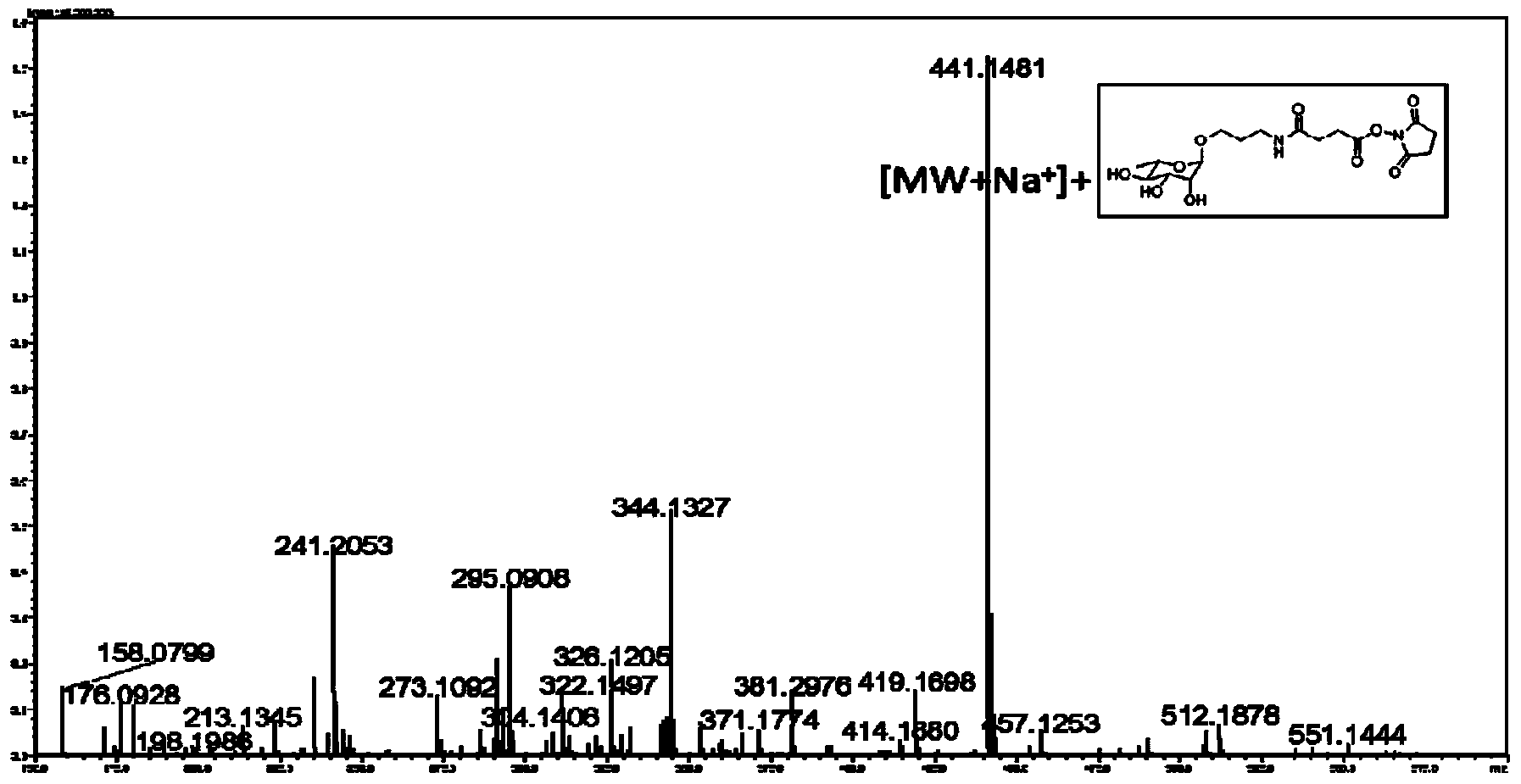
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Whole cell vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Staphylococcus antigen and vaccine
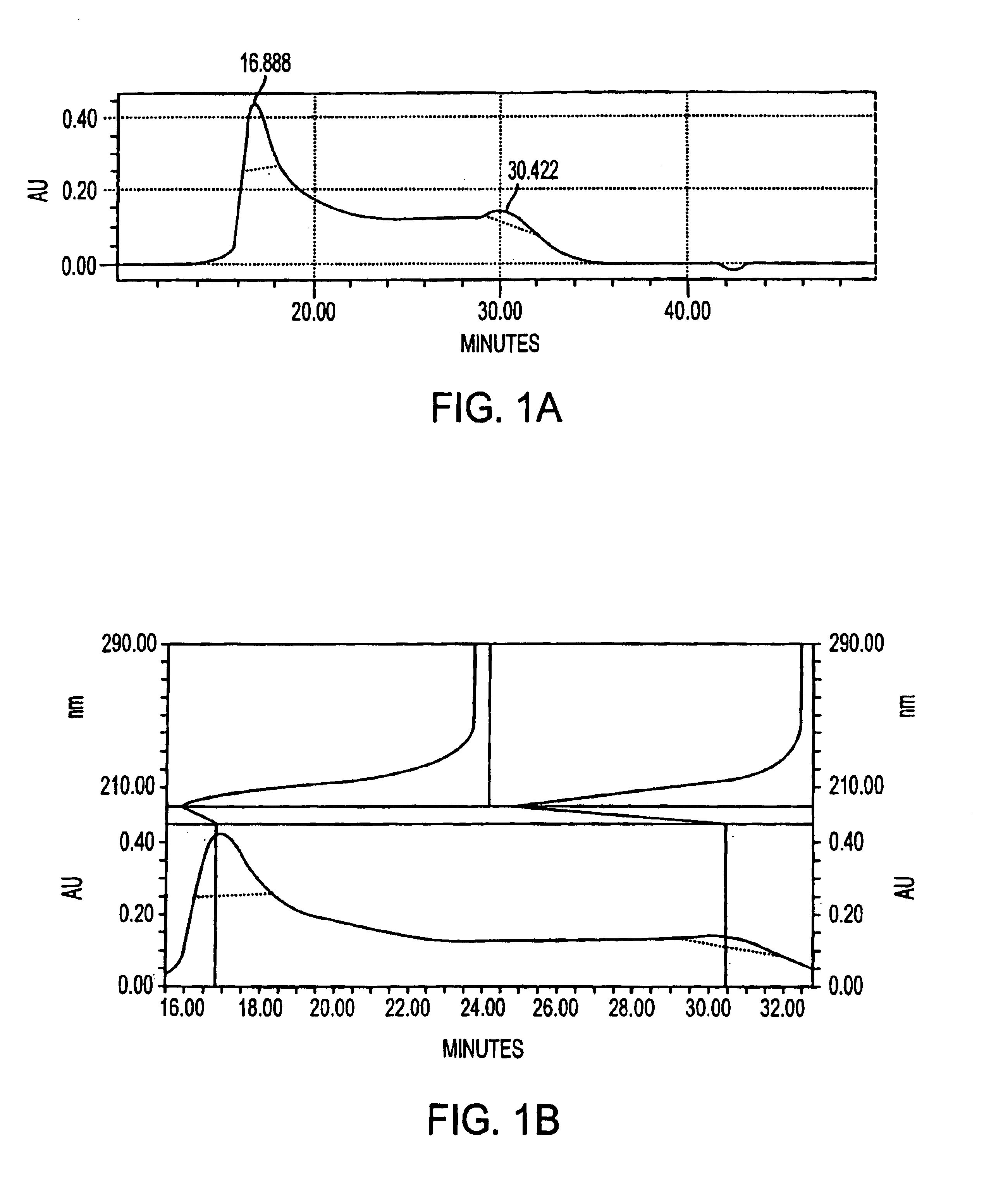
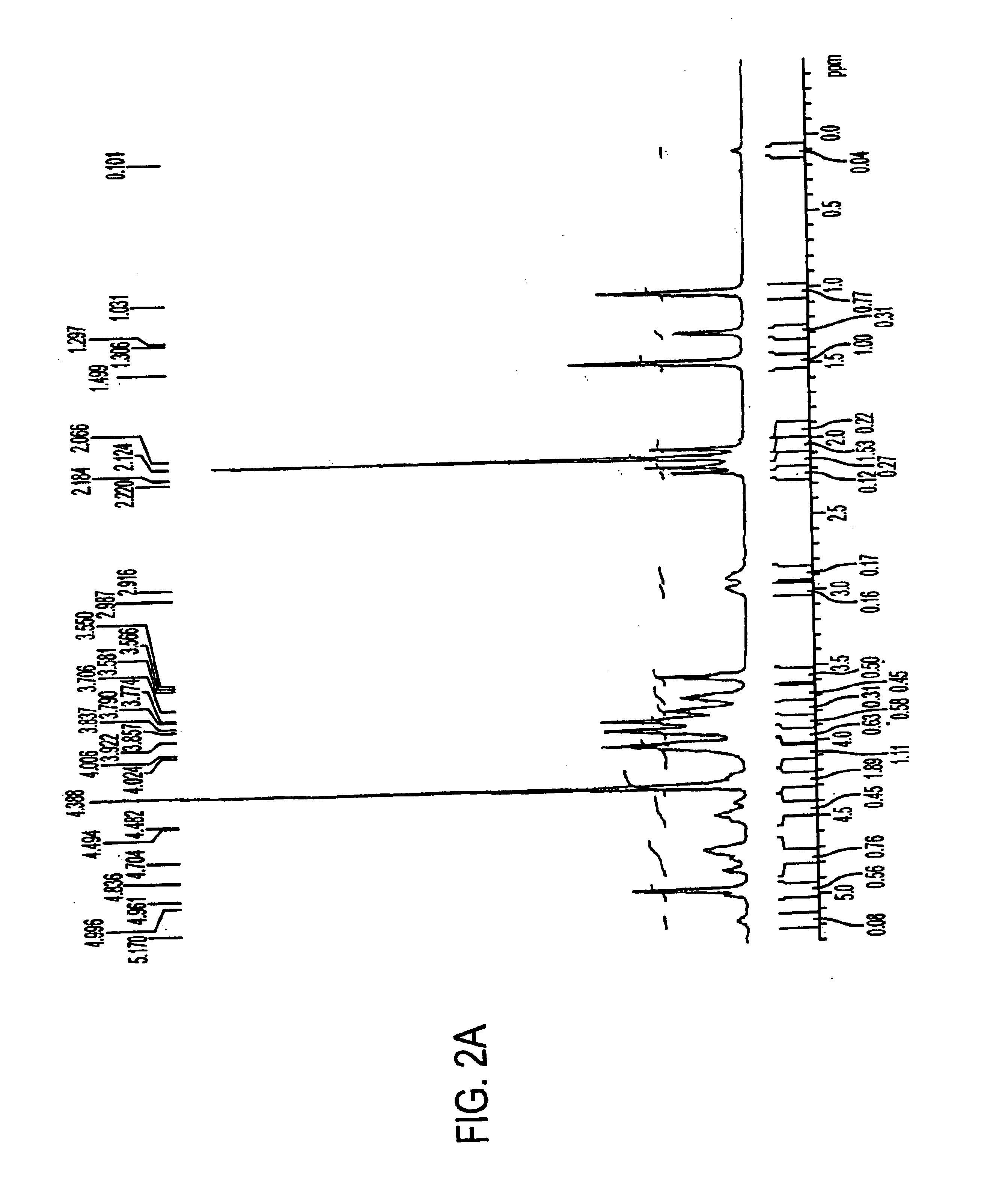
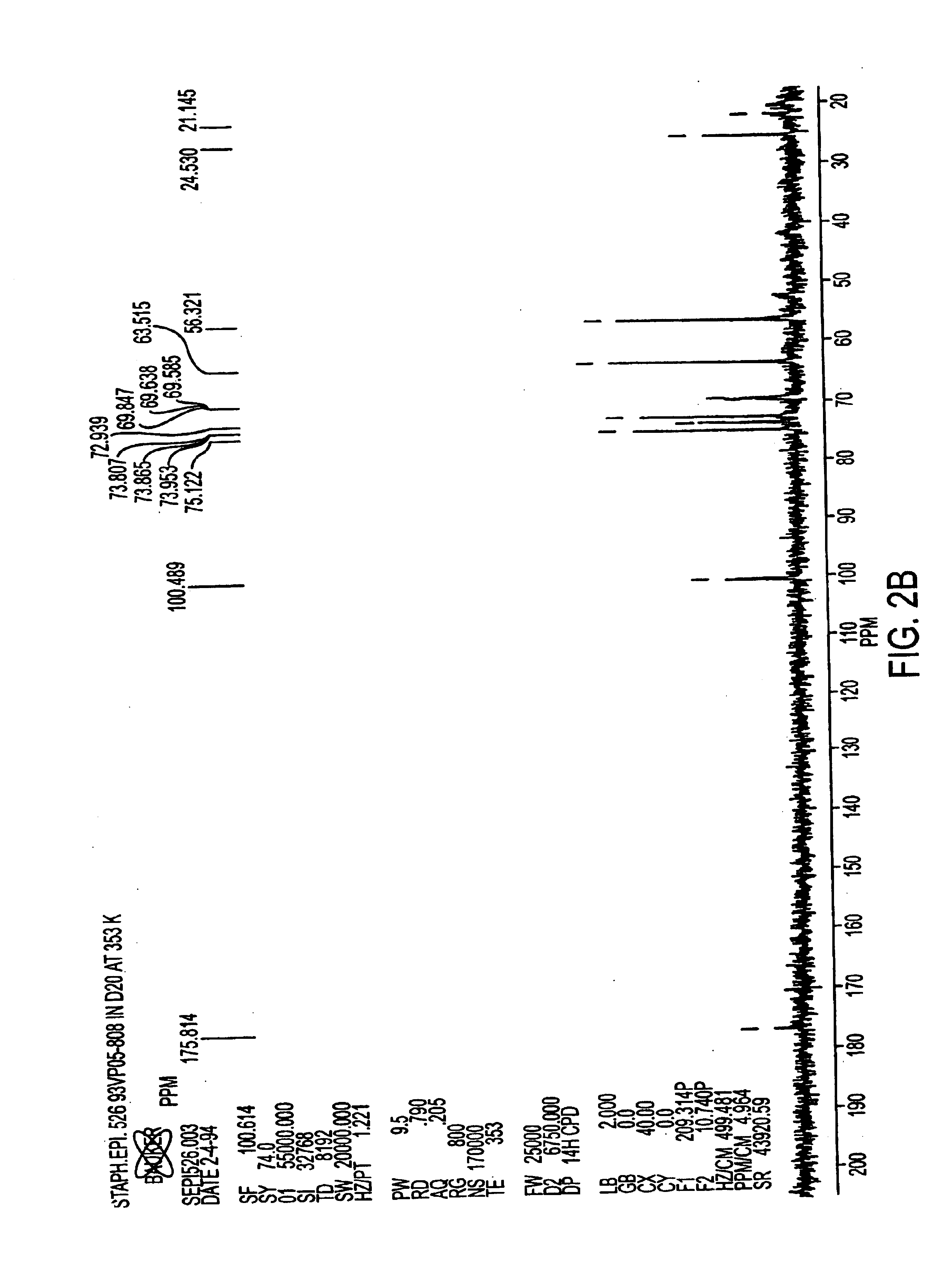
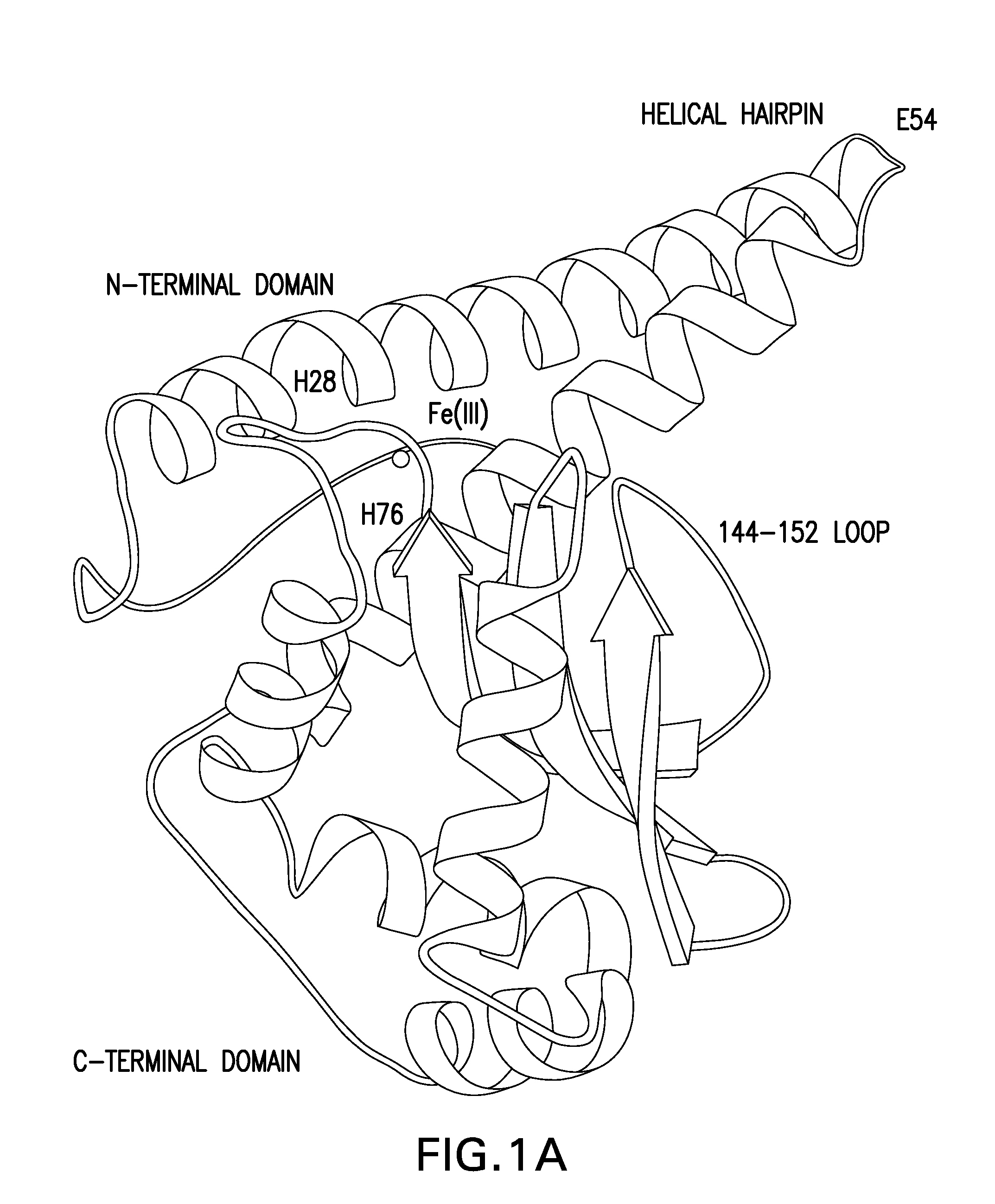
A negatively-charged Staphylococcus antigen contains amino acids and a N-acetylated hexosamine as a major carbohydrate component. The antigen is common to many coagulase-negative strains of Staphylococcus, including S. epidermidis, S. haemolyticus, and S. hominis. Staphylococcus strains that carry the antigen include many clinically significant strains of Staphylococcus. The antigen and antibodies to the antigen are useful in kits and assays for diagnosing Staphylococcus infection. Vaccines of the antigen and of whole cells that carry the antigen also are disclosed.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
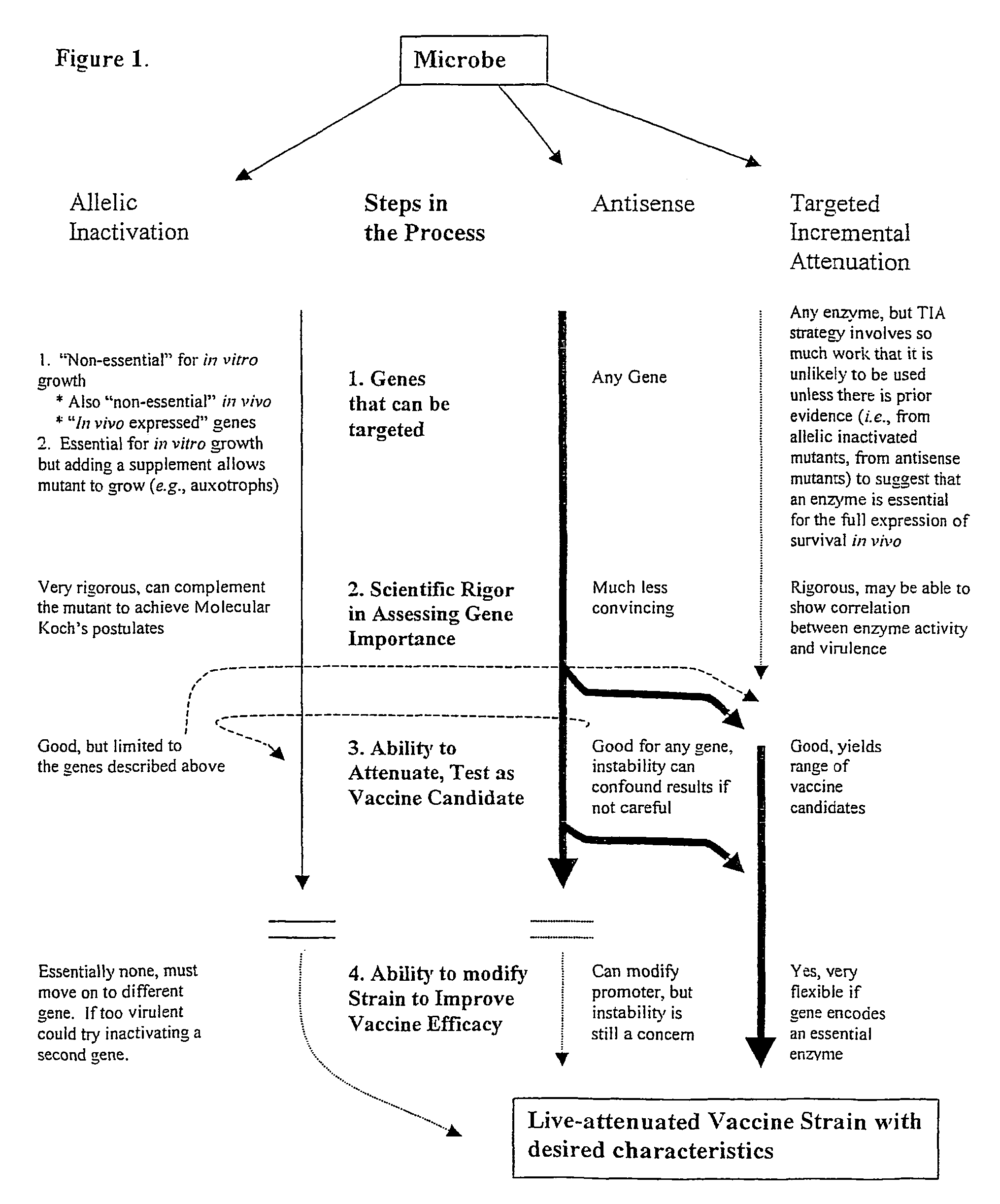
Methods of enhancing the immunogenicity of mycobacteria and compositions for the treatment of cancer, tuberculosis, and fibrosing lung diseases
InactiveUS20110243992A1Enhance recruitmentIncreased activationAntibacterial agentsBacteriaMycobacterium immunogenumCancer cell
Whole-cell vaccines and methods for enhancing the immunogenicity of cellular microorganisms for use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria or for use as vectors to express exogenous antigens and induce responses against other infectious agents or cancer cells. The present invention involves an additional method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme produced by the intracellular microbe is reduced by expressing a mutant copy of the enzyme, thereby modifying the microbe so that it increases immunogenicity.
Owner:VANDERBILT UNIV
Drug and tumor whole-cell vaccine for treating or preventing tumor, and preparation methods and applications of drug and whole-cell vaccine
InactiveCN102343086AImprove immunityAmplify specific immune responseAntibody medical ingredientsAntineoplastic agentsAntineoplastic ImmunotherapeuticWhite blood cell
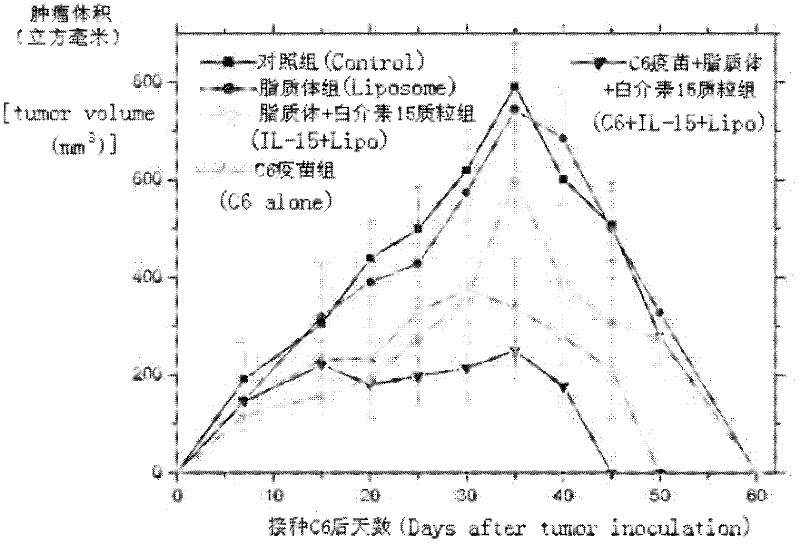
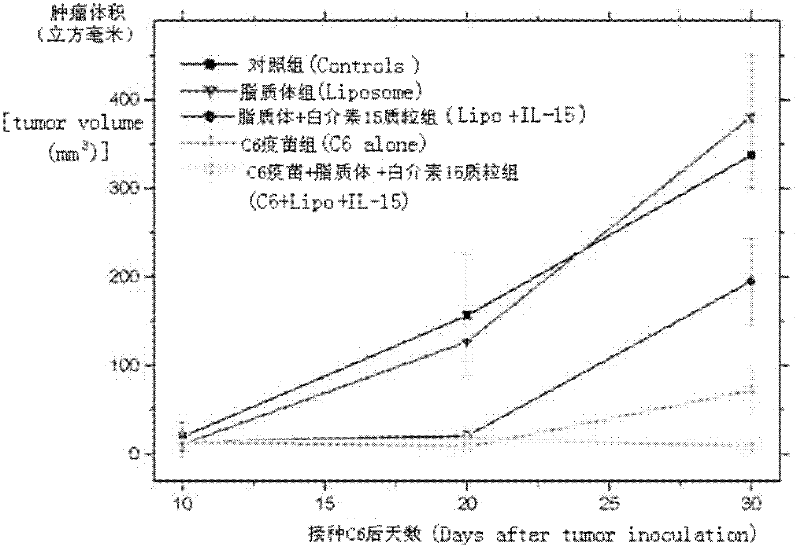
The invention relates to a drug and a tumor whole-cell vaccine for treating or preventing tumor, and preparation methods and applications of the drug and the whole-cell vaccine, and belongs to the fields of bioengineering and biological immunology. The invention provides a drug with good effect for treating or preventing tumor and a tumor whole-cell vaccine. The drug comprises the tumor whole-cell vaccine, a cation liposome, and recombinant plasmid for encoding interleukin-15 gene. The drug has the dual actions of tumor prevention immunization and anti-tumor immune treatment, and has durable action and obvious tumor prevention or treatment effect.
