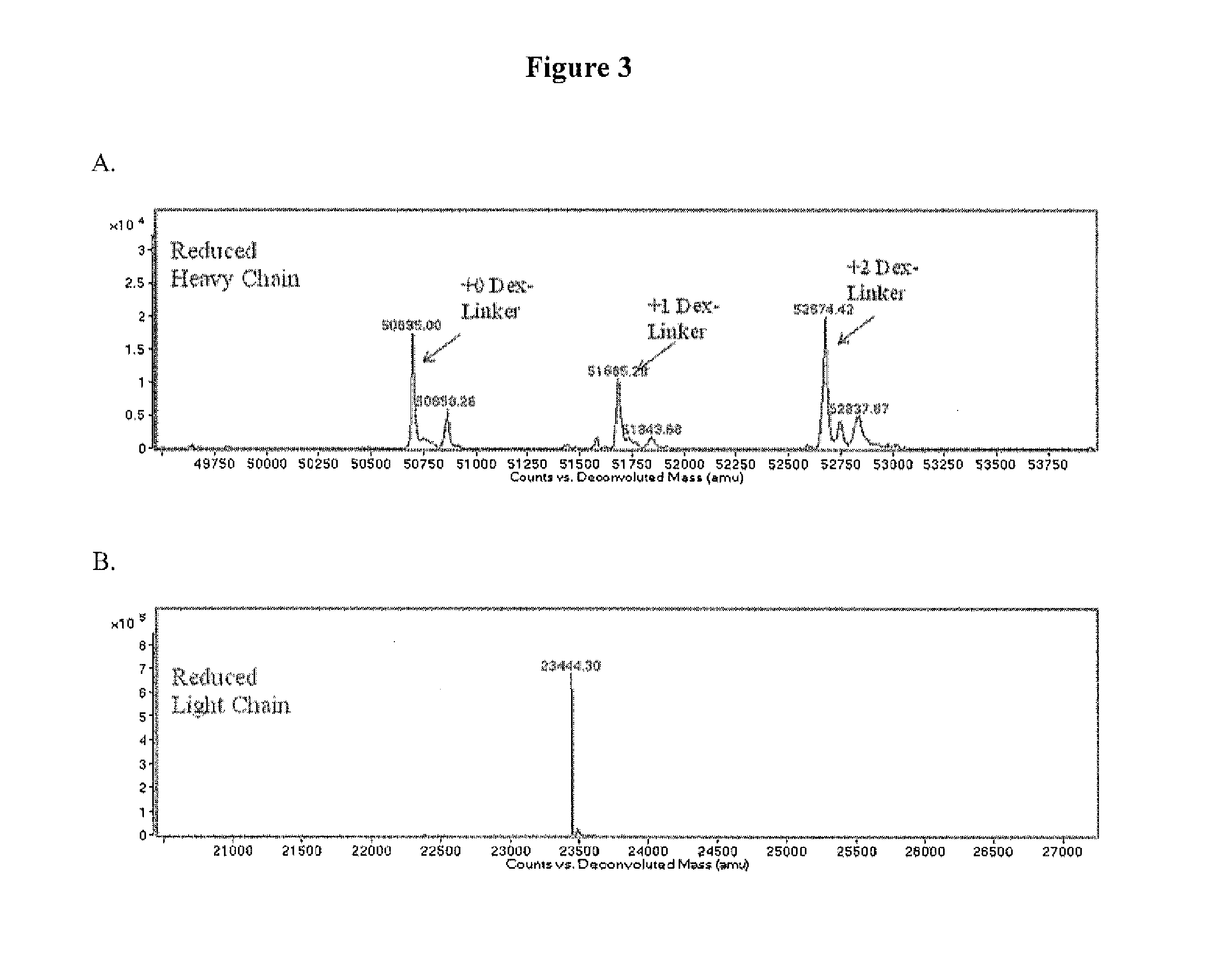
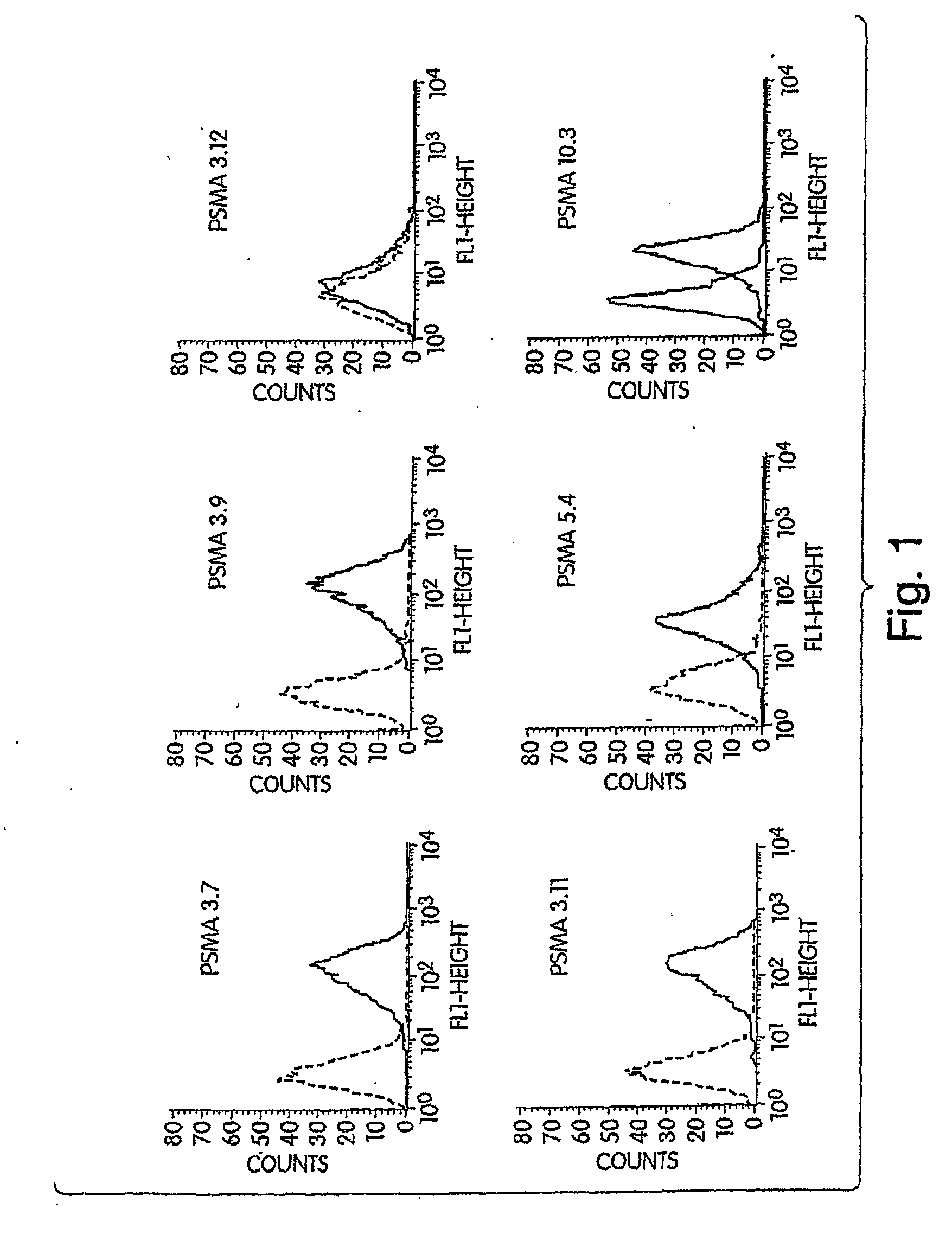
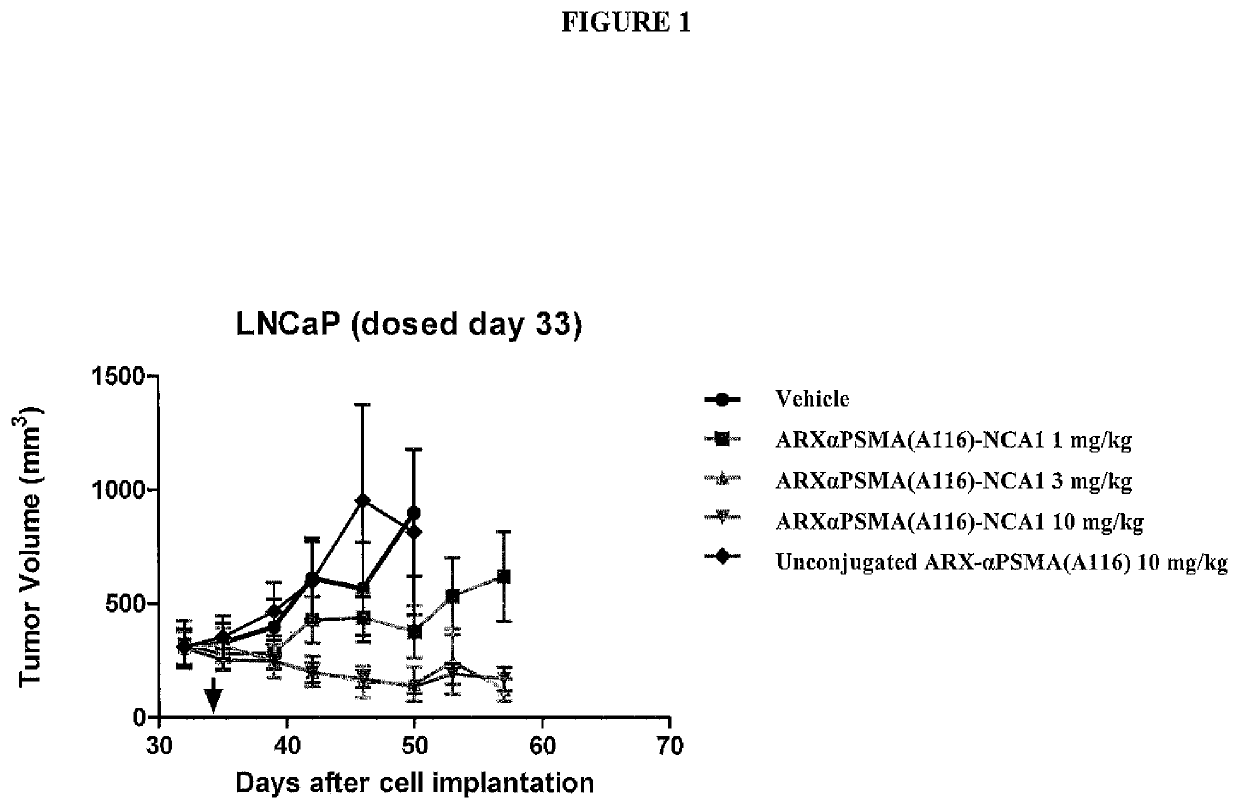
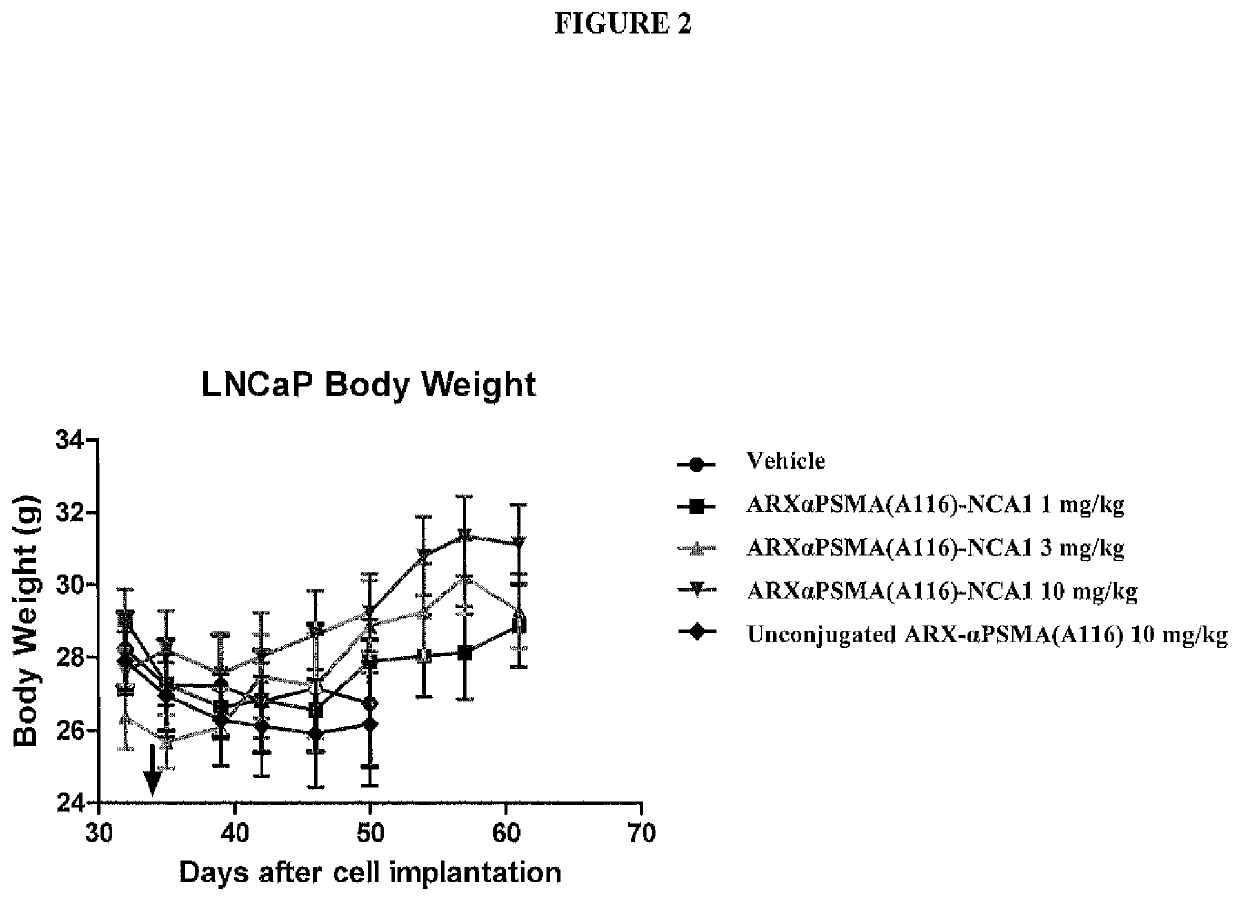
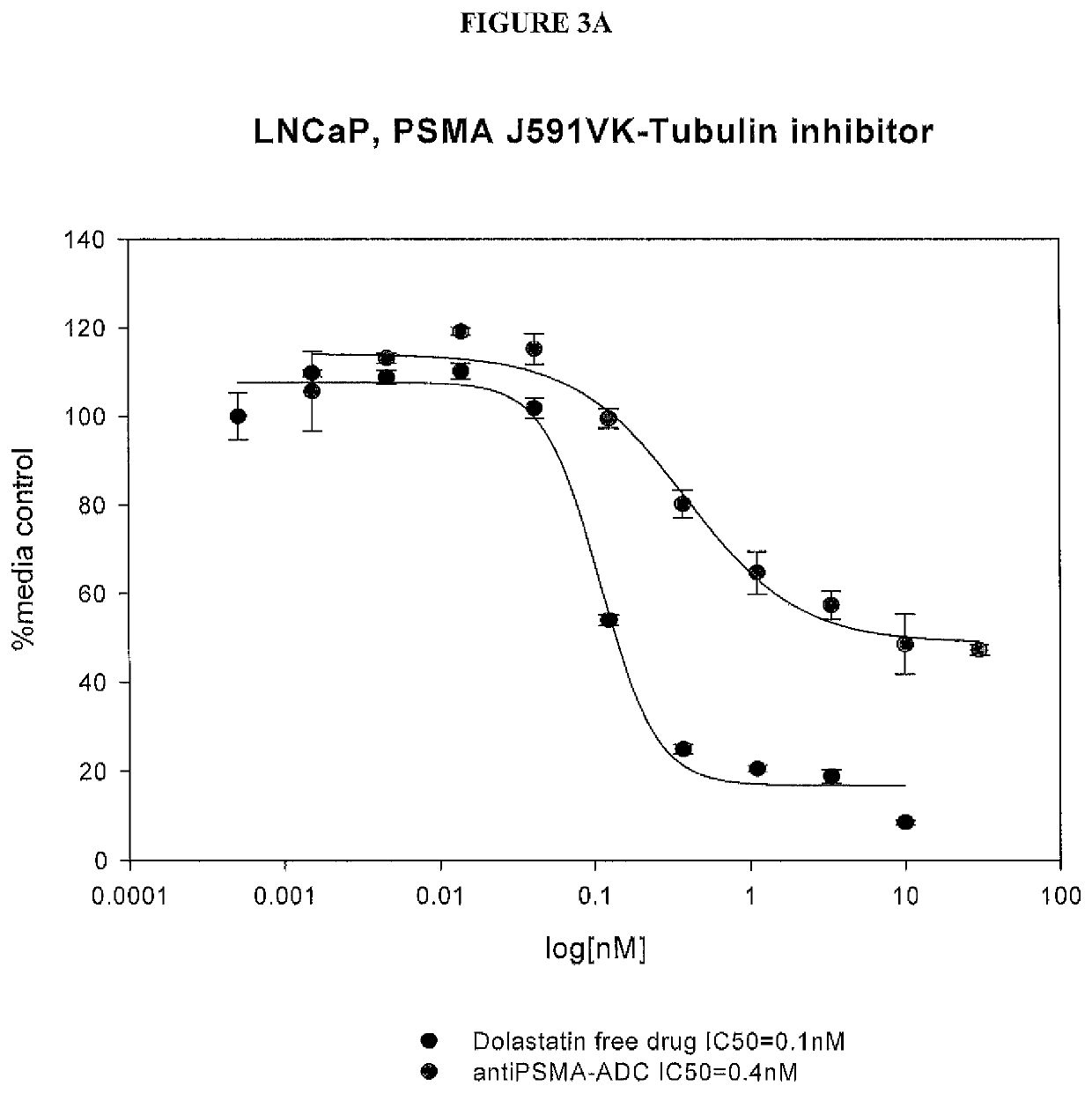
33 results about "PSMA Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunohistochemical analysis of PSMA using anti-PSMA Polyclonal Antibody (Product # PA5-32568) in Prostate Carcinoma Tissue. The recommened dilution for this antibody in immunohistochemistry applications is 1:100.
