Cancer therapy
A prostate cancer and vaccine technology, applied in the field of cancer therapy, can solve problems such as non-certification of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0239] Example 1 - The role of PD-1 in patients who previously received the PAP vaccine
[0240] Although DNA vaccines elicit antigen-specific CD8+ T cells in humans, persisting over a timescale of approximately years, the persistence of antigen-expressing tumors suggests that tumor escape mechanisms are at work. For example, data collected in human trials indicates that some patients show no signs of an immune response, and even those who do still show signs of disease progression. Therefore, to improve the immunological activity of DNA vaccines (eg, in the development of DNA vaccines for the treatment of prostate cancer), experiments were performed during the development of embodiments of the technology to collect data related to the mechanism of tumor resistance.
[0241] To assess the role of PD-1 in patients who previously received a DNA vaccine encoding PAP. Specifically, similar to previously reported studies, experiments were performed using a delayed-type hypersensit...
Embodiment 2
[0243] Example 2 - Results of co-administration of pembrolizumab and pTVG in patients with castration-resistant metastatic prostate cancer- Clinical results obtained with the HP DNA vaccine
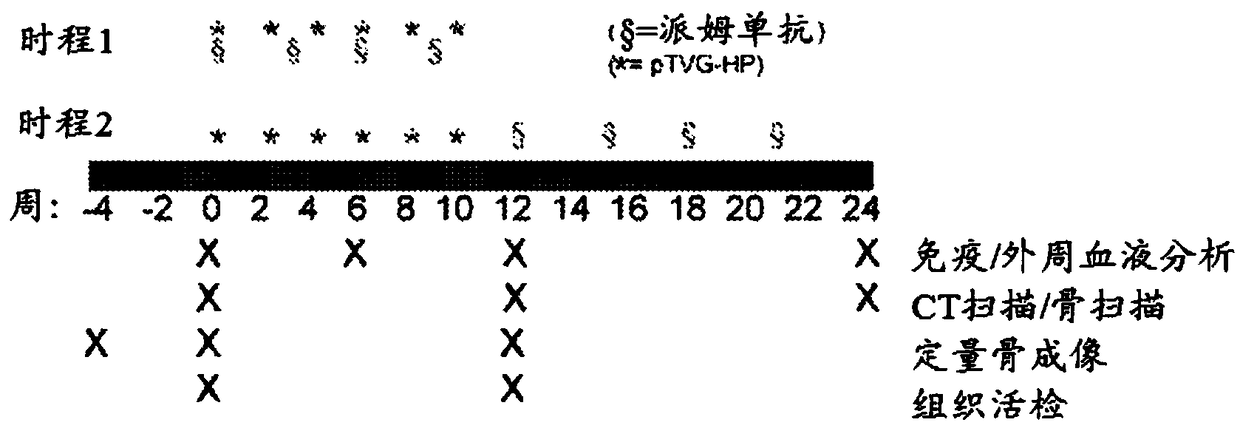
[0244] This example presents results obtained from co-administration of pembrolizumab and pTVG-HP DNA vaccine to patients with castration-resistant metastatic prostate cancer. Briefly, patients in the trial were divided into two groups. The first arm (concurrent arm) received concurrent administration of pembrolizumab and pTVG-HP DNA vaccine on day 1, followed by subsequent administration of pembrolizumab every 3 weeks thereafter, and subsequent administration of pTVG-HP DNA every 2 weeks thereafter Vaccine up to 10 weeks. The second cohort (sequential cohort) received pTVG-HP DNA vaccine every 2 weeks for a total of 10 weeks, followed by pembrolizumab every 3 weeks starting at week 12 until week 21. The schedule of administration is outlined in figure 1 middle. Results: Although tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com