Owner:SICHUAN UNIV
Fusion protein, recombinant vector, recombinant dendritic cell for transmembrane expression of novel coronavirus antigen S2, and application thereof
ActiveCN112409496APrevent intrusionImproving immunogenicitySsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeDendritic cell
The invention provides a fusion protein, a recombinant vector, recombinant dendritic cells for transmembrane expression of novel coronavirus antigen S2, and application thereof, and belongs to the technical field of whole-cell vaccines. The fusion protein comprises a CD4 signal peptide, a novel coronavirus antigen S2 protein, a Flag tag sequence and a CD4 transmembrane domain which are linked in sequence; According to the invention, transmembrane cell expression is independently carried out on S2, ADE risks possibly caused by other S protein epitopes are avoided. The cell vaccine constructed by the fusion protein provided by the invention can induce higher neutralizing antibody titer in a mouse body.
Owner:焦顺昌 +1
Pro-Apoptotic Bacteria and Compositions for Delivery and Expression of Antigens
InactiveUS20090325298A1Enhance antigen presentationImproves vaccine efficacyBacterial antigen ingredientsBacteriaCancer cellApoptosis
Whole-cell vaccines and methods for enhancing the immunogenicity of cellular microorganisms for use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria or for use as vectors to express exogenous antigens and induce responses against other infectious agents or cancer cells. The present invention involves an additional method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme produced by the intracellular microbe is reduced by expressing a mutant copy of the enzyme, thereby modifying the microbe so that it increases immunogenicity.
Owner:VANDERBILT UNIV +1
Pro-Apoptotic Bacterial Vaccines To Enhance Cellular Immune Responses
InactiveUS20120276144A1Diminishing intracellular survivalReduced activityAntibacterial agentsBacterial antigen ingredientsVaccine PotencyVaccine efficacy
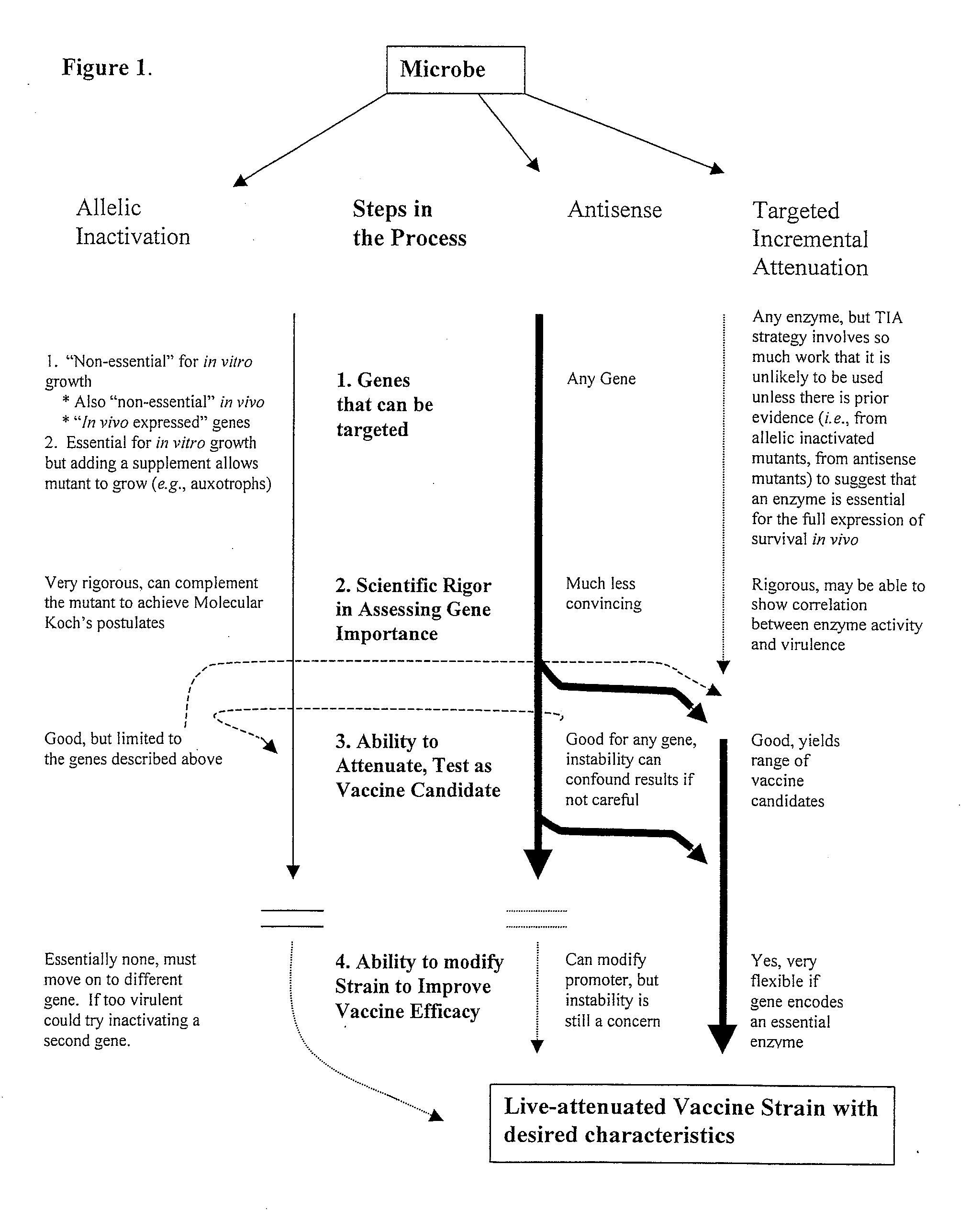
Whole-cell vaccines and methods for their use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria. The present invention involves a method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme is reduced, thereby modifying the microbe so that it increases immunogenicity. Also, the present invention provides a method of incrementally modifying enzyme activity to produce incrementally attenuated mutants of the microbe from which an effective vaccine candidate can be selected.