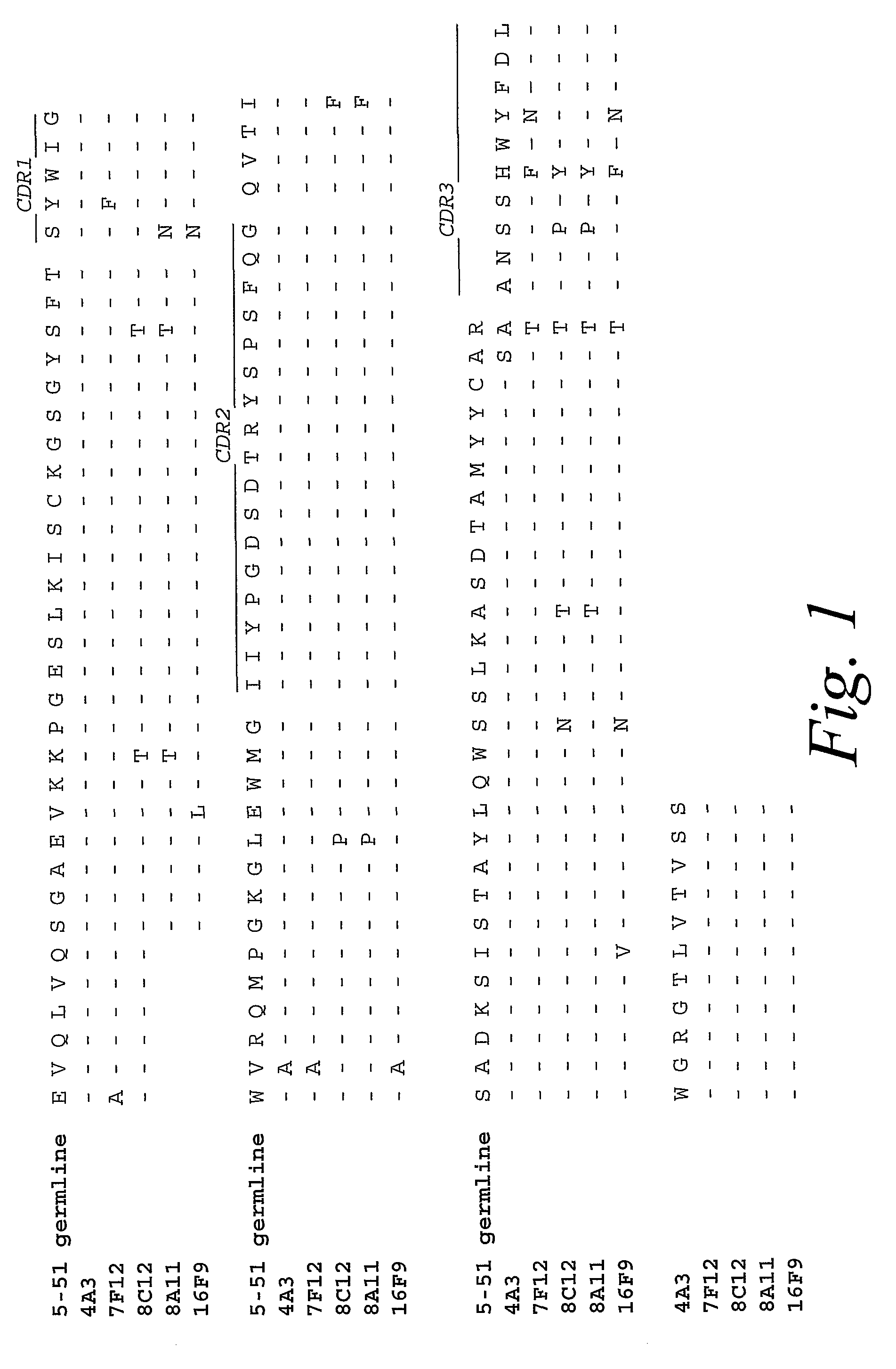
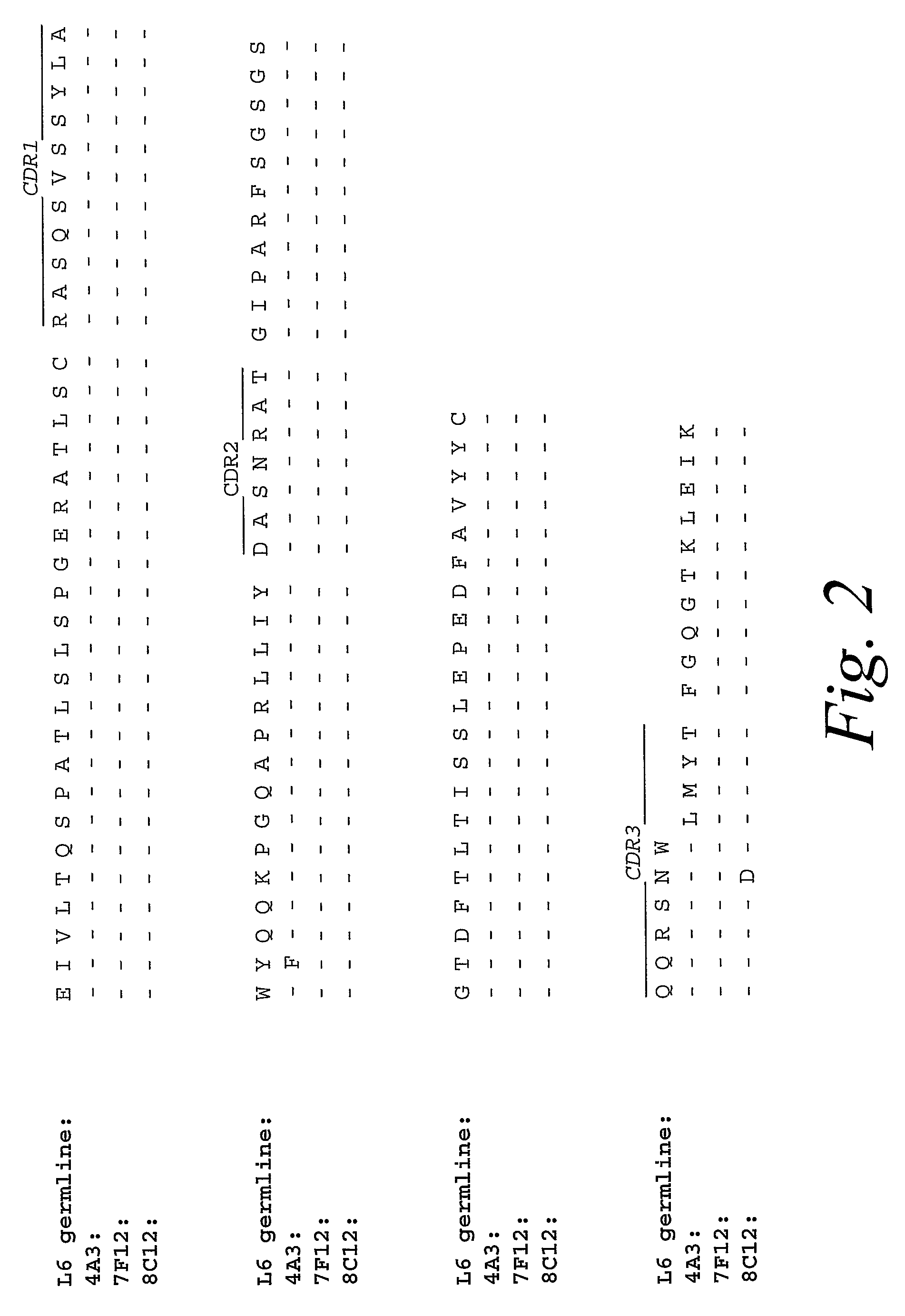
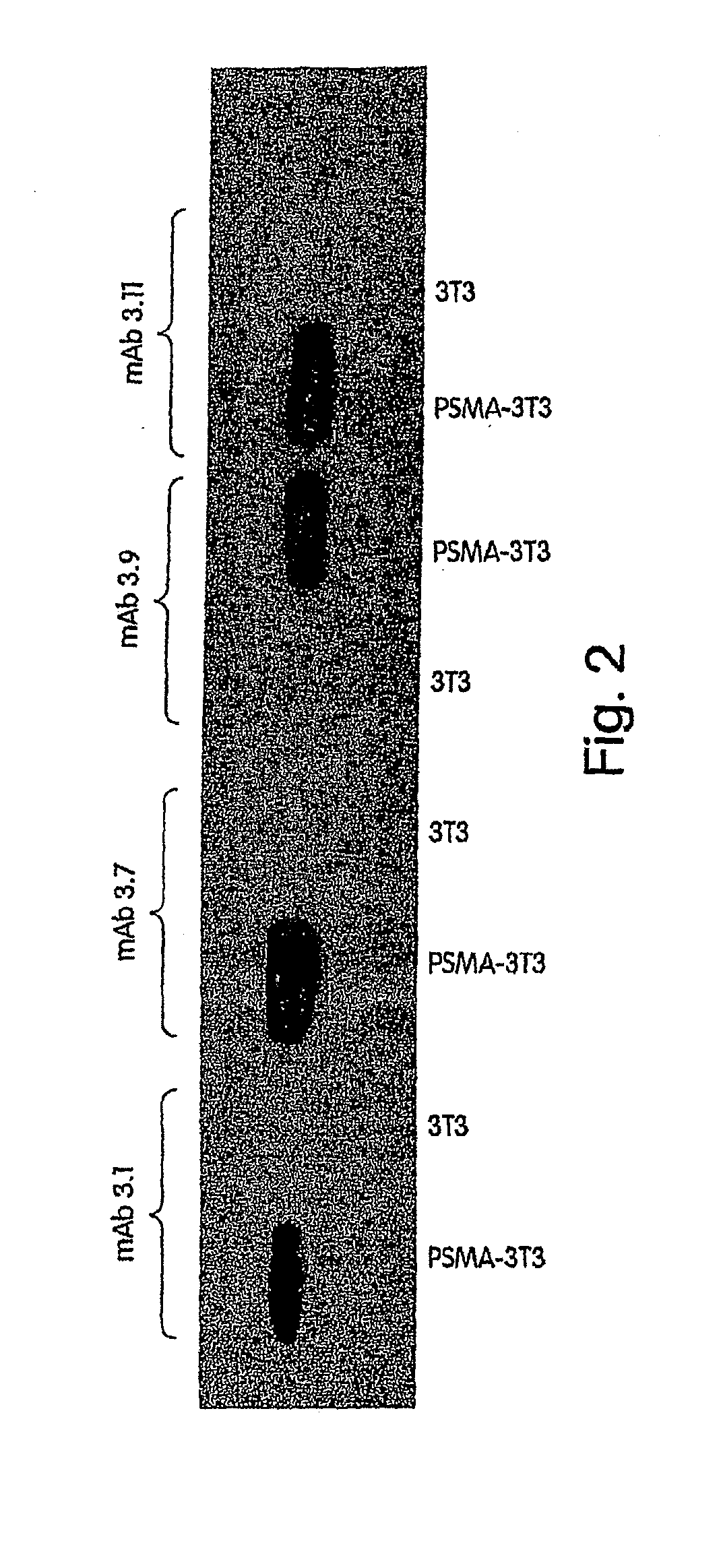
Monoclonal antibodies against prostate specific membrane antigen (PSMA) lacking in fucosyl residues
ActiveUS7875278B2Inhibit cell growthStrong cytotoxicityAnimal cellsAntibody ingredientsAntigenFucosylation
The invention pertains to anti-PSMA antibodies that lack fucosyl residues. The antibodies of the invention exhibit increased antibody-dependent cellular cytotoxicity (ADCC) activity as compared to the fucosylated form of the antibodies. The invention also provides host cells that express the anti-PSMA antibodies that lack fucosyl residues, wherein the host cells are deficient for a fucosyl transferase. Methods of using the antibodies to inhibit the growth of PSMA+ cells, such as tumor cells, are also provided.
