Anti-PD-L1/OX40 bispecific antibody preparation as well as preparation method and application thereof
A bispecific antibody and PD-L1 technology, applied in the direction of antibodies, anti-inflammatory agents, antibody medical components, etc., can solve problems such as the complexity of degradation pathways and the prediction of preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0148] II. Preparation of Formulations
[0149] The present invention provides a stable formulation comprising anti-PD-L1 / OX40 bispecific antibody protein. Anti-PD-L1 / OX40 bispecific antibody proteins used in formulations of the invention can be prepared using techniques known in the art for producing antibodies. For example, antibodies can be produced recombinantly. In a preferred embodiment, the antibodies of the invention are produced recombinantly in 293 cells or CHO cells.
[0150] Antibodies are now widely used as active ingredients of pharmaceuticals. Techniques for purifying therapeutic antibodies to pharmaceutical grade are well known in the art. For example, Tugcu et al. (Maximizing productivity of chromatography steps for purification of monoclonal antibodies, Biotechnology and Bioengineering 99 (2008) 599–613.) describe the use of ion exchange chromatography (anionic IEX and / or cationic CEX chromatography) of monoclonal antibodies following a protein A capture s...
Embodiment 1
[0196] Example 1. Preparation and purification of anti-PD-L1 / OX40 bispecific antibody
[0197] An anti-PD-L1 / OX40 bispecific antibody was obtained as described in PCT Application No. PCT / CN2020 / 073959. The antibody has a peptide chain #1 sequence of SEQ ID NO: 1 and a peptide chain #2 sequence of SEQ ID NO: 7, and is a bispecific antibody. PCT Application No. PCT / CN2020 / 073959 is hereby incorporated by reference in its entirety.
[0198] Briefly, antibodies are expressed recombinantly in CHO cells and purified by filtration, chromatography, virus inactivation, filtration, etc.
Embodiment 2
[0199] Embodiment 2.pH screening test
[0200] 2.1 Experimental steps
[0201] In this example, the effect of pH (5.0-7.0) on the stability of the purified anti-PD-L1 / OX40 bispecific antibody in Example 1 was investigated. A total of 5 pH values were designed, which were 5.0, 5.5, 6.0, 6.5 and 7.0.
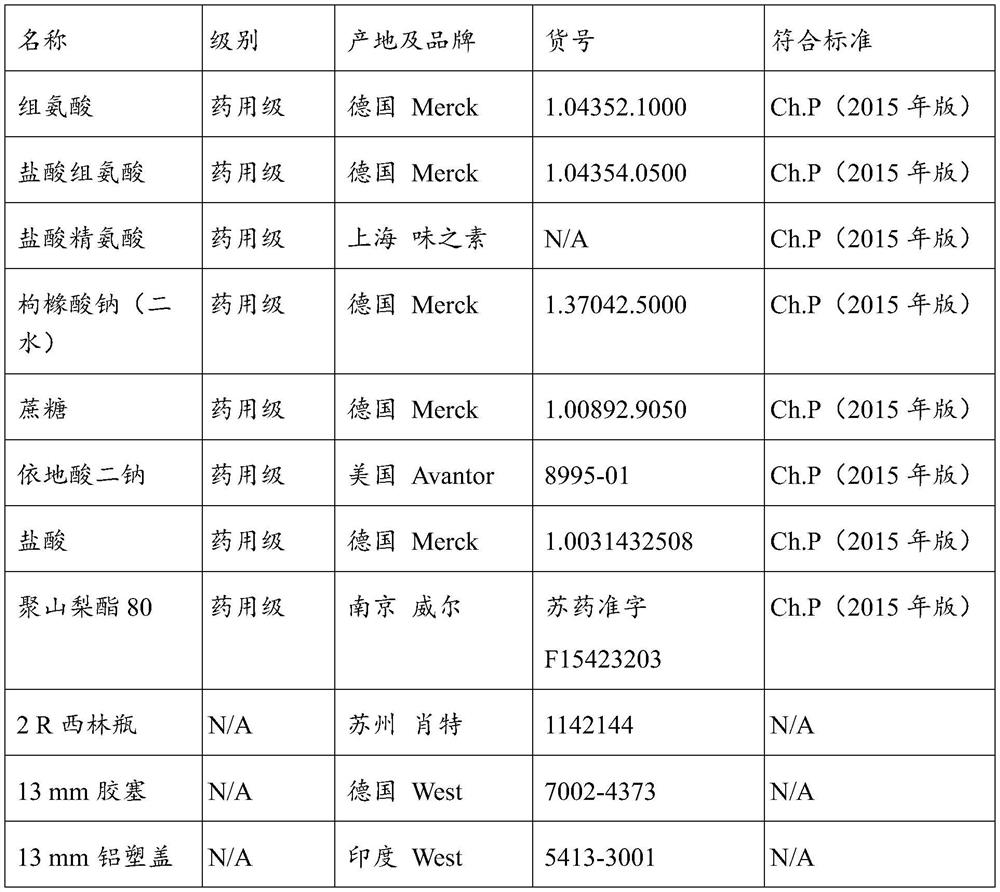
[0202] Prepare 10mM histidine, 5% (w / v) sorbitol buffer solution, adjust the pH to 5.0, 5.5, 6.0, 6.5 and 7.0 with hydrochloric acid, and replace the anti-PD-L1 / OX40 bispecific antibody ultrafiltration to In the above buffers with different pH values, adjust the protein content of the sample to about 30mg / ml; and add polysorbate 80 to make the final concentration about 0.2mg / ml; filter and pack into 2R vials, stopper and cap . The above samples were placed under the condition of 40°C±2°C for stability investigation, and the specific scheme is shown in the table.
[0203] Table 1. Pre-prescription study protocol
[0204]
[0205] Note: (1) √ indicates sampling at this poi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com