Owner:VANDERBILT UNIV
Vaccine composition and application thereof
InactiveCN106139137AReduce contentImprove immunityAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma
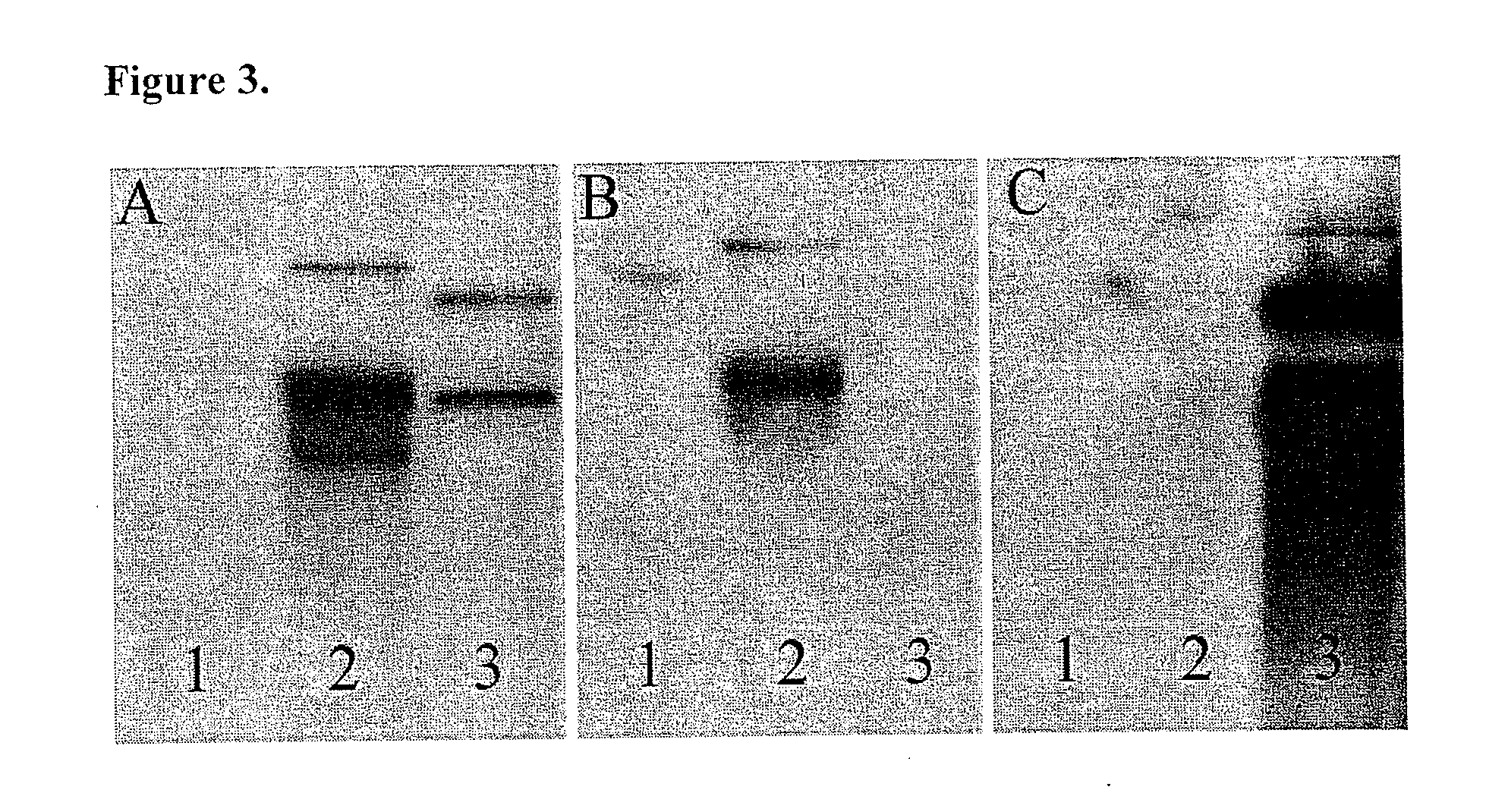
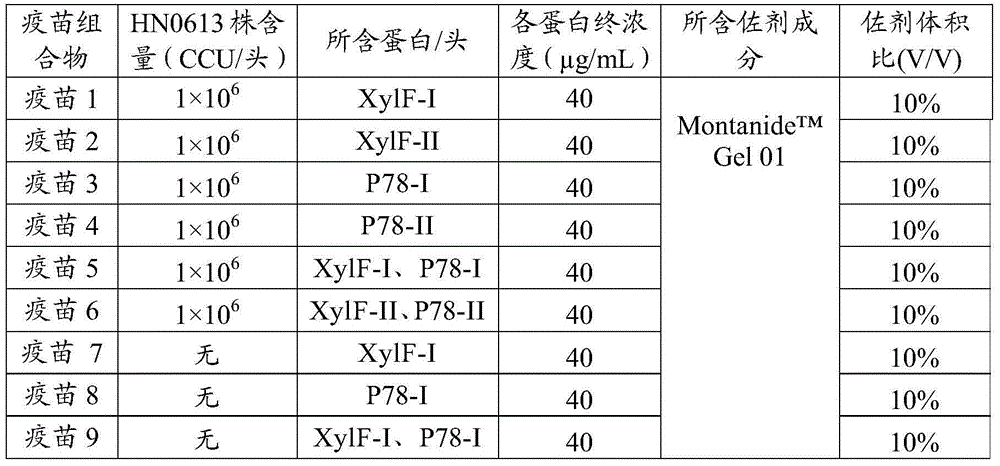
The invention provides a swine mycoplasma pneumonia vaccine composition, the vaccine composition includes swine pneumonia mycoplasma whole cell antigen and an immunogenic protein, and also comprises a veterinary-medicine-acceptable carrier, wherein the immunogenic protein comprises at least one of XylF and P78 proteins. The present invention also discloses the application of the vaccine composition in preparing of medicines for preventing and / or treating swine mycoplasma pneumonia. The content of the whole cell antigen and the immunogenic protein of the vaccine composition is decreased significantly compared with that of single use of a whole cell vaccine or a subunit vaccine for immunization, and immune effect is enhanced; protein part can be recombinationally expressed by means of genetic engineering in quantity, short time is taken, and the swine mycoplasma pneumonia vaccine composition can also be convenient for mass production. In addition, when an animal is immunized with the vaccine composition, the immune effects of the vaccine composition on the swine mycoplasma pneumonia antigen contained in the vaccine composition and other antigens are not affected.
Owner:PU LIKE BIO ENG
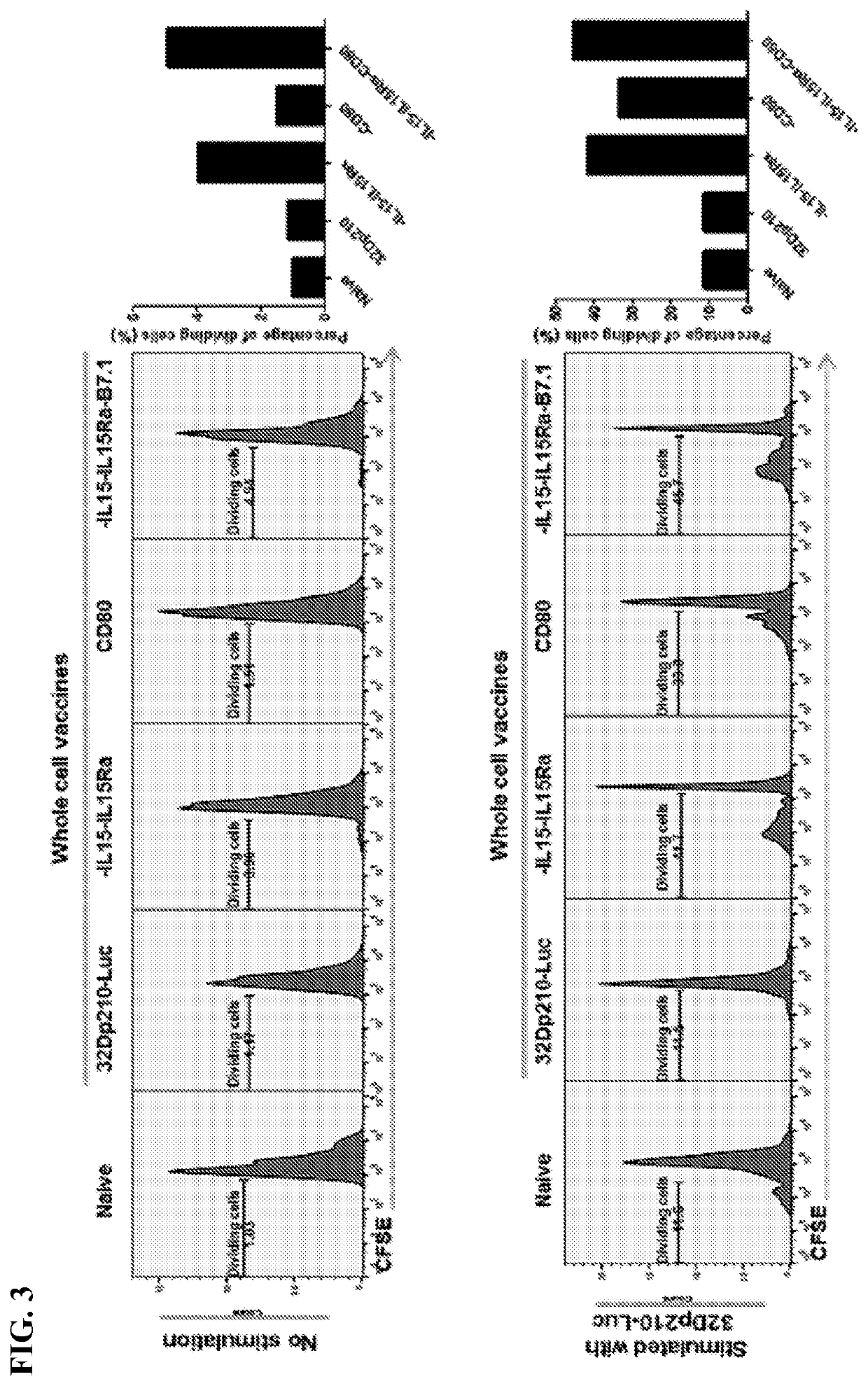
Autologous irradiated whole cell tumor vaccines lentivirally engineered to express cd80, il-15 and il-15 receptor alpha
PendingUS20200179447A1Extending remissionReduction in signPharmaceutical delivery mechanismMammal material medical ingredientsReceptorCD80
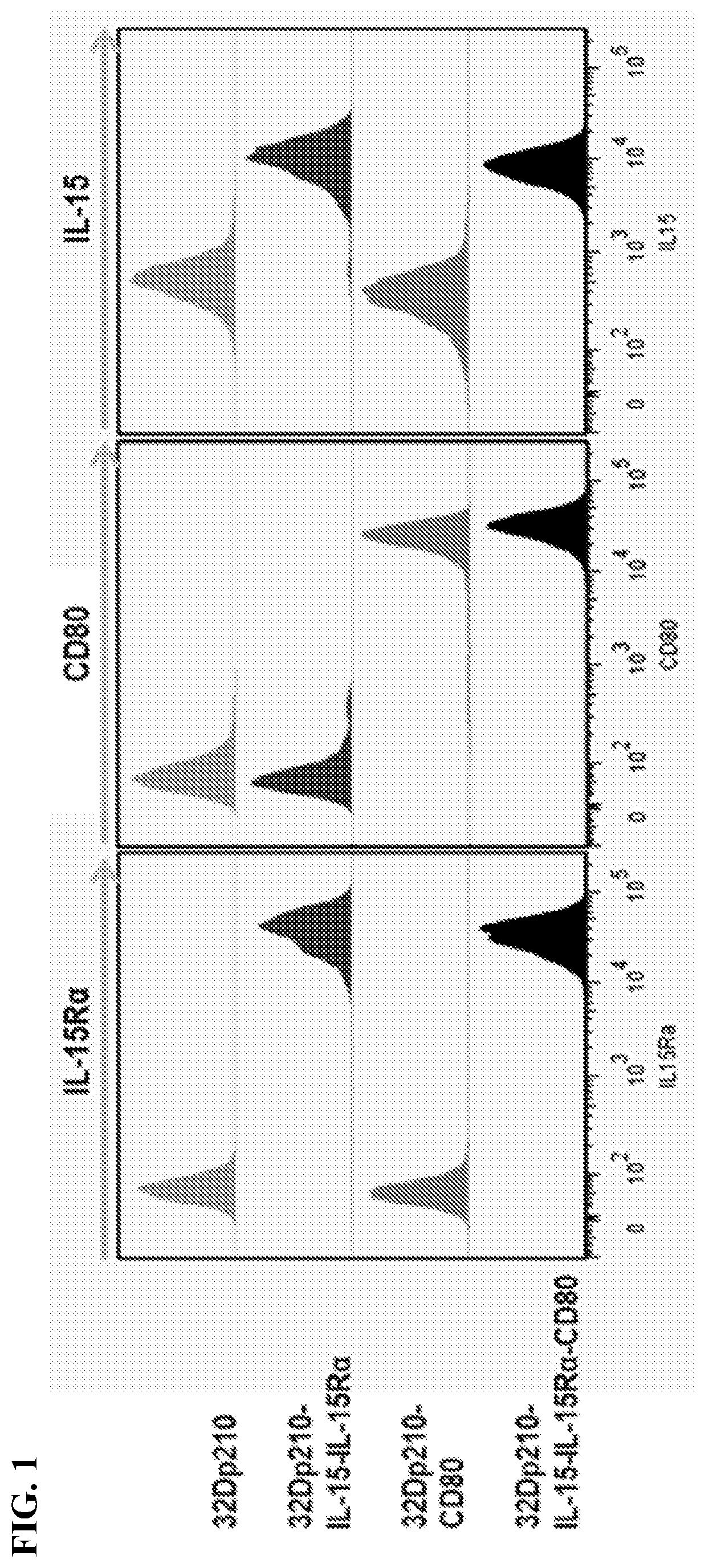
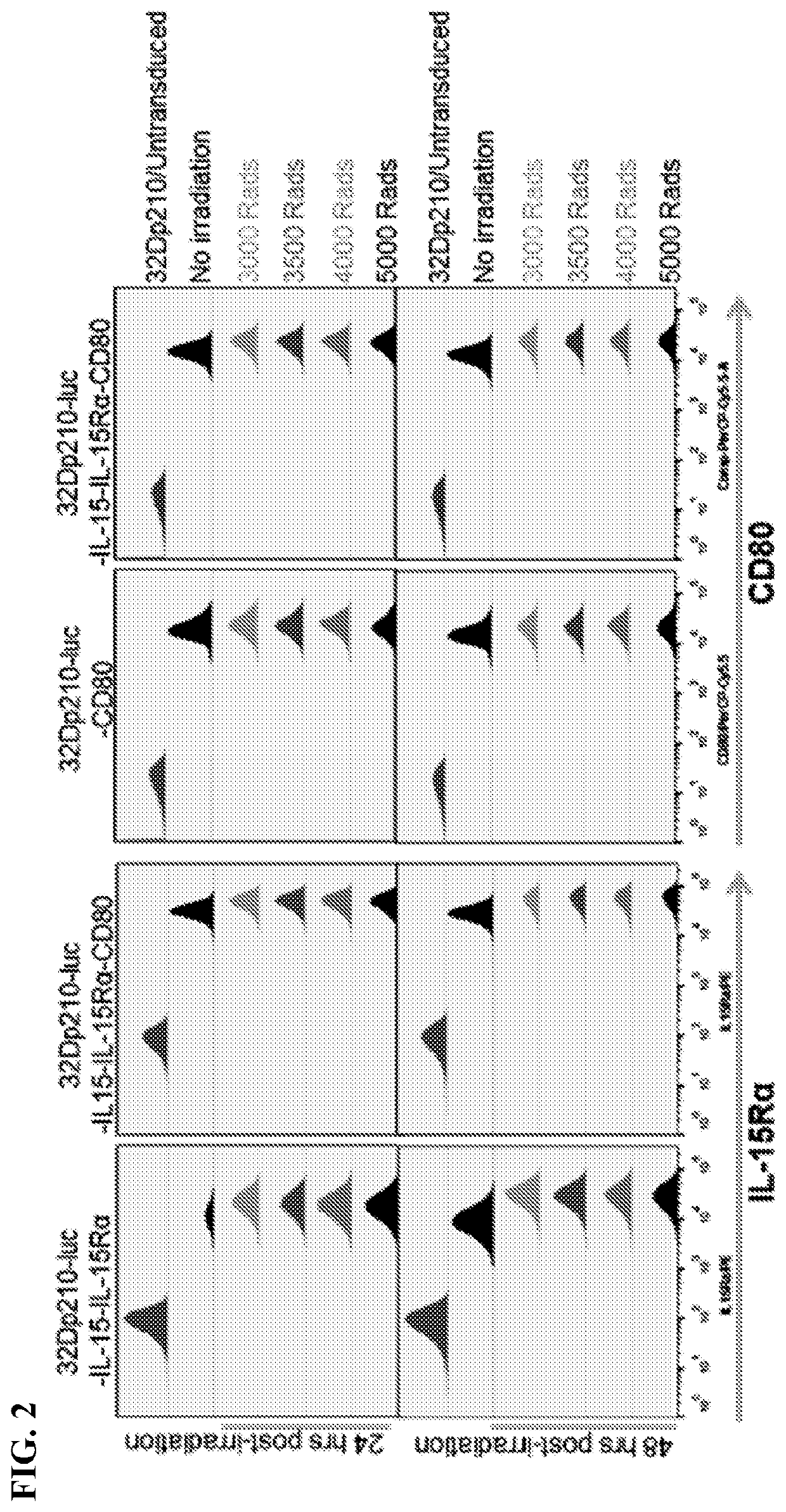
Provided herein, inter alia, are cell media compositions and whole cell vaccines comprising recombinant cells expressing IL-15, IL-15Rα, and CD80 capable of treating and preventing relapse in individuals diagnosed with or thought to have leukemia as well as methods for using the same.