Owner:ER SQUIBB & SONS INC +1
Compositions of PSMA antibodies
InactiveUS20080286284A1Effectiveness of treatmentOrganic active ingredientsFungiAntigen Binding FragmentCancers diagnosis
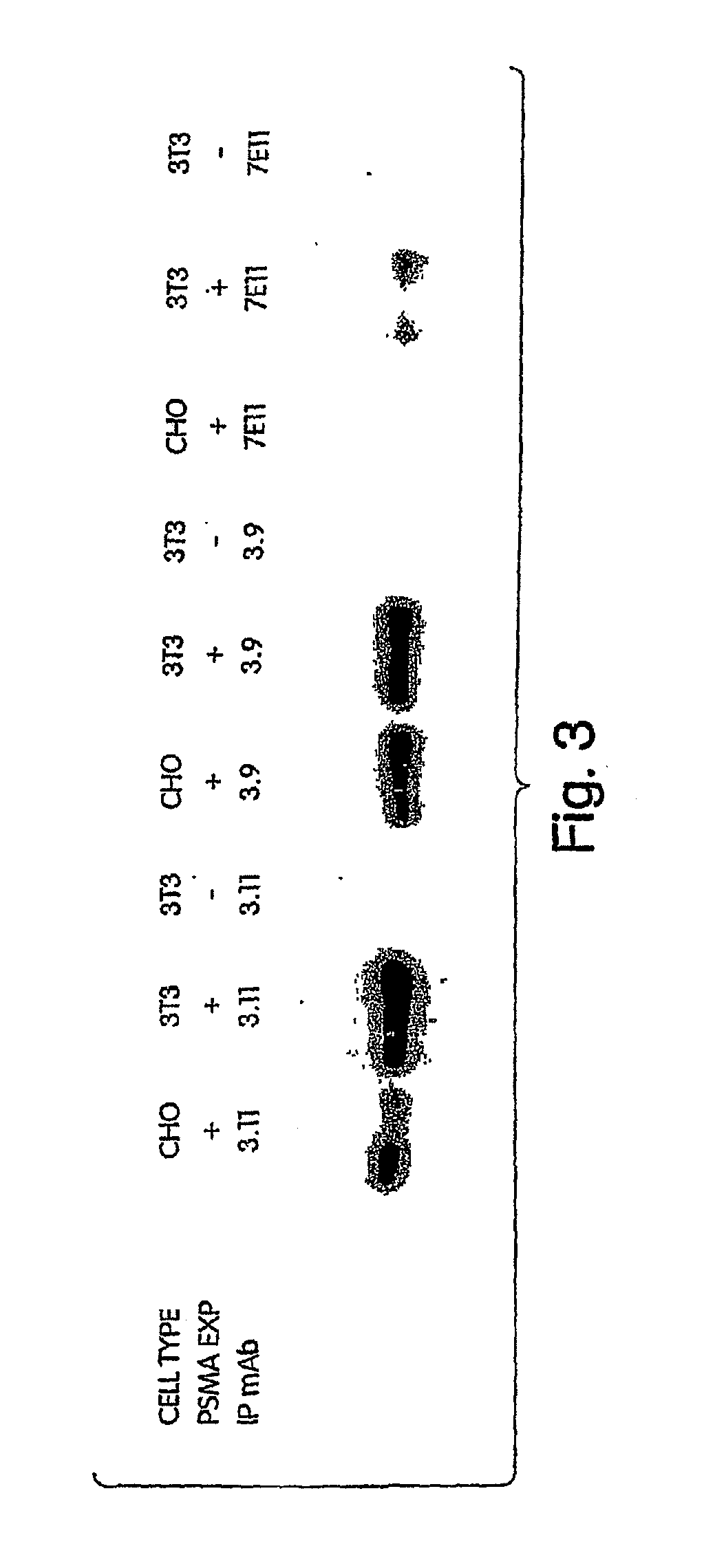
The invention includes antibodies or antigen-binding fragments thereof which bind specifically to conformational epitopes on the extracellular domain of PSMA, compositions containing one or a combination of such antibodies or antigen-binding fragments thereof, hybridoma cell lines that produce the antibodies, and methods of using the antibodies or antigen-binding fragments thereof for cancer diagnosis and treatment. The invention also includes oligomeric forms of PSMA proteins, compositions comprising the multimers, and antibodies that selectively bind to the multimers.
Owner:AMGEN FREMONT INC +1
PSMA antibodies and protein multimers
The invention includes antibodies or antigen-binding fragments thereof which bind specifically to conformational epitopes on the extracellular domain of PSMA, compositions containing one or a combination of such antibodies or antigen-binding fragments thereof, hybridoma cell lines that produce the antibodies, and methods of using the antibodies or antigen-binding fragments thereof for cancer diagnosis and treatment. The invention also includes oligomeric forms of PSMA proteins, compositions comprising the multimers, and antibodies that selectively bind to the multimers.
Owner:PSMA DEV COMPANY
Anti-PSMA Antibodies Conjugated to Nuclear Receptor Ligand Polypeptides
InactiveUS20150152187A1Delayed onset of actionProlong the action timeImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesPSMA Antibody
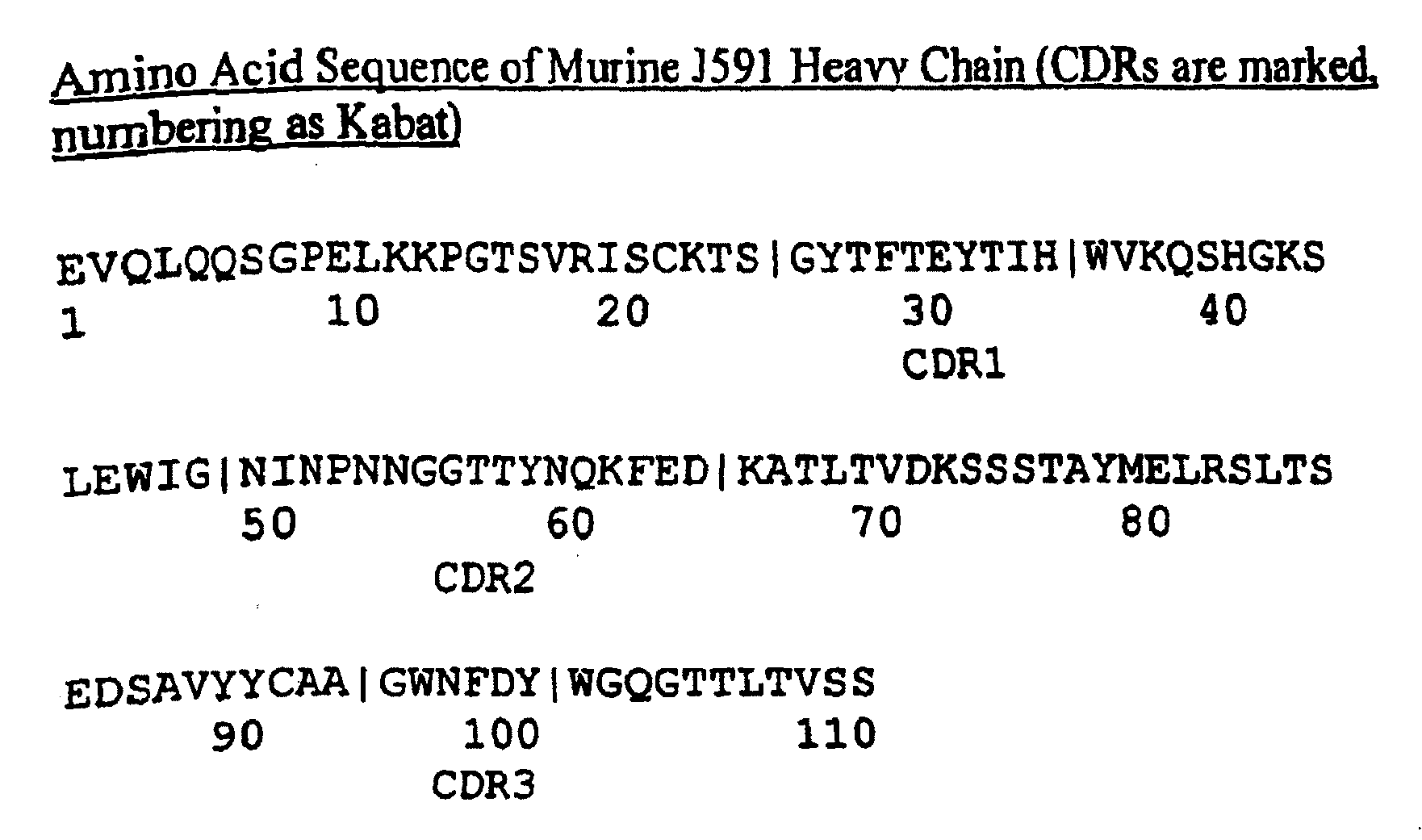
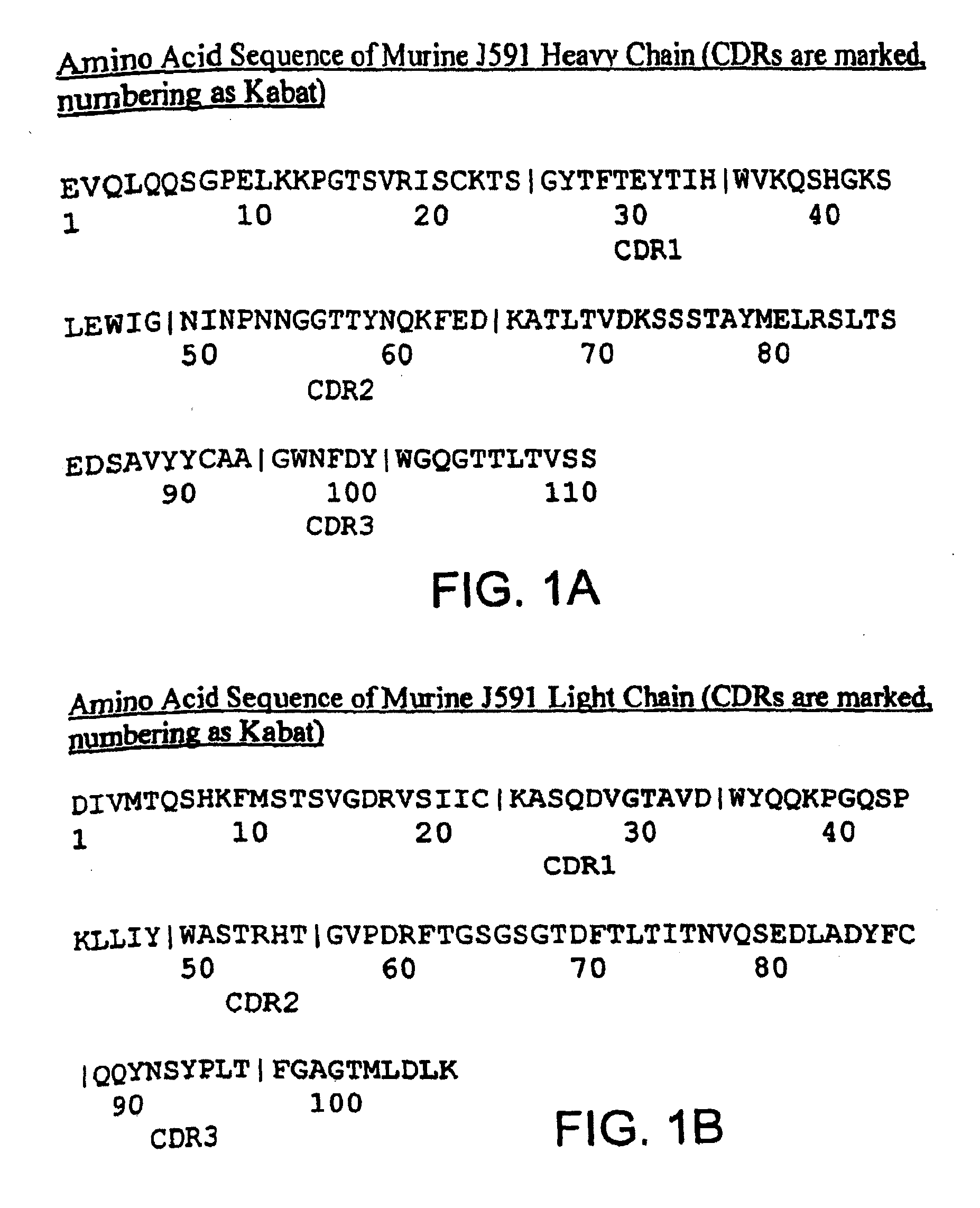
This invention relates to anti-prostate-specific membrane antigen antibodies (αPSMA) and αPSMA antibody—nuclear receptor ligand (NRL) conjugates comprising at least one non-naturally-encoded amino acid.