Owner:RGT UNIV OF CALIFORNIA
Vibrio anguillarum exuviae vaccine and method of preparing the same
InactiveCN101108247AOvercoming the disadvantages of structural destruction of bacterial surface antigensImprove protectionAntibacterial agentsAntibody medical ingredientsDiseaseBacteroides
The invention relates to a bacterium coreless vaccine used for the fish aquaculture, in particular to a Vibiro anguillarum coreless vaccine and the preparation method thereof. The Vibiro anguillarum is an engineering bacteria construction carrier and then turn, foster, filter and purify the carrier to prepare the coreless vaccine. The adoption of the Vibiro anguillarum coreless vaccine retains the complete antigenic determinant of the Vibiro anguillarum cell membrane, overcomes the defect of antigenic damage caused by the traditional physical and chemical vaccine inactivation way. By immunizing the fish by oral administration and injection, the invention can better protect the fish compared with the traditional inactive cell vaccine. In addition, the invention has commercial development value due to low cost and broad prospect in developing the polyvalent vaccine and preventing various diseases as a vaccine carrier.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
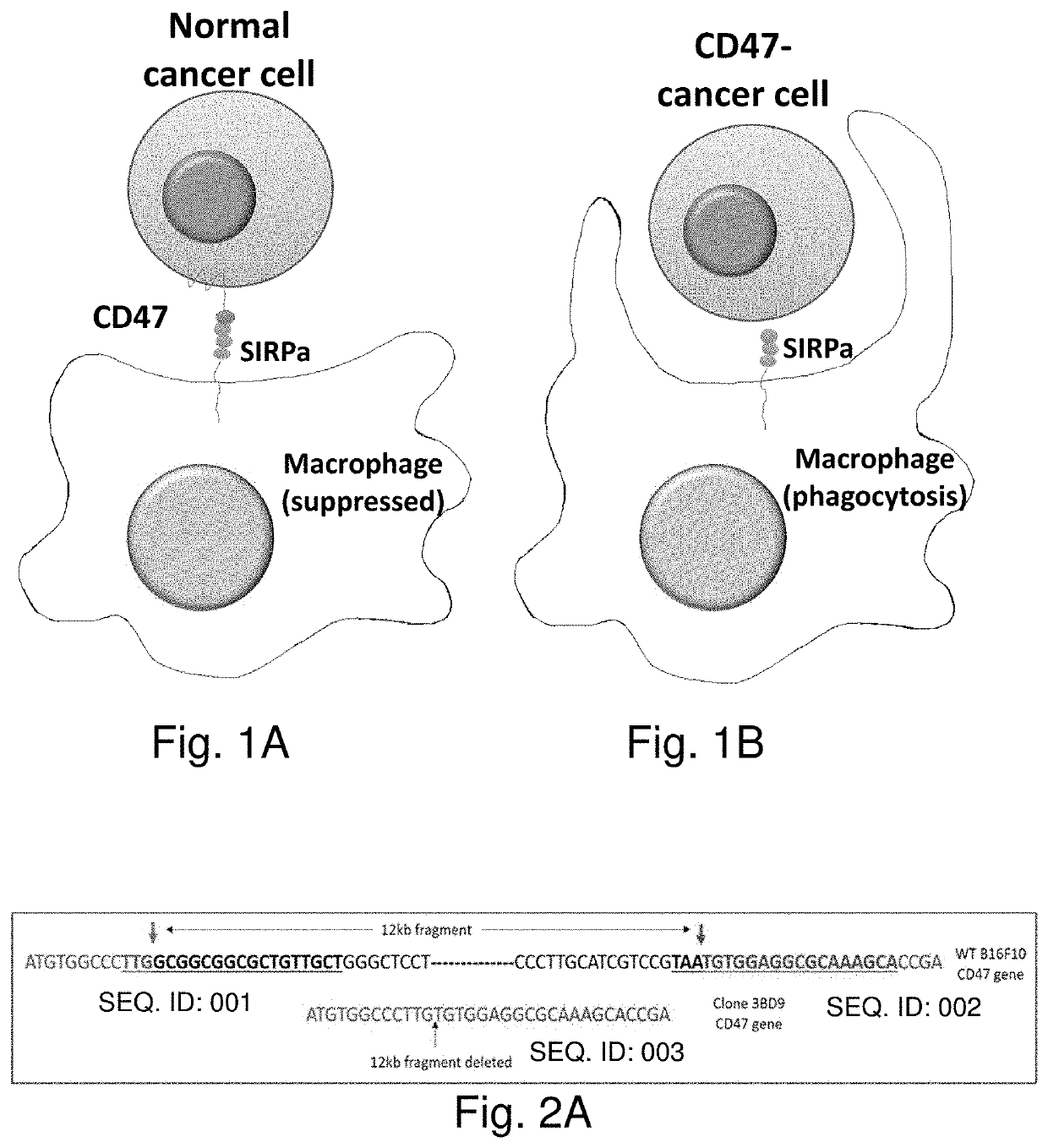
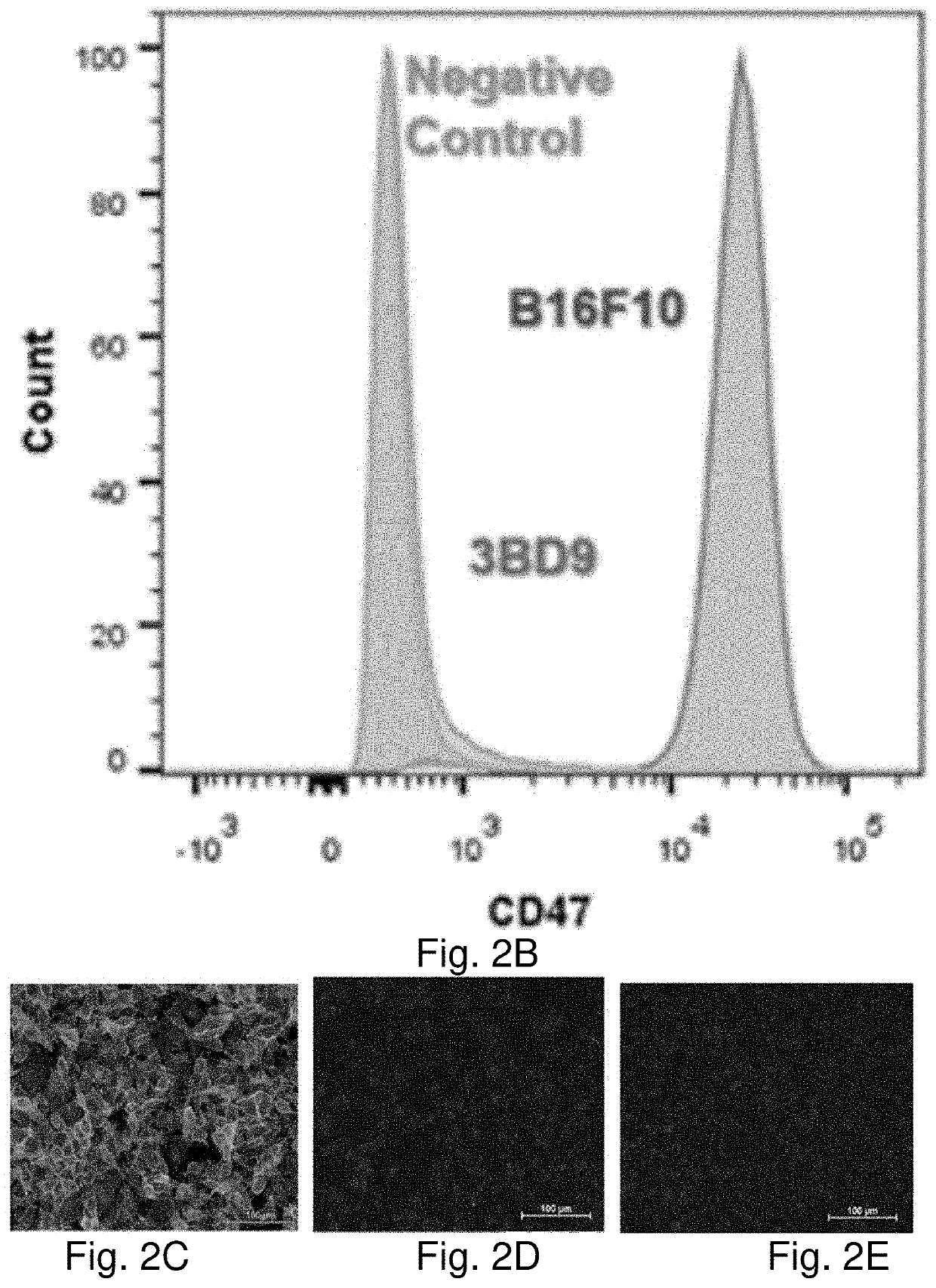
Genome edited cancer cell vaccines
PendingUS20200113986A1Reduce inhibitionIncrease immune recognitionGenetically modified cellsCulture processStage melanomaCytokine
A cancer vaccine technology is provided which knocks out expression of cell surface immune checkpoint proteins, to facilitate their processing by immune cells, and optionally by knocking-in the expression of cytokines to boost immune response. Non-replicating tumor cells lacking cell surface CD47 are highly effective immunizing agents against subcutaneous mouse melanoma. Whole-cell vaccines inhibited tumor growth, and immunophenotyping showed a dramatic increase in activated effector cell subsets and M1-type macrophages aided by a significant reduction in the tumor-associated macrophage and myeloid derived suppressor cell compartments. A remarkable downregulation of cell surface CD47 was observed in the tumors that did escape after vaccination with genetically modified cells, suggesting the intricate involvement of CD47 in a prophylactic situation. An effective vaccination strategy to increase tumor-specific immune response in solid tumors is provided to improve the outcome of cancer immunotherapy.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
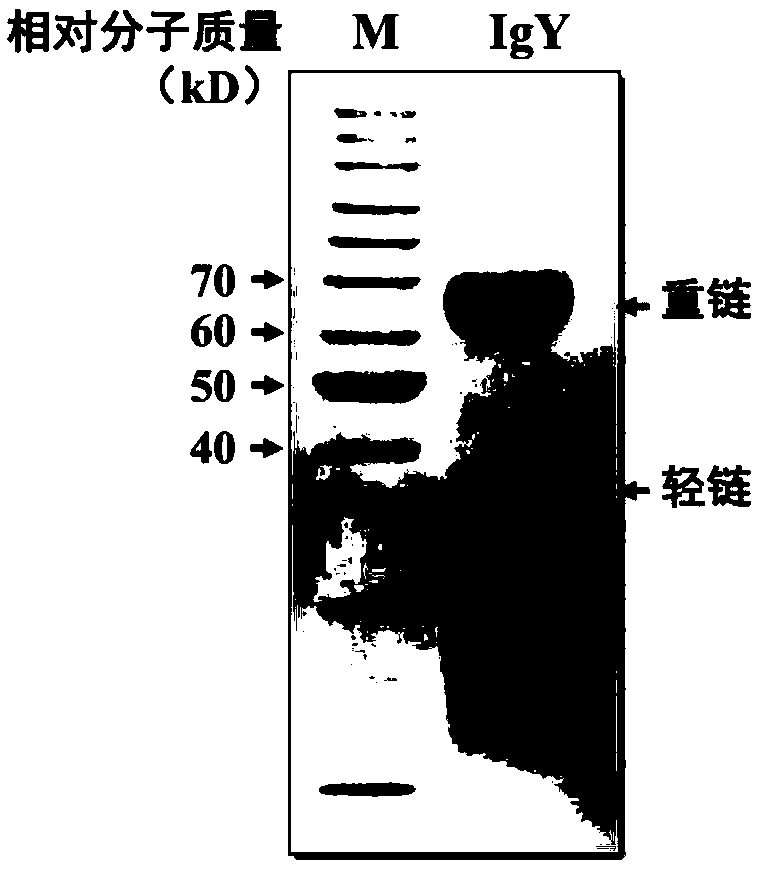
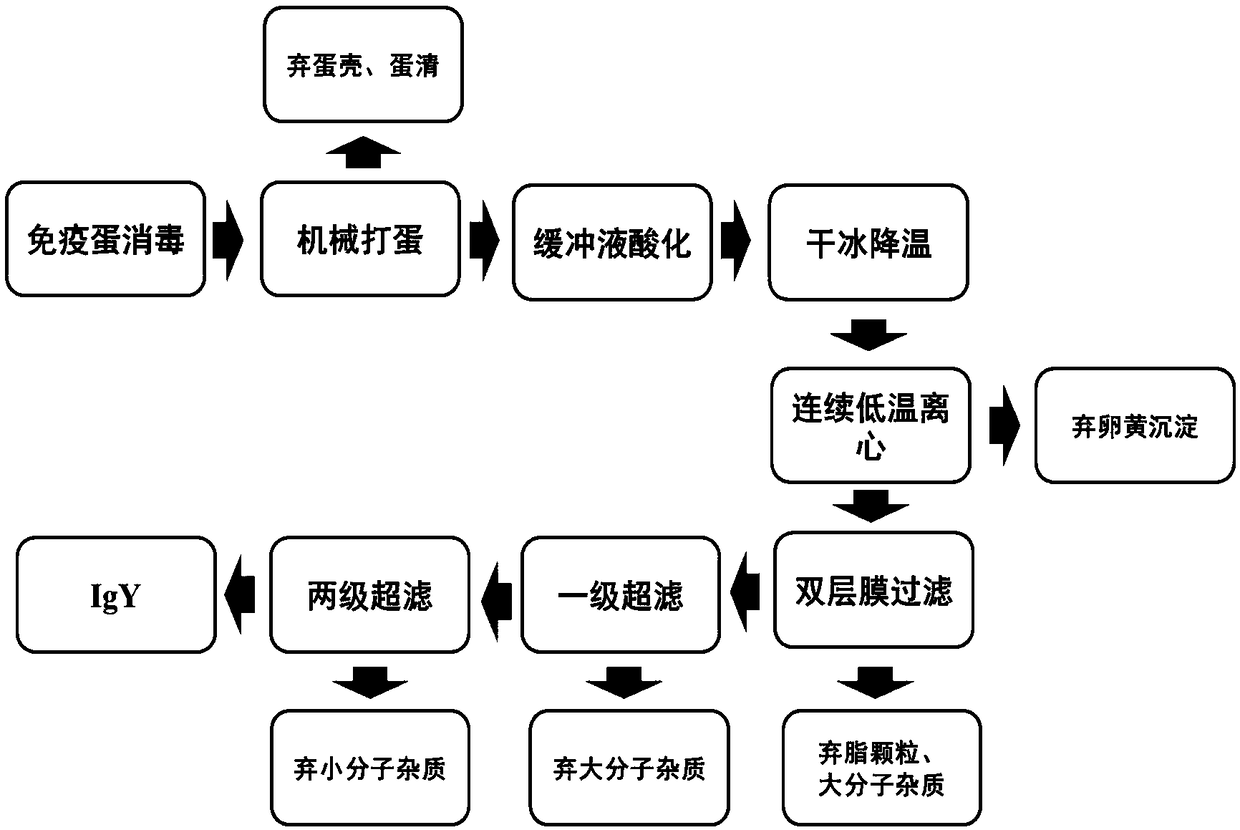
Fast extraction method of immunoglobulin Y, anti-influenza product prepared by immunoglobulin Y, and application of immunoglobulin Y
PendingCN108623679AAvoid infectionWide range of anti-virus typesAntibacterial agentsEgg immunoglobulinsEscherichia coliFiber
The invention discloses a fast extraction method of immunoglobulin Y, an anti-influenza product prepared by the immunoglobulin Y, and application of the immunoglobulin Y. The fast extraction method includes: using a tetravalent influenza vaccine comprising H1N1, H3N2, B(Victoria) and B(Yamagata) and an escherichia coli staphylococcus aureus whole-cell vaccine to immunize laying hens; mechanicallyand automatically separating eggs to obtain yolk, and using a citric acid-disodium hydrogen phosphate buffer solution dilute and stabilize yolk liquid pH; adding dry ice to fast lower system temperature; performing continuous high-speed low-temperature centrifuging to remove fat particles so as to obtain immunoglobulin Y supernate; allowing the immunoglobulin Y supernate to sequentially pass double filter membrane layers with pore diameters being 2-6 micrometers and 0.3-0.8 micrometers respectively, using a hollow fiber ultrafiltration membrane with the molecular weight cut-off being 300kD toremove impure protein, and processing with a hollow fiber ultrafiltration membrane with the molecular weight cut-off being 100kD to obtain concentrated immunoglobulin Y liquid; adding auxiliary materials to prepare into an oral and nasal mucosa spray. The method is high in automation level, low in energy consumption, high in yield, green and environmentally friendly, capable of achieving large-scale industrial production and the like.
Owner:CHENGDU ANTIK BIOTECH CO LTD
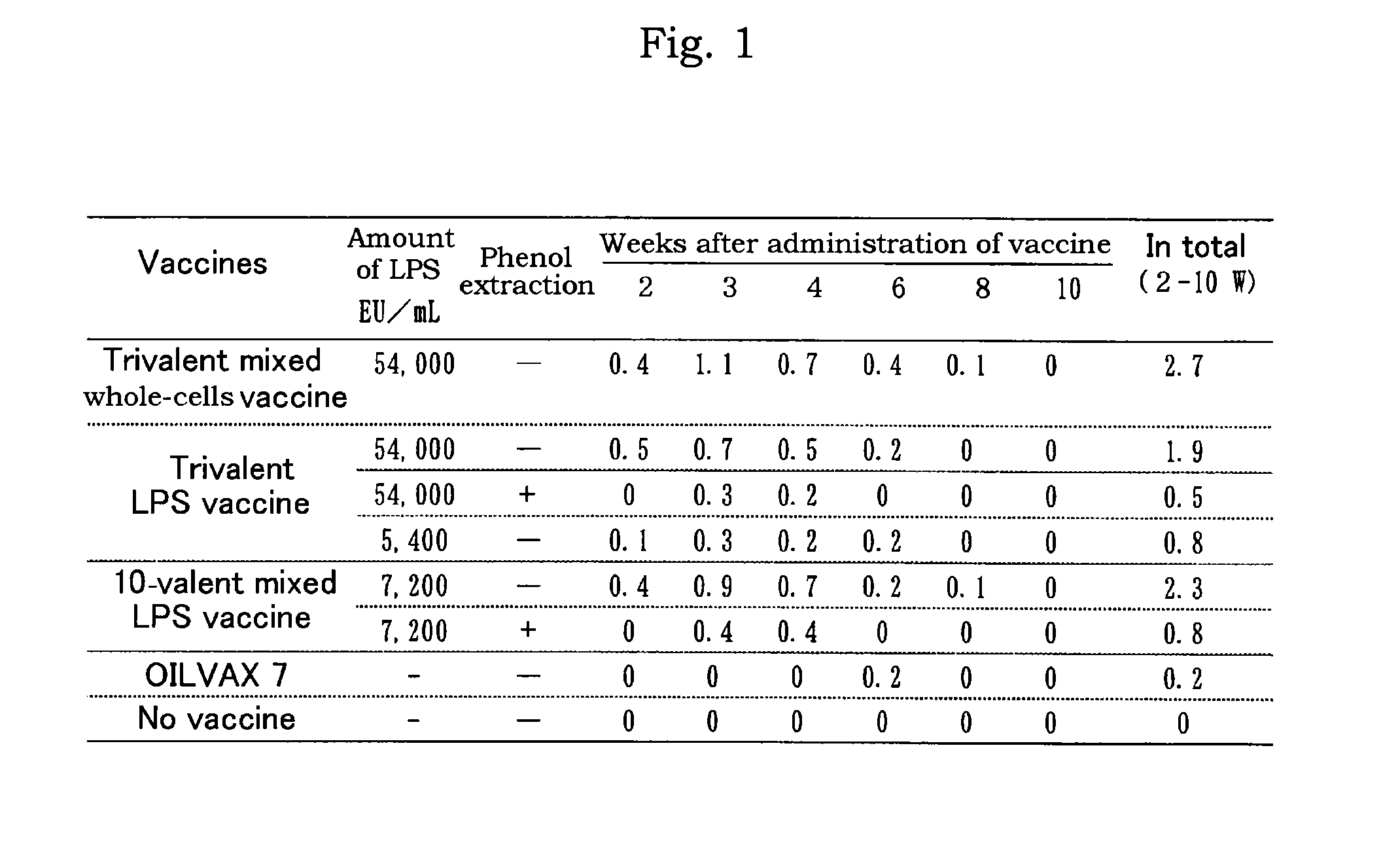
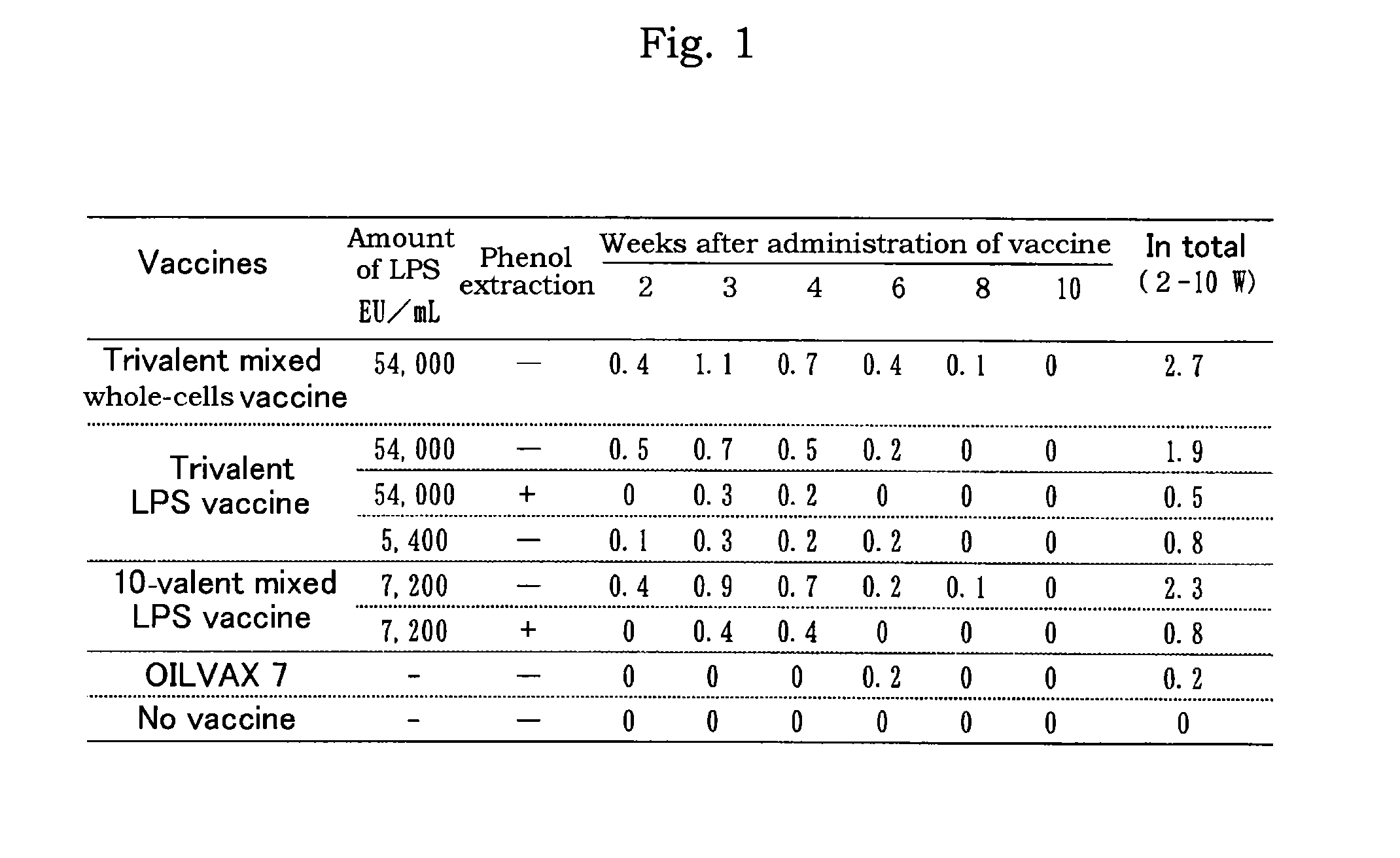
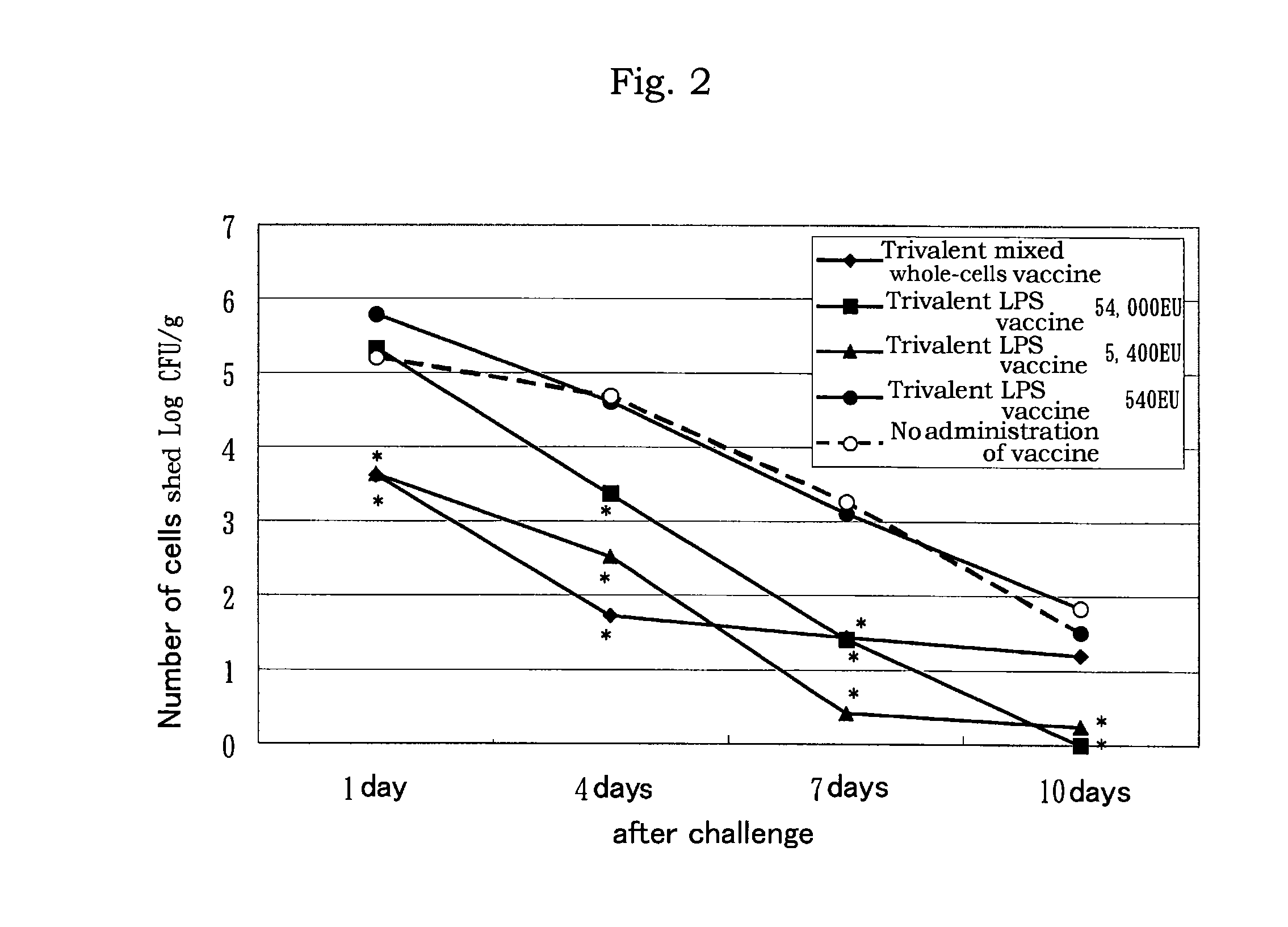
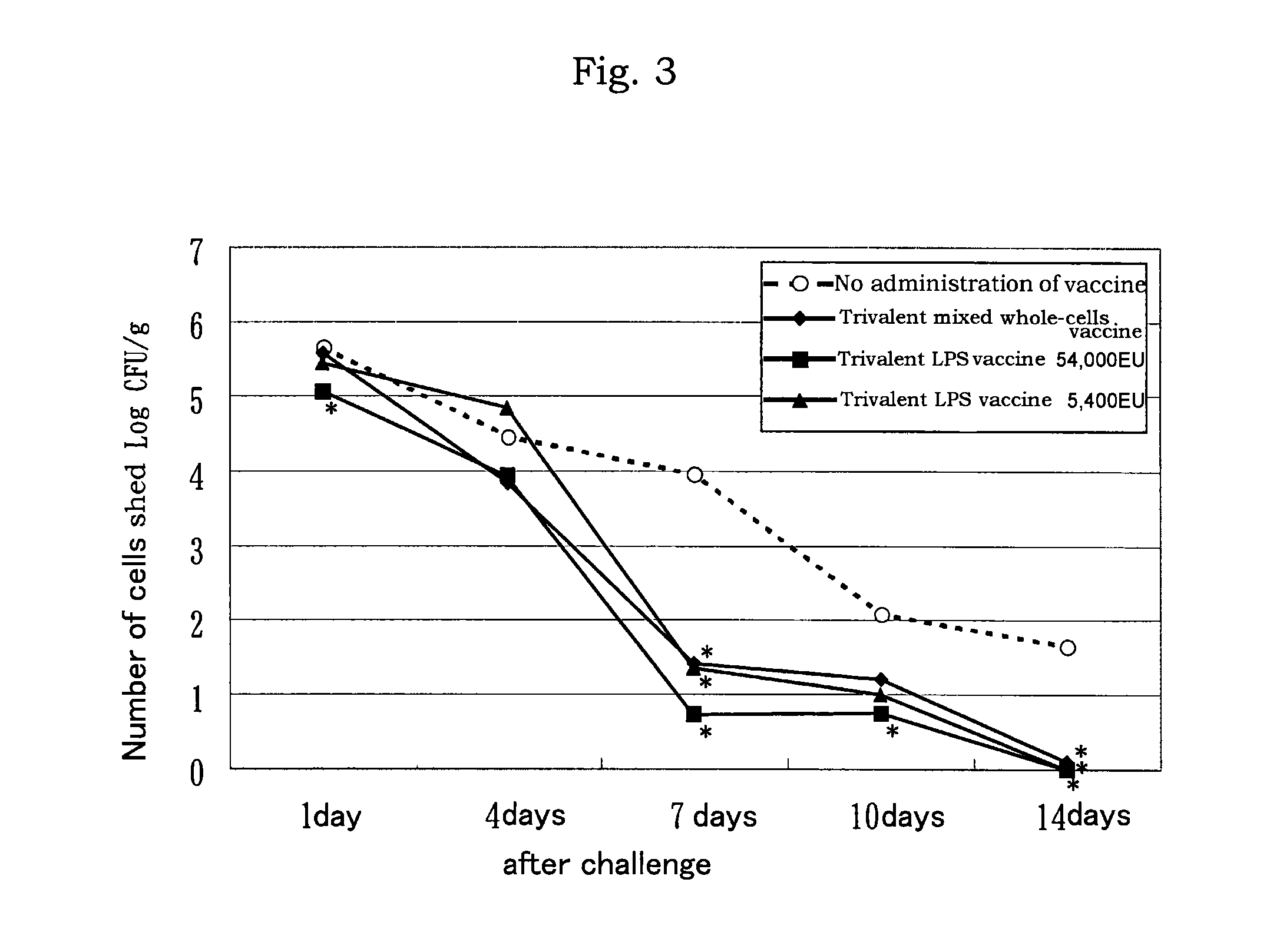
LPS vaccine
ActiveUS9345756B2Increase the number ofReduce injectionAntibacterial agentsBacterial antigen ingredientsMicrobiologyBULK ACTIVE INGREDIENT
A vaccine composition for birds comprising as an active ingredient a structure containing O-antigen derived from Gram-negative bacteria, provided that said structure does not contain a whole cell, and a process for preparing the same are provided. By using a structure containing O-antigen (e.g. lipopolysaccharide) derived from Gram-negative bacteria as an active ingredient in accordance with the present invention, alleviation of inoculation reaction and reduction in an amount of injection are attained as compared to the conventional whole-cells vaccine to thereby allow for the increase in the number of other antigens to be mixed therewith.
Owner:MEIJI ANIMAL HEALTH CO LTD
Pro-apoptotic bacterial vaccines to enhance cellular immune responses
InactiveUS8021671B2Improves vaccine efficacyDiminishing intracellular survivalAntibacterial agentsBiocideBacteroidesApoptosis