Owner:AMBRX
Psma antibodies and uses thereof
InactiveUS20110165081A1Peptide/protein ingredientsImmunological disordersDimerAntiendomysial antibodies
The invention includes stable multimeric, particularly dimeric, forms of PSMA protein, compositions and kits containing dimeric PSMA protein as well as methods of producing, purifying and using these compositions. Such methods include methods for eliciting or enhancing an immune response to cells expressing PSMA, including methods of producing antibodies to dimeric PSMA, as well as methods of treating cancer, such as prostate cancer.
Owner:PSMA DEV COMPANY +1
Prostate cancer diagnosis and treatment
InactiveUS7910693B2Facilitate the processIn-vivo radioactive preparationsPeptide/protein ingredientsProstate cancerAnti-PSMA Antibody
The present invention relates to novel mimetopes of anti-PSMA antibodies and their use for detecting, imaging, staging, treating and monitoring of prostate cancer, and / or metastatis thereof. The present invention also relates to novel pharmaceutical compositions for the treatment of prostate cancer. Furthermore the present invention relates to assay systems and kits for detecting, imaging, staging, treating and monitoring of prostate cancer, and / or metastasis thereof.
Owner:PROSCAN RX PHARMA
Kit for detecting metastatic castration resistant prostate cancer (mCRPC) drug resistance
ActiveCN105779599AIt has the function of guiding medicineGuaranteed PCR amplification requirementsMicrobiological testing/measurementAntigenFluorescence
The invention provides a kit for detecting metastatic castration resistant prostate cancer (mCRPC) drug resistance. The kit is characterized by comprising an antibody-oligonucleotide probe obtained by independently coupling PSA (prostate specific antigen) and PSMA (prostate specific membrane antigen) antibodies with different oligonucleotides; an amplification primer and a buffer solution for performing PCR (polymerase chain reaction) amplification on the antibody-oligonucleotide probe; and a fluorescent probe. By detecting the expression quantity of PSA and PSMA antigens, the kit can analyze the relative activity of AR signals, thereby detecting the mCRPC drug resistance of a patient.
Owner:SHANGHAI MAJORBIO BIO PHARM TECH
Methods of treating prostate cancer with Anti-prostate specific membrane antigen antibodies
InactiveUS20090280120A1Relieve painReduce needImmunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersAntigen Binding FragmentOncology
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
Prostate-Specific Membrane Antigen Antibody Drug Conjugates
ActiveUS20150152190A1Ability to increase and prolongGood treatment effectAntibody ingredientsPharmaceutical non-active ingredientsAntigenDrug conjugation
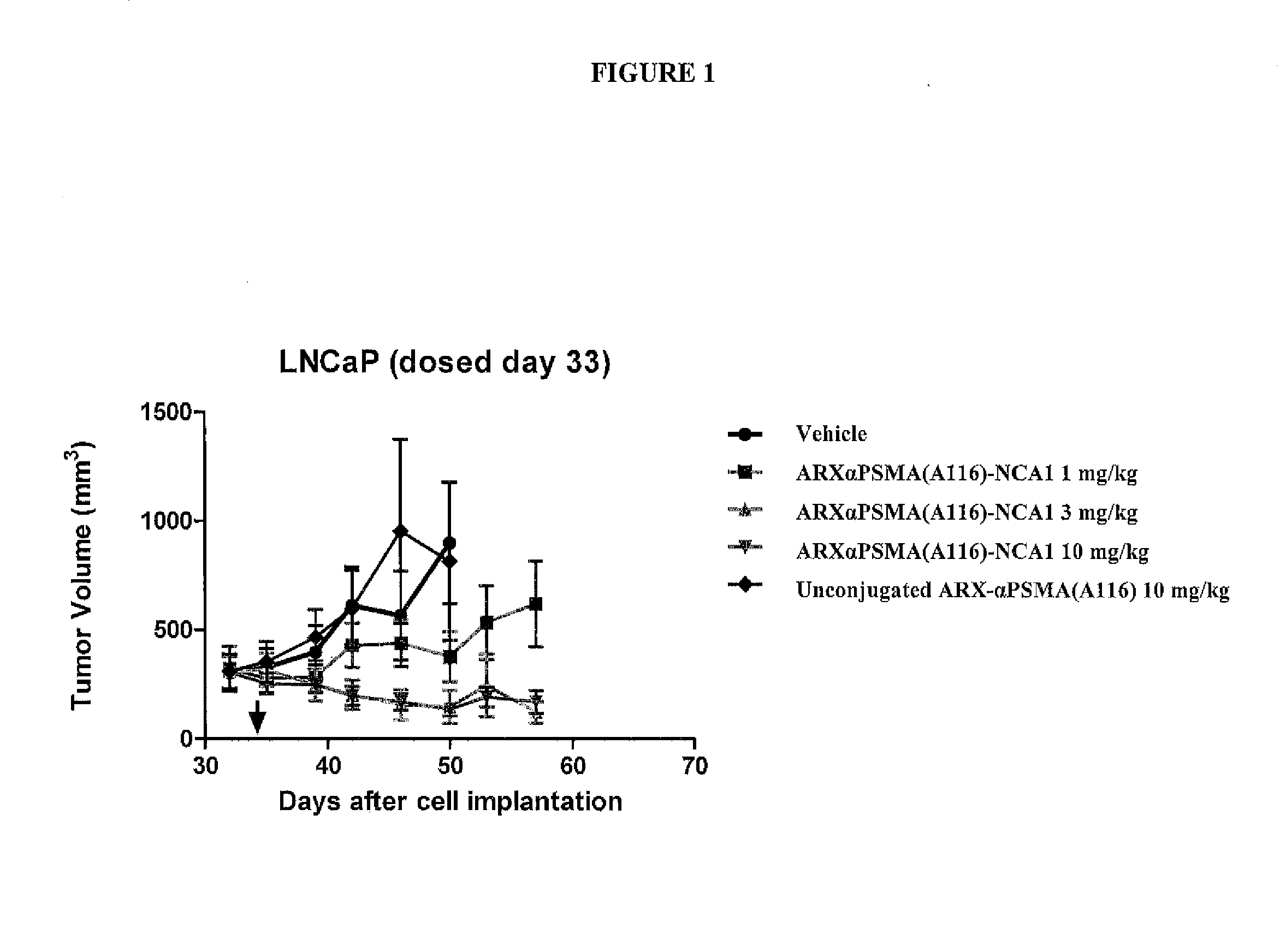
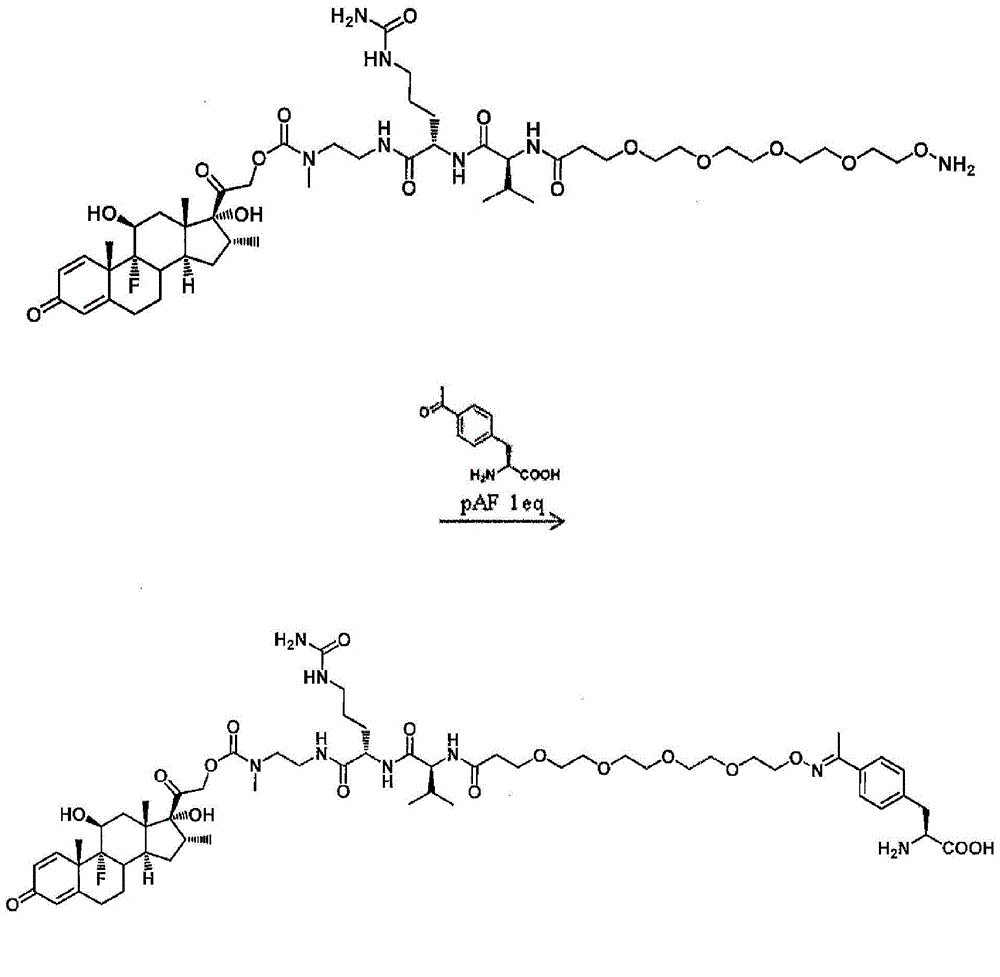
This invention relates to prostate-specific membrane antigen (PSMA) antibodies and antibody drug conjugates comprising at least one non-naturally-encoded amino acid. Disclosed herein are αPSMA antibodies with one or more non-naturally encoded amino acids and further disclosed are antibody drug conjugates wherein the αPSMA antibodies of the invention are conjugated to one or more toxins. Also disclosed herein are non-natural amino acid dolastatin analogs that are further modified post-translationally, methods for effecting such modifications, and methods for purifying such dolastatin analogs. Typically, the modified dolastatin analogs include at least one oxime, carbonyl, dicarbonyl, and / or hydroxylamine group. Further disclosed are methods for using such non-natural amino acid antibody drug conjugates, dolastatin analogs, and modified non-natural amino acid dolastatin analogs, including therapeutic, diagnostic, and other biotechnology uses.
Owner:AMBRX
Anti-psma antibodies conjugated to nuclear receptor ligand polypeptides
InactiveCN104619350AImmunoglobulins against cell receptors/antigens/surface-determinantsPharmaceutical non-active ingredientsAntigenProstate specific membrane
This invention relates to anti-prostate-specific membrane antigen antibodies (alphaPSMA) and alphaPSMA antibody - nuclear receptor ligand (NRL) conjugates comprising at least one non-naturally-encoded amino acid.