Whole-cell vaccines and methods for their use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria. The present invention involves a method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme is reduced, thereby modifying the microbe so that it increases immunogenicity. Also, the present invention provides a method of incrementally modifying enzyme activity to produce incrementally attenuated mutants of the microbe from which an effective vaccine candidate can be selected.
Owner:VANDERBILT UNIV +1
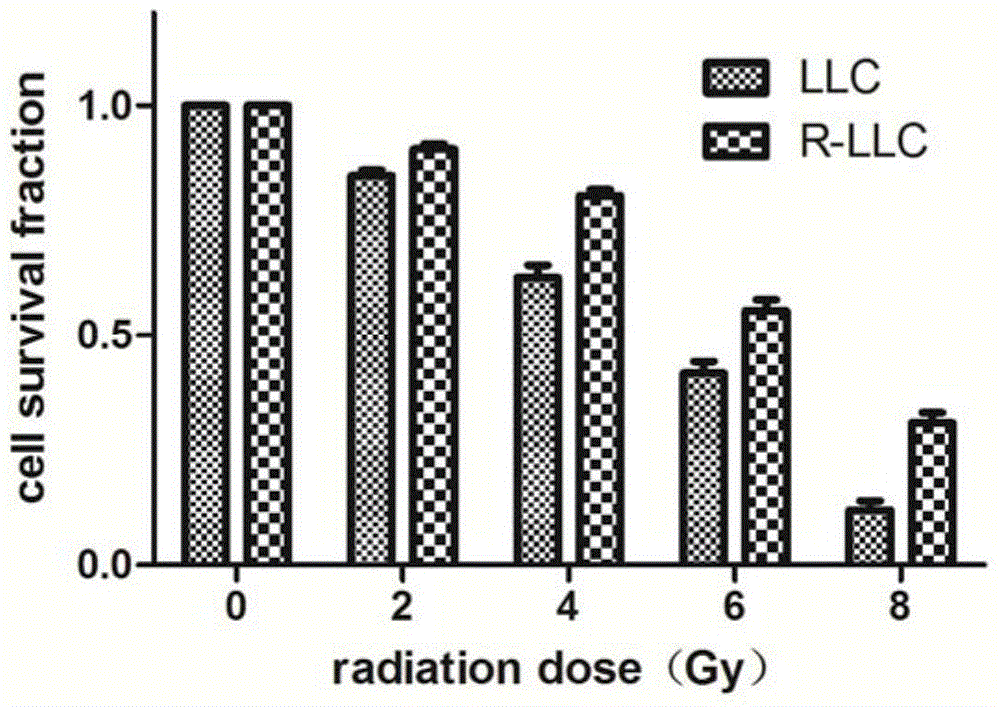
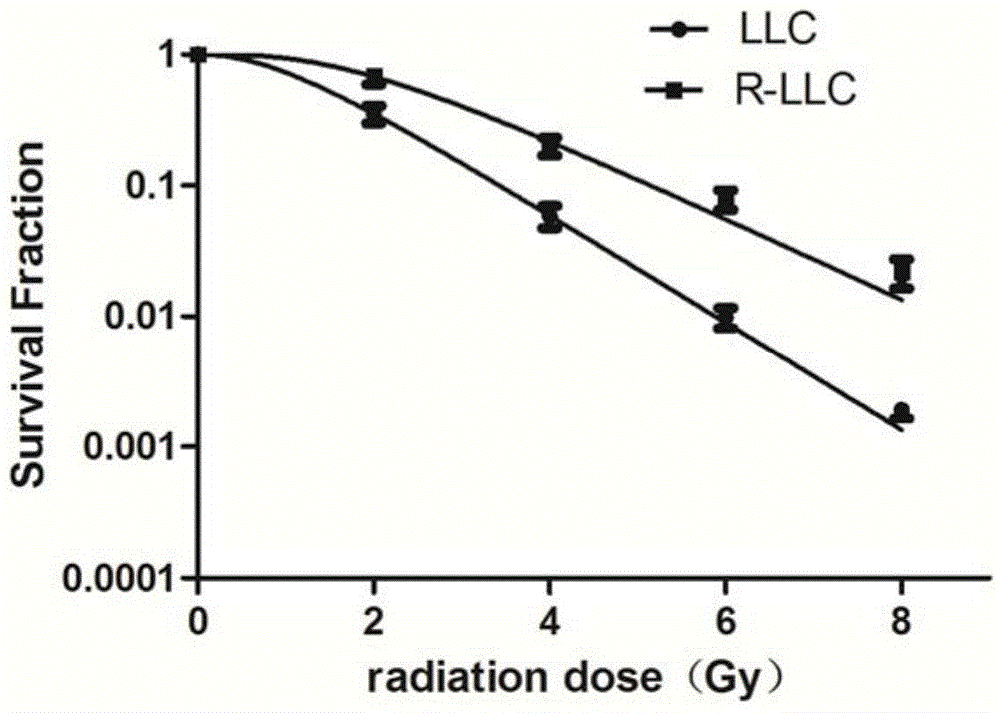
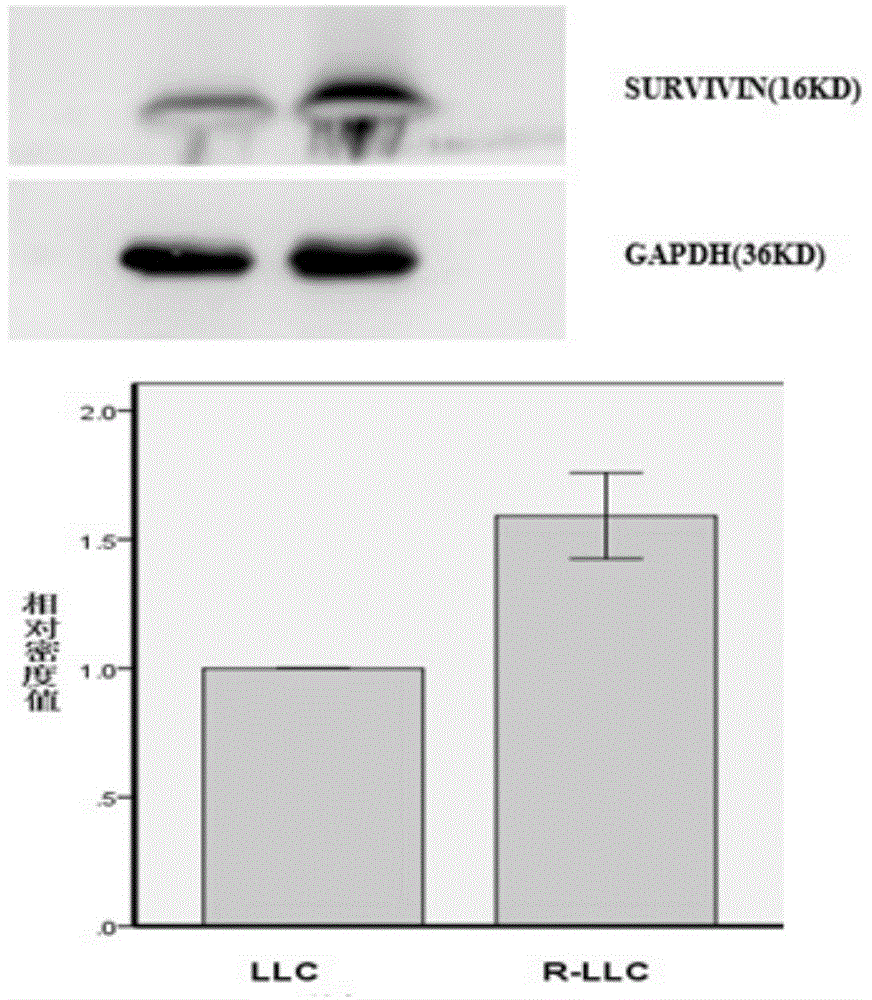
Radioresistant lung cancer whole-cell vaccine, and preparation method and application thereof
ActiveCN104946592AIncreased radiation resistanceGrowth inhibitionMicrobiological testing/measurementTumor/cancer cellsAbnormal tissue growthLymphatic Spread
The invention relates to a radioresistant lung cancer whole-cell vaccine, and a preparation method and application thereof, and particularly establishes a radioresistant lung cancer cell strain with obviously higher radioresistance, of which the collection number is CCTCC No.C201537. The animal experiment proves that the lung cancer whole-cell vaccine prepared by using the cell strain has the obvious actions on inhibiting mouse lung cancer tumors from growth, promoting transplantation tumor apoptosis and lowering the expression of the transplantation tumor immunologic escape PDL-1 protein; and when being combined with CpG ODN, the lung cancer whole-cell vaccine has more obvious actions on tumor inhibition, and part of mouse tumors completely disappear, thereby obtaining permanent specific antitumor effects. The invention provides a new idea and method for treating lung cancers and preventing postoperative metastasis and relapse.
Owner:JINSHAN HOSPITAL FUDAN UNIV
Rhamnose modified tumor whole-cell vaccine
InactiveCN103705915AGood anticancer effectGrowth inhibitionAntibody medical ingredientsAntineoplastic agentsSide effectWilms' tumor
The invention discloses a rhamnose modified tumor whole-cell vaccine consisting of complete tumor cells, wherein the complete tumor cells have rhamnose antigens on surfaces through a chemical method and lose differentiation and proliferation capacities through inactivation treatment. The tumor vaccine disclosed by the invention is simple in preparation, good in stability and low in cost, can overcome the defects of poor effect, easy relapse and great side effect of an existing tumor treatment method, is safer and more effective to treat tumors, is theoretically suitable for treatment of various malignant tumors and cancers of human, and has very broad application prospects.
Owner:SHANDONG UNIV
Quick extraction method for egg yolk antibody, prepared anti-burn, anti-scald and anti-infection product and application of egg yolk antibody
InactiveCN108640988ANo painNo drug resistanceAntibacterial agentsEgg immunoglobulinsFiberEscherichia coli
The invention discloses a quick extraction technology for an egg yolk antibody, a prepared anti-burn, anti-scald and anti-infection product and application of the egg yolk antibody. The technology comprises the steps that whole-cell vaccines of pseudomonas aeruginosa, escherichia coli and staphylococcus aureus are used for conducting immunization on laying hens; eggs are automatically and mechanically separated to obtain egg yolk, a citric acid-sodium hydrogen phosphate buffer solution is diluted, and the pH value of an egg yolk solution is stabilized; dry ice is added for quickly lowering thesystem temperature; continuous high-speed low-temperature centrifugation is conducted, fat particles are removal, and an egg yolk antibody supernatant is obtained; the egg yolk antibody supernatant passes 2-6micron and 0.3-0.8micron double-layer filter membranes and a hollow fiber ultrafiltration membrane with cut-off molecular weight of 300 kD in sequence to remove miscellaneous protein and finally passes through a hollow fiber ultrafiltration membrane with cut-off molecular weight of 100 kD to obtain a concentrated egg yolk antibody solution; ingredients are added to prepare a skin externalspray agent. The technology has the advantages that the automation degree is high, the energy consumption is low, the yield is high, the environment is protected, and large-scale industrialized production can be achieved.
Owner:CHENGDU ANTIK BIOTECH CO LTD
Bordetella parapertussis whole-cell vaccine composition
ActiveUS8465754B2Preventing whooping coughImprove and stabilize qualityAntibacterial agentsBacterial antigen ingredientsBordetella pertussis DNABordetella parapertussis infection
An objective of the present invention is to provide a whole-cell bacterial vaccine composition for preventing whooping cough caused by Bordetella parapertussis, comprising whole cells, whole-cell homogenate, or cell lysate of B. parapertussis as an immunogen, and methods for producing them.
Owner:DAIICHI SANKYO CO LTD
Lps vaccine
ActiveUS20150086590A1Reduce swellingIncrease the number ofAntibacterial agentsBacterial antigen ingredientsMicrobiologyBULK ACTIVE INGREDIENT
A vaccine composition for birds comprising as an active ingredient a structure containing O-antigen derived from Gram-negative bacteria, provided that said structure does not contain a whole cell, and a process for preparing the same are provided. By using a structure containing O-antigen (e.g. lipopolysaccharide) derived from Gram-negative bacteria as an active ingredient in accordance with the present invention, alleviation of inoculation reaction and reduction in an amount of injection are attained as compared to the conventional whole-cells vaccine to thereby allow for the increase in the number of other antigens to be mixed therewith.
Owner:MEIJI ANIMAL HEALTH CO LTD
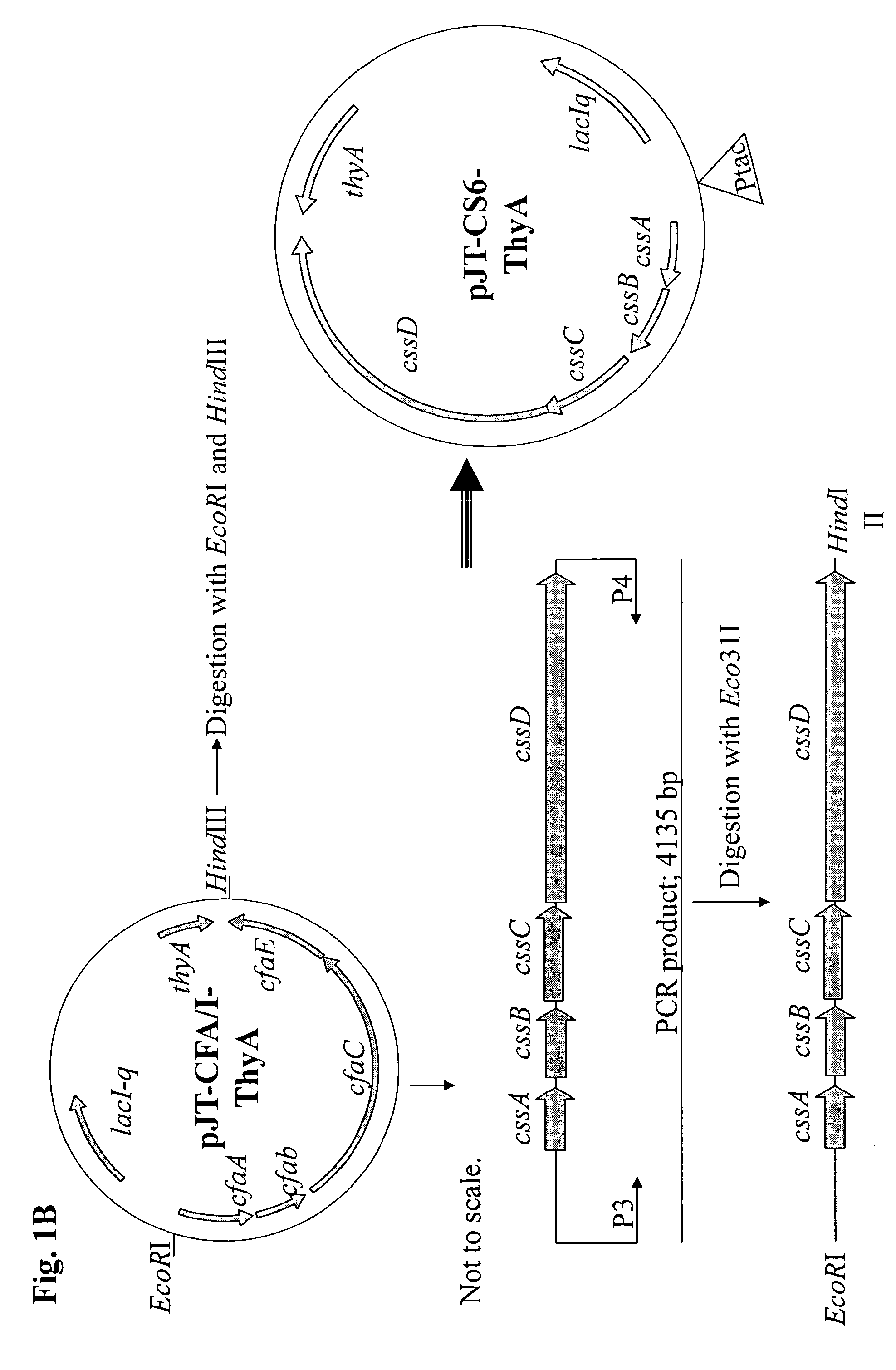
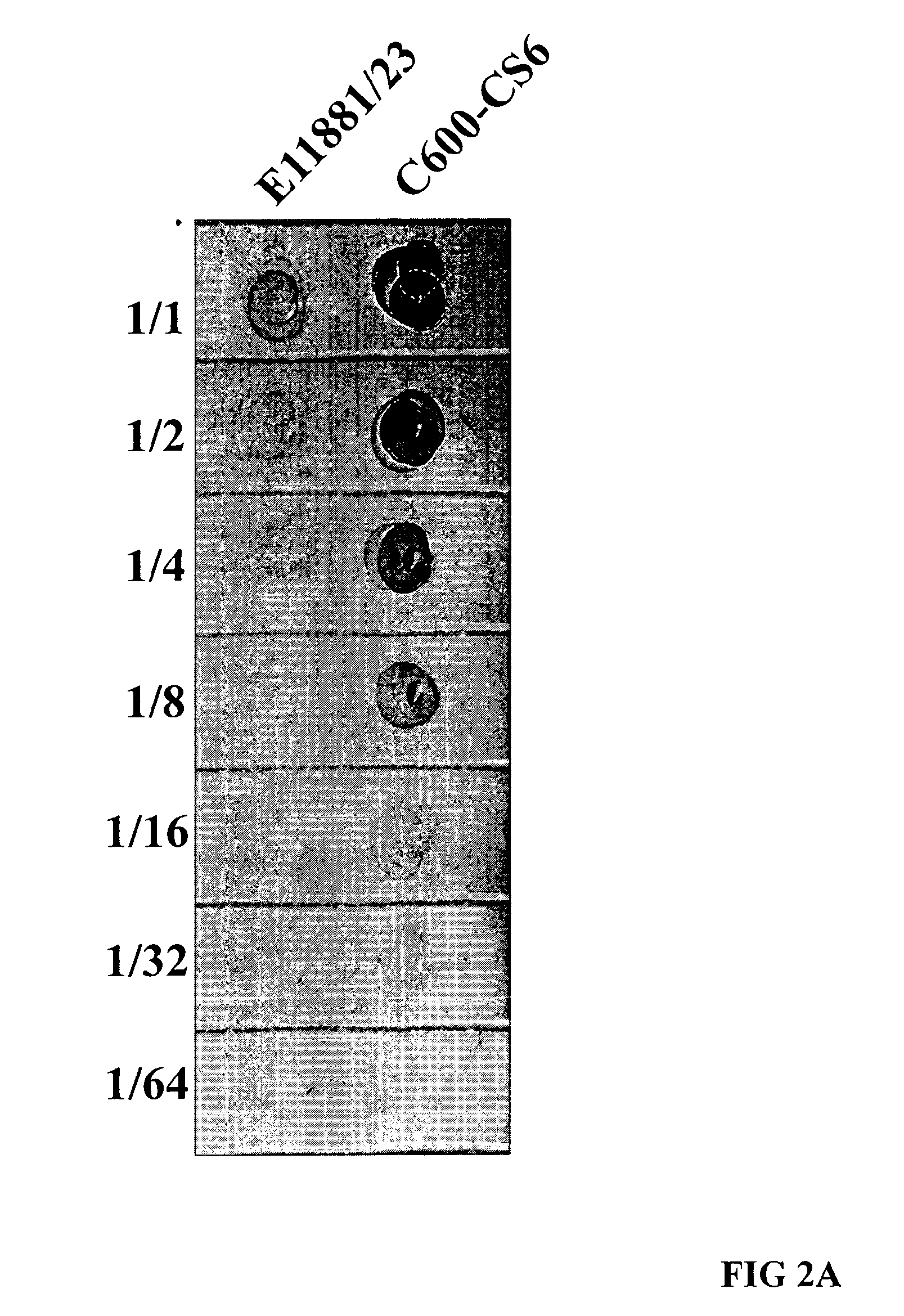
Method for increasing ETEC CS6 antigen presentation on cell surface and products obtainable thereof
A method for increasing the presentation of ETEC CS6 antigen on cell surface, comprising the step of contacting cells expressing said antigen with an aqueous solution comprising 0.6-2.2 percent phenol by weight, such that the presentation of said antigen is increased by at least 100%. A method for the manufacture of a killed whole cell vaccine for immunization against CS6-expressing ETEC. Cells and vaccines obtainable by the above methods.
Owner:SCANDINAVIAN BIOPHARMA HLDG
Vaccine for fish and application
The invention relates to a vaccine for fish, in particular to a whole-cell vaccine prepared from Aeromonas caviae or a vaccine prepared from exotoxin of the Aeromonas caviae, and application thereof in preventing fish diseases. A strain is obtained through the separation from a focus of southern catfish, is the Aeromonas caviae through the identification, is named DKN-1, and was preserved in China Center for Type Culture Collection on Sep 12th, 2007, and a preserving number of the strain is CCTCC NO: M207146. The use of the vaccine can stimulate fish bodies to produce the immunity against ulcer syndrome, and strengthen the resistance against diseases.
Owner:TONGWEI
Somatic whole-cell vaccine composition for treating chronic diseases and preparation method thereof
ActiveCN109125717APrevent relapseGood curative effectCell dissociation methodsMetabolism disorderDiseaseLife quality
The invention discloses a somatic whole-cell vaccine composition for treating chronic diseases. The composition includes somatic cells, monoclonal antibodies, cell factors, a cell cracking agent and normal saline. The invention also discloses a preparation method of a somatic whole-cell vaccine for treating the chronic diseases, and the method includes the steps of collecting, separation, extraction, culturing, amplification, collecting, splitting decomposition, purification and agent preparation. The vaccine is prepared by utilizing variational cells in blood of the human body and can stimulate the human body to carry out a specific immunity reaction on variational antigens, correspondingly immune cells and the monoclonal antibodies both of which have a specific lethal effect are generated and circularly identify and kill the variational cells carrying the variational antigens in the human body, metabolic waste generated by the variational cells are removed, and the purposes of preventing relapse of the diseases and improving the living quality are achieved. At present, it is proved by experiments that the vaccine has a great therapeutic effect on patients suffering from diabetesand skin diseases.
Owner:JIANGSU SUPERBIO LIFE SCI CO LTD
A fusion protein, recombinant vector, recombinant dendritic cell and application thereof for transmembrane expression of novel coronavirus antigen s2
ActiveCN112409496BPrevent intrusionImproving immunogenicitySsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeDendritic cell
The invention provides a fusion protein, a recombinant vector, a recombinant dendritic cell and its application for transmembrane expression of novel coronavirus antigen S2, belonging to the technical field of whole cell vaccines. The fusion protein includes CD4 signal peptide linked in sequence , novel coronavirus antigen S2 protein, Flag tag sequence and CD4 transmembrane domain; the present invention expresses S2 alone across the membrane, avoiding the risk of ADE that may be caused by other S protein epitopes, and the fusion protein construction provided by the present invention The cellular vaccine can induce higher neutralizing antibody titers in mice.
Owner:GENERAL HOSPITAL OF PLA +1
Selectively Split Whole Cell Vaccines
ActiveCN102648003BSimple and cheap to manufactureFreed from fatal experimental pneumoniaAntibacterial agentsBacterial antigen ingredientsImmunopotencyImmunity
The present invention provides immunogenic compositions having an inactivated, whole-cell S. pneumoniae, multiplex immunity-inducing fraction and methods of making immunogenic compositions by The immunogenic composition is prepared by selectively lysing a whole-cell bacterial preparation in such a way that the soluble fraction that primarily induces an antibody response and the cellular fraction that primarily induces an antibody-independent response are retained in the immunogenic composition.
Owner:CHILDRENS MEDICAL CENT CORP +2
A kind of radioresistance lung cancer whole cell vaccine and its preparation method and application
ActiveCN104946592BIncreased radiation resistanceGrowth inhibitionMicrobiological testing/measurementTumor/cancer cellsApoptosisTreatment of lung cancer
The present invention relates to a radiation-resistant lung cancer whole-cell vaccine and its preparation method and application. Specifically, the present invention establishes a radiation-resistant lung cancer cell line with significantly improved radiation resistance. The preservation number is CCTCC C201537. The cell line is used to A lung cancer whole-cell vaccine was prepared, and animal experiments confirmed that the lung cancer whole-cell vaccine had the effects of significantly inhibiting the growth of mouse lung cancer tumors, promoting apoptosis of transplanted tumor cells, and reducing the expression of PDL-1 protein in immune escape of transplanted tumors, and combined with CpG ODN The anti-tumor effect is more significant, and the tumors of some mice completely disappear, and a durable and specific anti-tumor effect is obtained. The invention provides new ideas and methods for the treatment of lung cancer and the prevention of postoperative metastasis and recurrence.