Owner:AMBRX
Treatment of Proliferative Disorders Using Antibodies to PSMA
InactiveUS20100291113A1Block enzymatic activityLimitation to intakeOrganic active ingredientsIn-vivo radioactive preparationsExtracellular StructureCancer therapy
Methods of treating cancer in a patient are provided. In some embodiments the method comprises administering an antibody that is capable of binding to the extracellular domain of PSMA. In some embodiments, the method comprises restricting folate intake by the patient. Methods of monitoring cancer therapy are provided as well as kits for treating cancer and kits for monitoring cancer therapy.
Owner:CORNELL UNIVERSITY
Anti-psma antibodies, bispecific antigen-binding molecules that bind psma and cd3, and uses thereof
ActiveCN108137700AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAntiendomysial antibodies
The present disclosure provides antibodies that bind to prostate- specific membrane antigen (PSMA), bispecific antibodies that bind to PSMA and CD3, and methods of using the same. According to certainembodiments, the antibodies of the disclosure bind human PSMA with high affinity and bind CD3 to induce human T cell proliferation. The disclosure includes antibodies that bind PSMA and CD3 and induce T cell-mediated killing of PSMA-expressing tumor cells. According to certain embodiments, the present disclosure provides bispecific antigen-binding molecules comprising a first antigen- binding domain that specifically binds human CD3, and a second antigen-binding molecule that specifically binds human PSMA. In certain embodiments, the bispecific antigen-binding molecules of the present disclosure are capable of inhibiting the growth of prostate tumors expressing PSMA. The antibodies and bispecific antigen- binding molecules of the disclosure are useful for the treatment of diseases and disorders in which an upregulated or induced targeted immune response is desired and / or therapeutically beneficial. For example, the antibodies of the disclosure are useful for the treatment of various cancers.
Owner:REGENERON PHARM INC
Pyrrolobenzodiazepine—anti-PSMA antibody conjugates
ActiveUS10751346B2Organic active ingredientsImmunoglobulins against animals/humansDimerAntiendomysial antibodies
Owner:MEDIMMUNE LTD
Kit for detecting prostatic cancer based on liquid biopsy
ActiveCN106970221AEfficient enrichmentEasy to distinguishBiological testingFluorescenceTUMOUR DETECTION
The invention provides a kit for detecting prostatic cancer based on liquid biopsy, comprising a staining enhancement solution for enhancing staining effect and specific antibodies with a fluorescent staining marker, wherein the specific antibodies include PSA (prostate specific antigen), CD45 antibody and PSMA (prostate specific membrane antigen); the staining enhancement solution comprises a surfactant having a concentration of 0.001-1 mg / mL. The kit herein is capable of effectively enriching target cells, determining whether the enriched target cells are derived from a patient with early prostatic cancer, and typing CTCs (circulating tumor cells); in addition, double-tumor-marker detection increases detection sensitivity, and detection accuracy is guaranteed through further CEP8 detection; furthermore, the staining effect is enhanced through the staining enhancement solution, each of various antibodies with a fluorescent staining marker can bind with a target cell, the target cells can be stained more effectively, fluorescence is greater, and the boundary is vivid.
Owner:上海美吉医学检验有限公司
Psma binding antibody and use thereof
ActiveCN108699157AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody medical ingredientsAntiendomysial antibodiesSquamous Carcinomas
The present invention provides a novel PSMA binding antibody termed 10B3 and pharmaceutical and diagnostic uses of the antibody 10B3. The PSMA antibody 10B3 does not cross-compete with the state of the art PMSA binding antibody J591 and has a reduced induction of antigen shift compared to J591 and a unique reactivity with squamous cell carcinoma (SCC) cells of different origin.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS +1
Pyrrolobenzodiazepine-anti-PSMA antibody conjugates
ActiveUS10736903B2Organic active ingredientsImmunoglobulins against animals/humansDimerAntiendomysial antibodies
Owner:MEDIMMUNE LTD
PSMA Binding Antibody and Uses Therof
ActiveUS20190022205A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody medical ingredientsAntiendomysial antibodiesSquamous Carcinomas
The present invention provides a novel PSMA binding antibody termed 10B3 and pharmaceutical and diagnostic uses of the antibody 10B3. The PSMA antibody 10B3 does not cross-compete with the state of the art PMSA binding antibody J591 and has a reduced induction of antigen shift compared to J591 and a unique reactivity with squamous cell carcinoma (SCC) cells of different origin.
Owner:UNIV TUBINGEN +1
Antibody therapeutics that bind psma
There is disclosed compositions and methods relating to or derived from anti-PSMA antibodies. More specifically, there is disclosed fully human antibodies that bind PSMA, PSMA-antibody binding fragments and derivatives of such antibodies, and PSMA-binding polypeptides comprising such fragments. Further still, there is disclosed nucleic acids encoding such antibodies, antibody fragments and derivatives and polypeptides, cells comprising such polynucleotides, methods of making such antibodies, antibody fragments and derivatives and polypeptides, and methods of using such antibodies, antibody fragments and derivatives and polypeptides, including methods of treating a disease. There is further disclosed targeted therapies for treatment of prostate cancer having the disclosed anti-PSMA antibodies and fragments thereof targeting the therapeutic agent or cell.
Owner:SORRENTO THERAPEUTICS INC
Antibodies against prostate specific membrane antigen
InactiveUS8629247B2Reduce the overall heightPromote cell deathAntibody ingredientsImmunoglobulinsProstate specific membraneAntigen Binding Fragment
Antibodies (Ab) and antigen binding fragments capable of binding to prostate specific membrane antigen and which may be used for diagnostic and therapeutic purposes are provided herein. Formulation anti-PSMA antibodies which are stable under extreme storage condition are also provided.
Owner:PROSCAN RX PHARMA
Prostate cancer diagnosis and treatment
InactiveUS20090131277A1Symptoms improvedDecrease and prevents and delayIn-vivo radioactive preparationsPeptide/protein ingredientsLymphatic SpreadProstate cancer
The present invention relates to novel mimetopes of anti-PSMA antibodies and their use for detecting, imaging, staging, treating and monitoring of prostate cancer, and / or metastatis thereof. The present invention also relates to novel pharmaceutical compositions for the treatment of prostate cancer. Furthermore the present invention relates to assay systems and kits for detecting, imaging, staging, treating and monitoring of prostate cancer, and / or metastasis thereof.
Owner:PROSCAN RX PHARMA
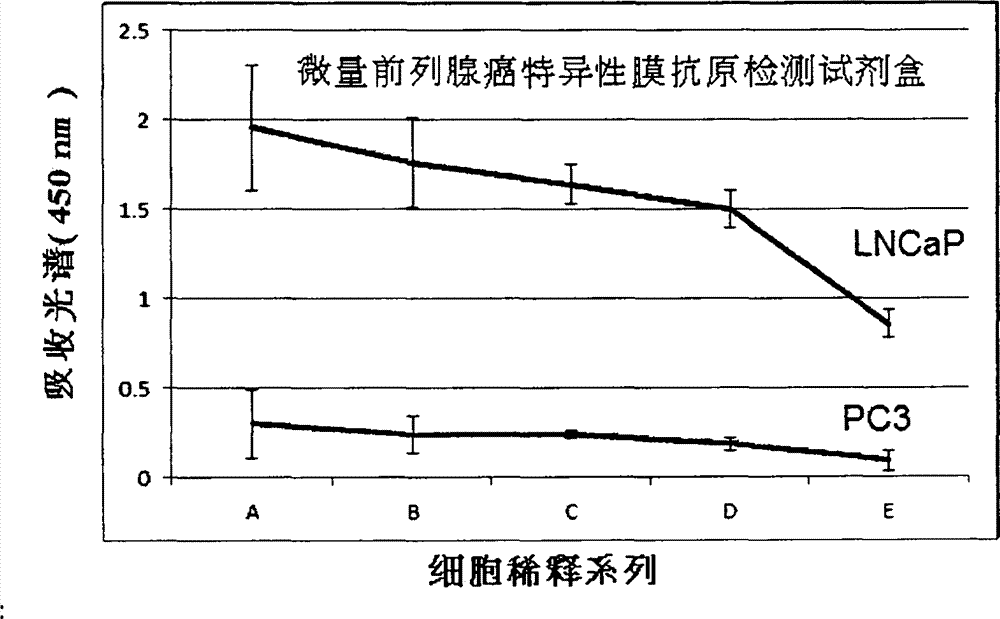
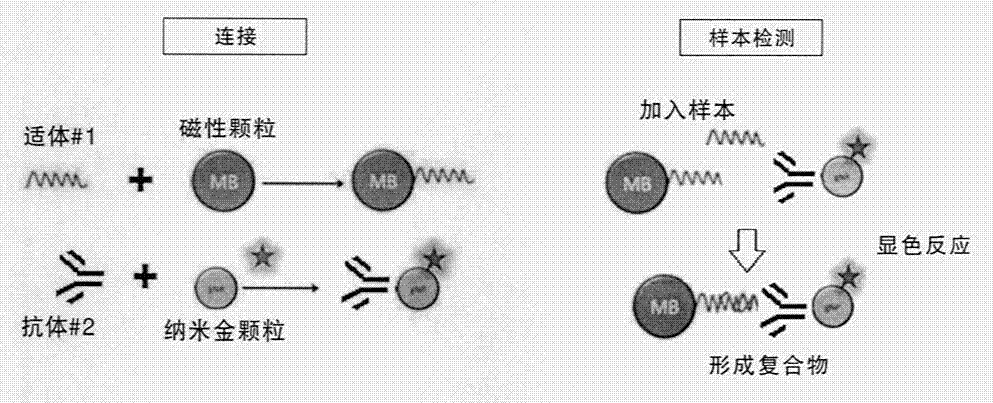
Method for detecting tiny amount of prostate cancer specific membrane antigen in circulating blood, and kit thereof
The invention provides a method for detecting a tiny amount of a prostate cancer specific membrane antigen in circulating blood. The method comprises the following steps: respectively mixing a magnetic particle coupled specific PSMA (prostate specific membrane antigen) aptamer and a nanogold particle coupled specific PSMA antibody with a sample to be detected, detecting, and identifying and / or quantifying a target molecule (PSMA). The invention also relates to a pretreatment agent of the blood sample to be detected, and a detection kit which are used for the method. The method has the advantages of simple use, sensitivity, strong specificity, good repeatability, fast result obtaining, and no complex apparatuses or special skills, and still keeps practical sensitivity and specificity when the sample to be detected is directly detected without precise purification, so the detection operation is greatly convenient, and practical industrial application of the method is popularized.
Owner:SUZHOU YOULIN BIO TECH
Antibodies against prostate specific membrane antigen
InactiveUS20120093719A1Reduce the overall heightPromote cell deathRadioactive preparation carriersAntibody ingredientsProstate specific membraneAntigen Binding Fragment
Antibodies (Ab) and antigen binding fragments capable of binding to prostate specific membrane antigen and which may be used for diagnostic and therapeutic purposes are provided herein. Formulation anti-PSMA antibodies which are stable under extreme storage condition are also provided.
Owner:PROSCAN RX PHARMA
Humanized Anti-prostate-specific membrane antigen (PSMA) antibody drug conjugates
PendingCN111989138AIncrease or decrease decompositionImprove bindingOrganic active ingredientsDipeptide ingredientsDiseaseAntiendomysial antibodies
The invention relates to prostate specific membrane antigen humanized antibodies (anti-PSMA) and anti-PSMA antibody drug conjugates. The invention also relates to methods and compositions for using anti-PSMA antibody drug conjugates in inhibiting, preventing or treating PSMA related diseases or cancers.
Owner:AMBRX
Prostate-specific membrane antigen antibody drug conjugates
ActiveUS10800856B2Increase doseAvoid toxicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenDrug conjugation
This invention relates to prostate-specific membrane antigen (PSMA) antibodies and antibody drug conjugates comprising at least one non-naturally-encoded amino acid. Disclosed herein are αPSMA antibodies with one or more non-naturally encoded amino acids and further disclosed are antibody drug conjugates wherein the αPSMA antibodies of the invention are conjugated to one or more toxins. Also disclosed herein are non-natural amino acid dolastatin analogs that are further modified post-translationally, methods for effecting such modifications, and methods for purifying such dolastatin analogs. Typically, the modified dolastatin analogs include at least one oxime, carbonyl, dicarbonyl, and / or hydroxylamine group. Further disclosed are methods for using such non-natural amino acid antibody drug conjugates, dolastatin analogs, and modified non-natural amino acid dolastatin analogs, including therapeutic, diagnostic, and other biotechnology uses.
Owner:AMBRX
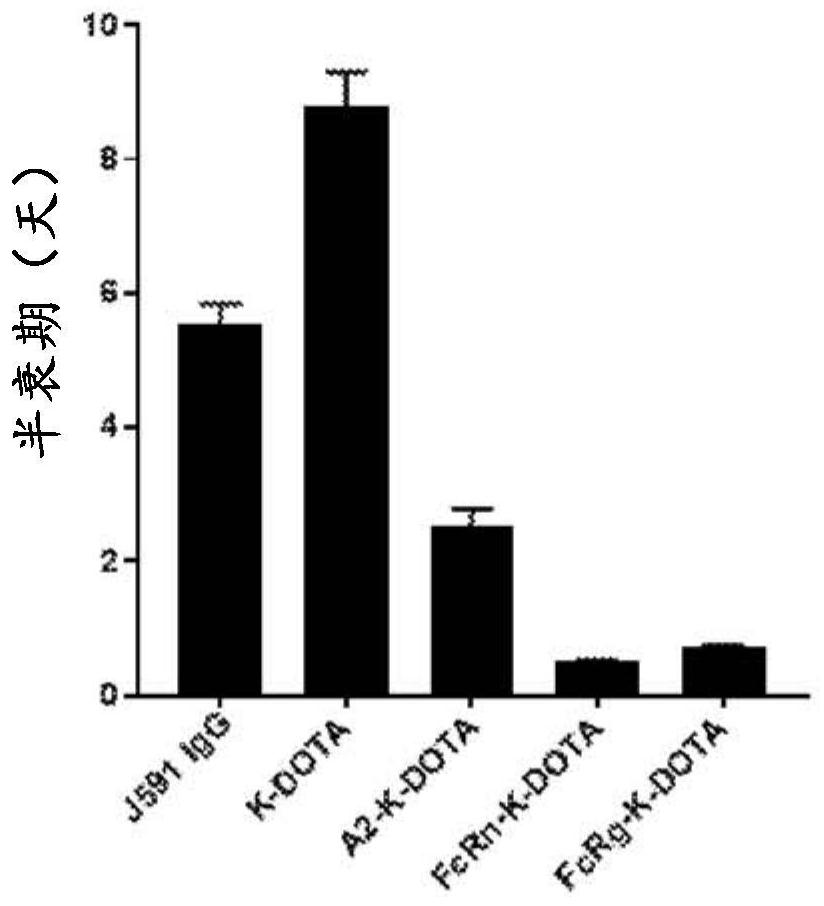
Antibodies for binding to PSMA with reduced affinity for neonatal Fc receptor
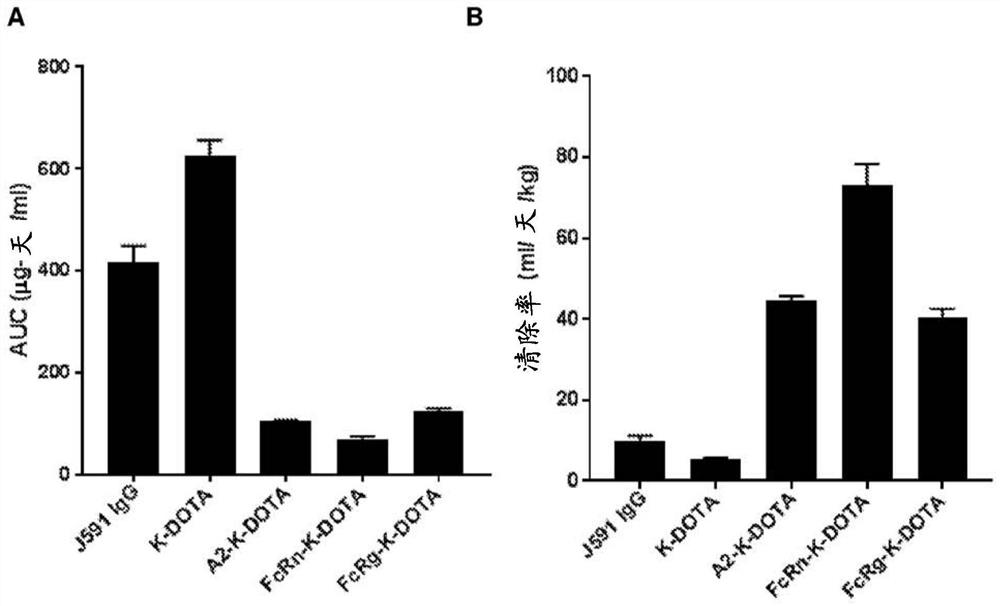
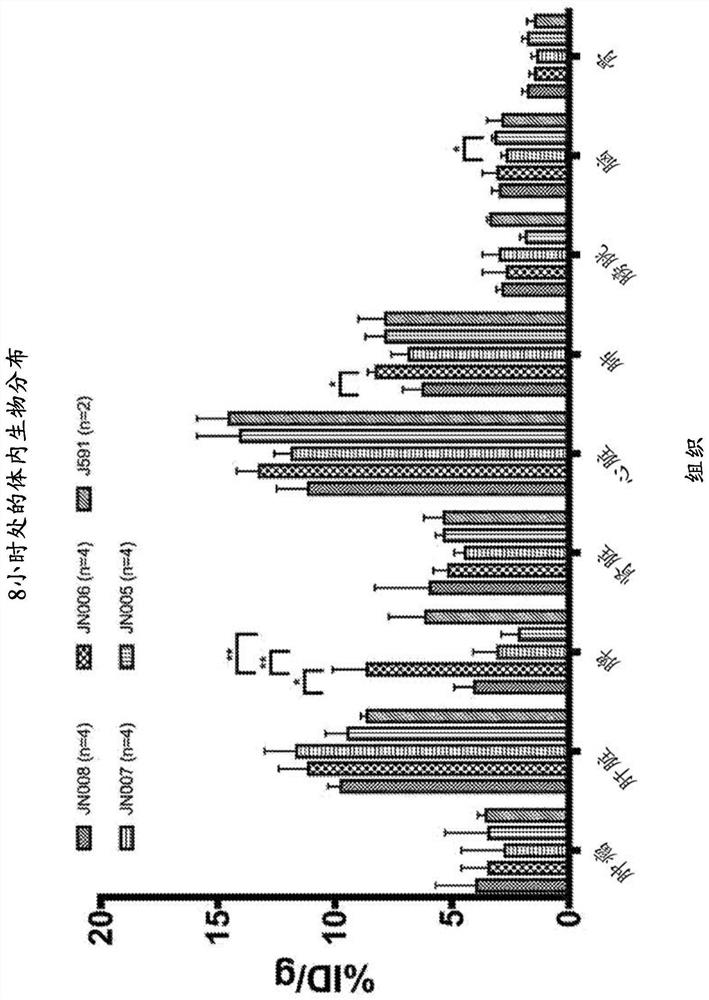
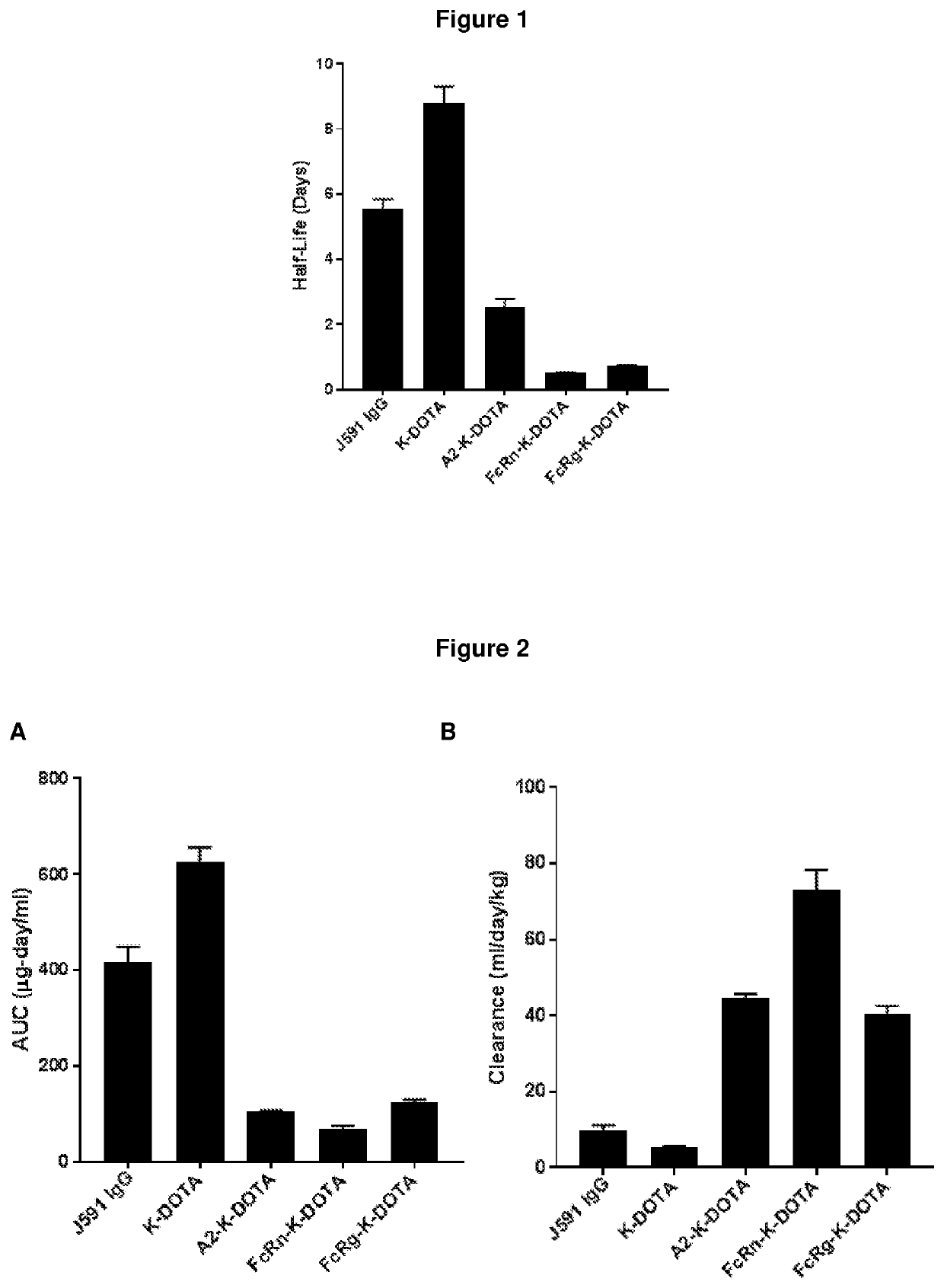
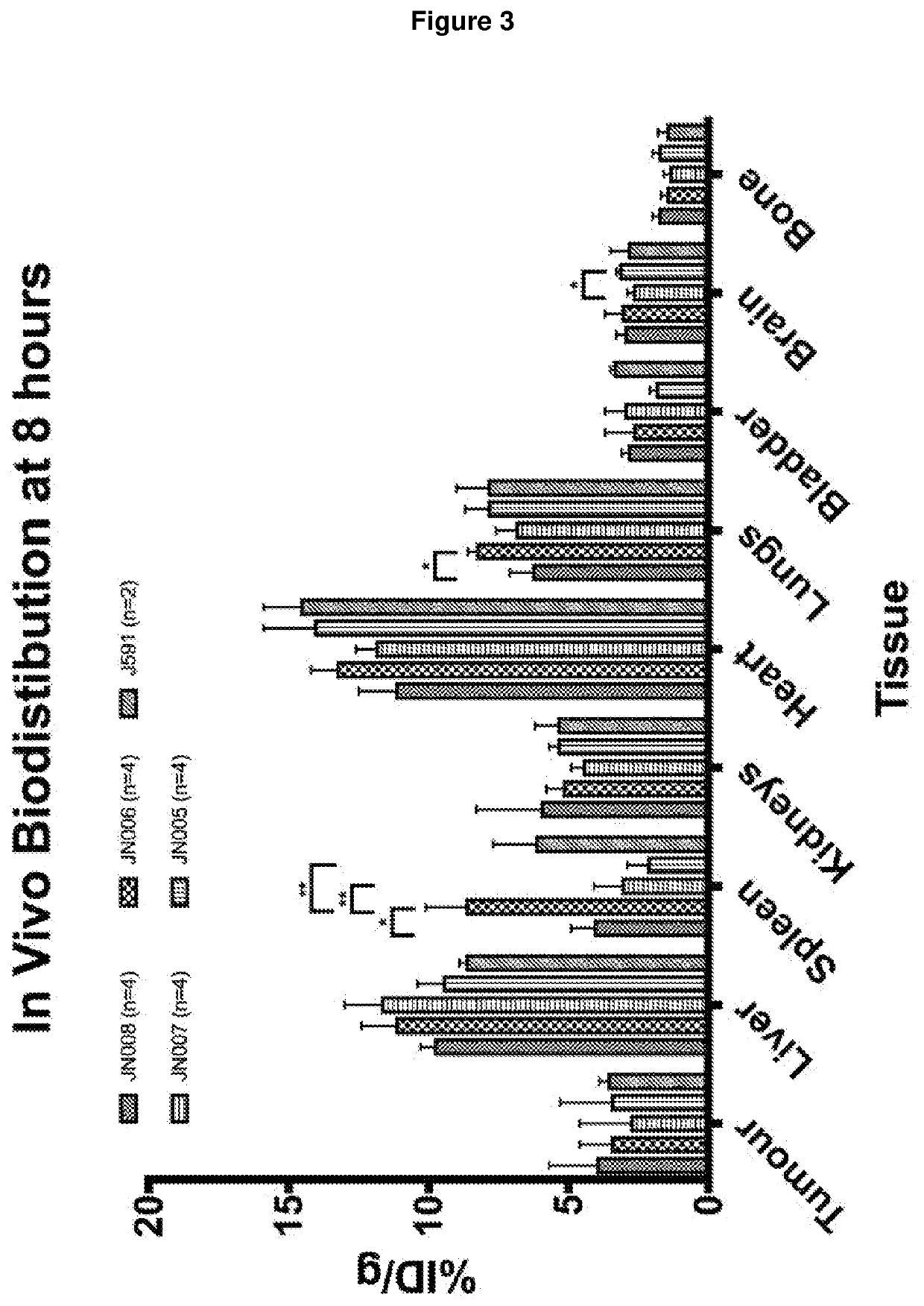
PendingCN114555641AReduced serum half-lifeQuick clearImmunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersAntiendomysial antibodiesAmino acid substitution
The present invention relates to anti-PSMA antibodies comprising a heavy chain constant region containing one or more amino acid substitutions as compared to wild-type IgG wherein the one or more amino acid substitutions reduce the affinity of the antibody for neonatal Fc receptor (FcRn) as compared to an IgG-based wild-type antibody, thereby reducing the serum half-life of the modified antibody. The one or more amino acid modifications, which have the effect of reducing FcRn binding, are selected from the group consisting of positions His310, His433, His435, His436, Ile253. The antibodies of the invention are particularly suitable for use in radioimmunotherapy.
Owner:泰利斯制药(创新)有限公司
Antibody therapeutics that bind PSMA