Owner:JINSHAN HOSPITAL FUDAN UNIV
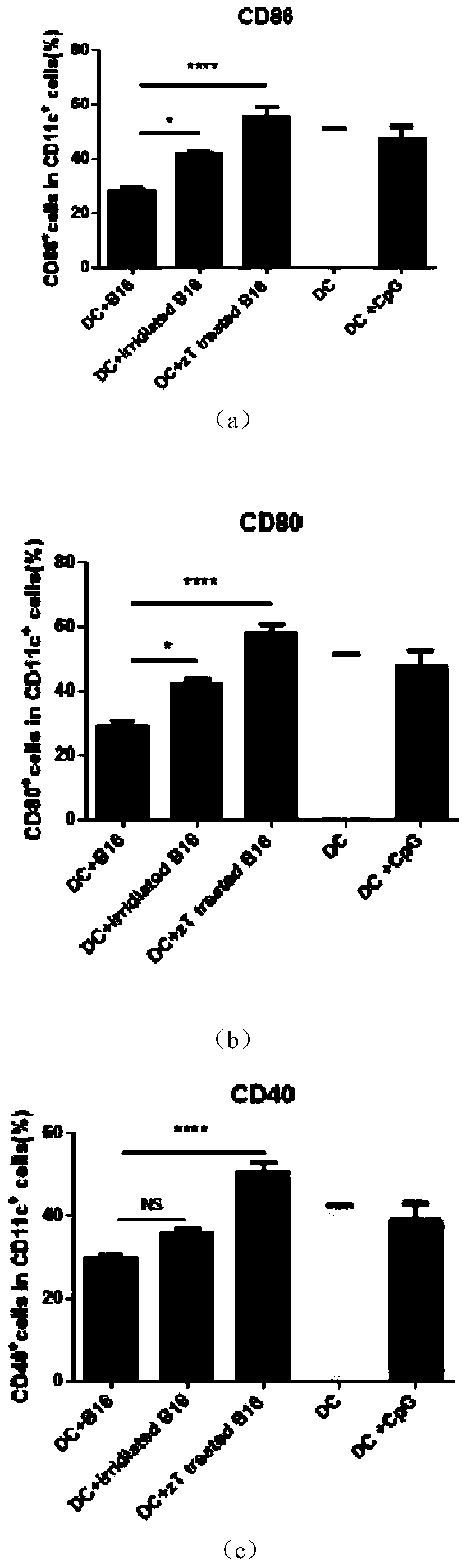
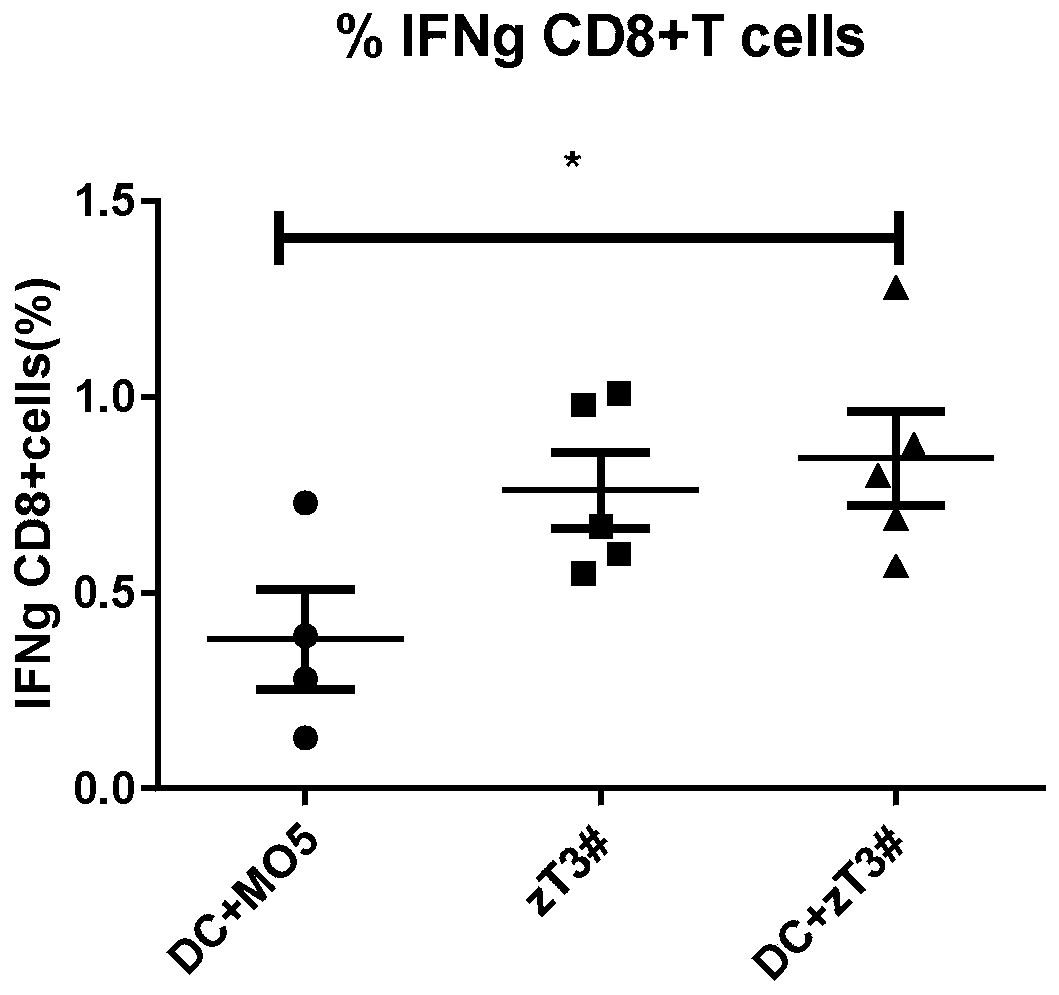
Whole-cell vaccine for inhibiting or preventing melanoma, and preparation method for whole-cell vaccine
ActiveCN110694055AImprove immune killing abilityIncreased activationGenetically modified cellsCancer antigen ingredientsDendritic cellStage melanoma
The invention relates to a whole-cell vaccine for inhibiting or preventing melanoma, and a preparation method for the whole-cell vaccine, in particular to a preparation method for a tumour vaccine which has a function of improving an immune response and is co-cultured by a new antigen cell and a dendritic cell, and application. The vaccine is characterized in that an MLH-1 gene with a DNA (deoxyribonucleic acid) repairing function is knocked out in a melanoma cell, so that a tumour cell generates mutation in a cell replication process so as to express a non-autologous antigen easy in immune cell recognition. After the mutated tumour cell is processed, the mutated tumour cell and a DC (Dendritic cell) are co-cultured, and therefore, costimulatory proteins expressed on the surface of the DCare obviously improved. The whole-cell vaccine prepared by the method obviously improves the immune response in the body of a mouse, in-vivo IFN-G (interferon-G) and TNF-A (Tumour necrosis factor-a) is improved than a non-injected vaccine group, and therefore, the whole-cell vaccine performs an important function of treating the tumor.
Owner:SHANGHAI UNIV
Vaccination of sex reversed hybrid tilapia (Oreochromis niloticus x O. aureus) with an inactivated Vibrio vulnificus vaccine
InactiveUS8420072B2Control process safetyReduce bacterial loadAntibacterial agentsBiocideDiseaseHeterologous
Vibrio vulnificus can cause infections in aquaculture-raised fish and is considered an opportunistic human pathogen. We isolated V. vulnificus from diseased hybrid tilapia (Oreochromis niloticus X O. aureus) cultured in a North American water reuse aquaculture facility. We have characterized the isolate using biochemical and molecular methods, developed a disease infection model, and determined that formalin-inactivated whole-cell vaccine provides protection against V. vulnificus. The V. vulnificus isolate was determined to be biotype 1, 16S rRNA type B, vcg type C, and vvhA type 2. Fish vaccinated with the formalin-inactivated whole-cell vaccine responded to vaccination as measured by agglutinating antibody titer. In two separate trials, vaccinated tilapia exhibited relative percent survival of 73 and 60% following challenge with the homologous isolate. In additional trials, vaccinated tilapia exhibited survival values of up to 87.5% following challenge with a heterologous isolate. Use of a mineral oil adjuvant enhanced protection.
Owner:UNITED STATES OF AMERICA
Method for increasing etec cs6 antigen presentation on cell surface and products obtainable thereof
Owner:SCANDINAVIAN BIOPHARMA HLDG
Vaccination of Sex Reversed Hybrid Tilapia (Oreochromis niloticus X O. aureus) With an Inactivated Vibrio vulnificus Vaccine
InactiveUS20120244190A1Control process safetyReduce bacterial loadAntibacterial agentsBiocideDiseaseHeterologous
Vibrio vulnificus can cause infections in aquaculture-raised fish and is considered an opportunistic human pathogen. We isolated V. vulnificus from diseased hybrid tilapia (Oreochromis niloticus X O. aureus) cultured in a North American water reuse aquaculture facility. We have characterized the isolate using biochemical and molecular methods, developed a disease infection model, and determined that formalin-inactivated whole-cell vaccine provides protection against V. vulnificus. The V. vulnificus isolate was determined to be biotype 1, 16S rRNA type B, vcg type C, and vvhA type 2. Fish vaccinated with the formalin-inactivated whole-cell vaccine responded to vaccination as measured by agglutinating antibody titer. In two separate trials, vaccinated tilapia exhibited relative percent survival of 73 and 60% following challenge with the homologous isolate. In additional trials, vaccinated tilapia exhibited survival values of up to 87.5% following challenge with a heterologous isolate. Use of a mineral oil adjuvant enhanced protection.
Owner:UNITED STATES OF AMERICA
Whole-cell vaccine, and preparation method and application thereof
PendingCN112336852AGood application effectImprove practicalityCancer antigen ingredientsCarrier-bound antigen/hapten ingredientsAgonistTGE VACCINE
The invention relates to the technical field of crossing of immunochemistry and cytobiology, and provides a whole-cell vaccine, and a preparation method and application thereof. The whole-cell vaccinecomprises tumor cells, TLR7 small-molecule agonist molecules and coupling chain molecules, the whole-cell vaccine obtained by coupling the TLR7 small-molecule agonist molecules and the tumor cells through the coupling chain molecules can recognize all antigens on the surfaces of the tumor cells, the vaccine also can be specifically recognize a three-dimensional spatial configuration of the wholetumor cells, the vaccine can be effectively enhance the application effect and practicability in immunotherapy for preventing tumorigenesis, and the wide application of a tumorigenesis immunotherapy method is improved.
Owner:SHENZHEN UNIV
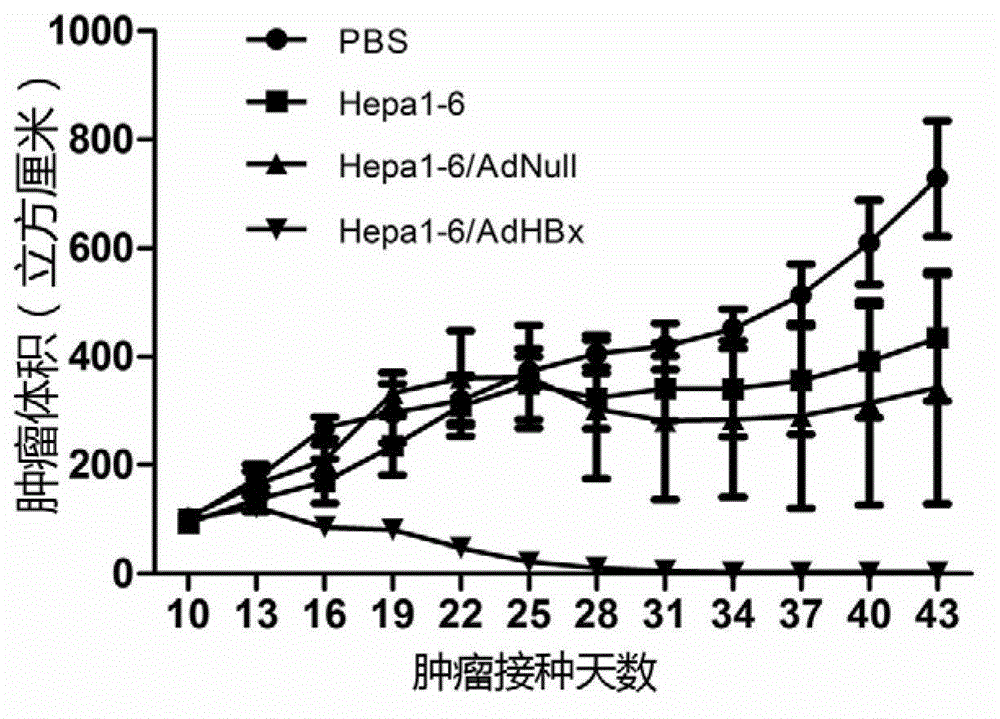
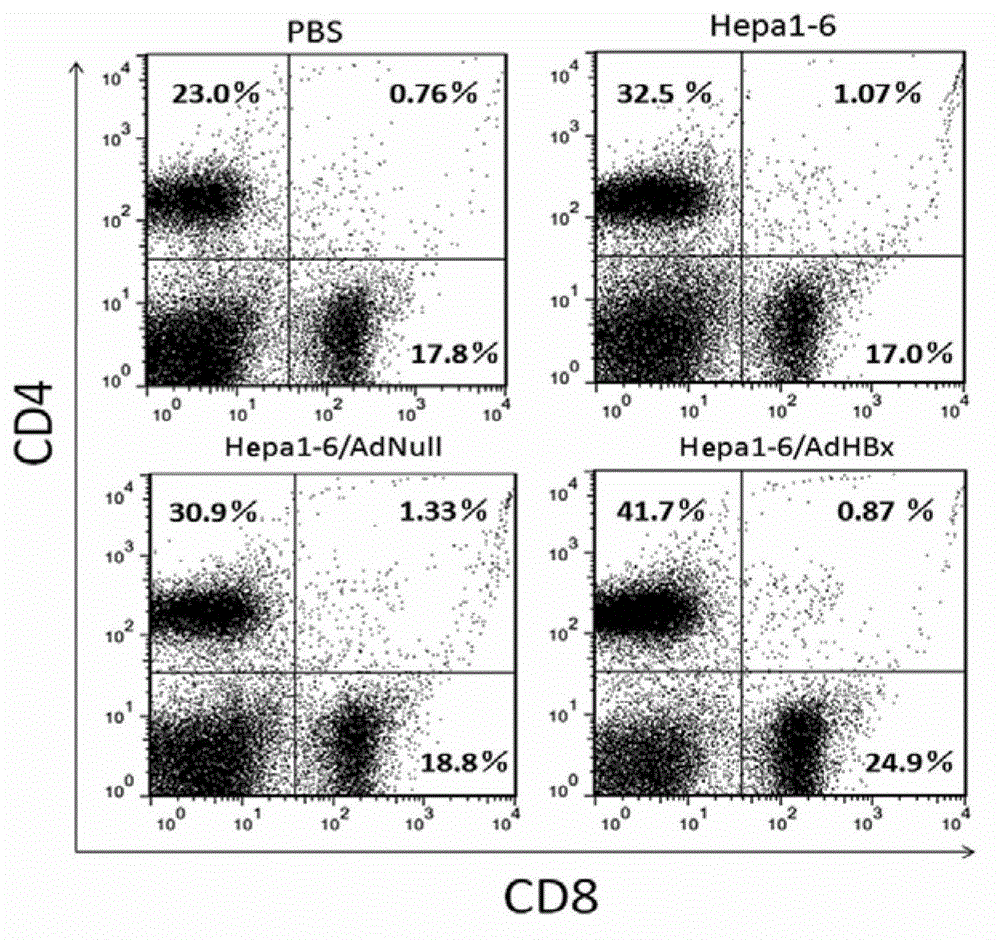
Anti-liver cancer whole cell vaccine modified by hbx and its preparation method and application
ActiveCN103230600BGood anti-liver cancer effectGenetic material ingredientsDigestive systemActive componentHepatitis B Virus-X
The invention belongs to the technical field of cellular immunotherapy, and specifically relates to a hepatitis B virus X protein (HBx) gene modified liver-cancer-inhibiting whole-cell vaccine. The invention aims at solving a technical problem of providing a novel gene-modified liver-cancer-inhibiting whole-cell vaccine which can be used for treating HBV-related liver cancer. The invention provides an HBx-modified liver-cancer-inhibiting whole-cell vaccine. The active components of the vaccine comprise liver cancer cells modified by liver cancer antigen gene, wherein the liver cancer antigen gene is HBx. The invention also provides a preparation method of the liver-cancer-inhibiting whole-cell vaccine. The liver-cancer-inhibiting whole-cell vaccine provided by the invention can be used in targeted killing of HBx-positive liver cancer cells, and shows good liver-cancer-inhibiting effect.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com