There is disclosed compositions and methods relating to or derived from anti-PSMA antibodies. More specifically, there is disclosed fully human antibodies that bind PSMA, PSMA-antibody binding fragments and derivatives of such antibodies, and PSMA-binding polypeptides comprising such fragments. Further still, there is disclosed nucleic acids encoding such antibodies, antibody fragments and derivatives and polypeptides, cells comprising such polynucleotides, methods of making such antibodies, antibody fragments and derivatives and polypeptides, and methods of using such antibodies, antibody fragments and derivatives and polypeptides, including methods of treating a disease. There is further disclosed targeted therapies for treatment of prostate cancer having the disclosed anti-PSMA antibodies and fragments thereof targeting the therapeutic agent or cell.
Owner:SORRENTO THERAPEUTICS INC
A kit for detecting prostate cancer based on liquid biopsy
ActiveCN106970221BEfficient enrichmentEasy to distinguishBiological testingFluorescenceTUMOUR DETECTION
Owner:上海美吉医学检验有限公司
Antibodies for binding psma with reduced affinity for the neonatal fc receptor
PendingUS20220323619A1Reducing serum half-lifeLow affinityImmunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersAntiendomysial antibodiesHeavy chain
The invention relates to anti-PSMA antibodies comprising a heavy chain constant region comprising one or more amino acid substitutions compared to a wild-type IgG, wherein the one or more amino acid substitutions reduce the affinity of the antibody for the neonatal Fc receptor (FcRn), thereby reducing the serum half-life of the modified antibody compared to a wild-type antibody of class IgG. The one or more amino acid modification having the effect of reducing FcRn binding is selected from positions His310, His433, His435, His436, Ile253. Antibodies of the present invention are particularly suited for use in radioimmunotherapy.
Owner:TELIX INT PTY LTD
Heterodimeric antibodies that bind prostate specific membrane antigen (PSMA) and cd3
PendingUS20220119525A1Good choiceMinimized reactivityHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesProstate specific membrane
Provided herein are novel antigen binding domains and antibodies (e.g., heterodimeric antibodies) that bind Prostate Specific Membrane Antigen (PSMA). In exemplary embodiments, the anti-PSMA antibodies also bind CD3. Such antibodies that bind PSMA and CD3 are useful, for example in the treatment of PSMA-related cancer.
Owner:XENCOR
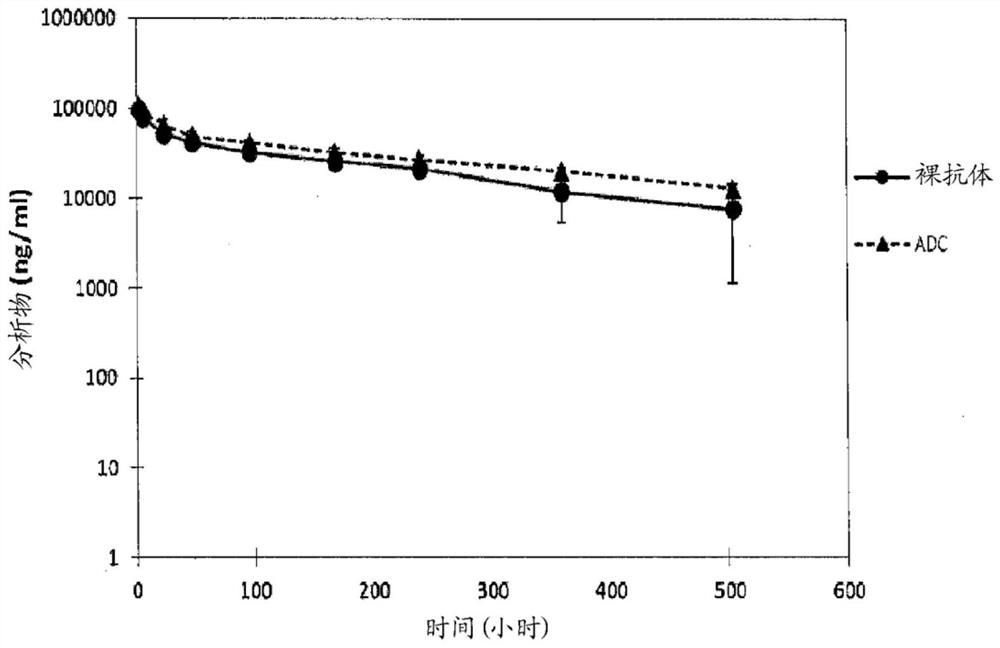
Combination therapy with an Anti-psma antibody-drug conjugate
InactiveUS20200129638A1Improve homogeneityIncreased durabilityOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDrug conjugationAntiendomysial antibodies
The present disclosure relates to combination therapies for the treatment of pathological conditions, such as cancer. In particular, the present disclosure relates to combination therapies comprising treatment with an Antibody Drug Conjugate (ADC) and a secondary agent.
Owner:ADC THERAPEUTICS SA +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com