Somatic whole-cell vaccine composition for treating chronic diseases and preparation method thereof
A whole-cell vaccine, a technology for chronic diseases, applied in the field of autologous whole-cell vaccine formulations for the treatment of chronic diseases and the field of preparation thereof, can solve problems such as no obvious breakthrough, and achieve the effects of preventing disease recurrence, improving curative effects, and improving quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
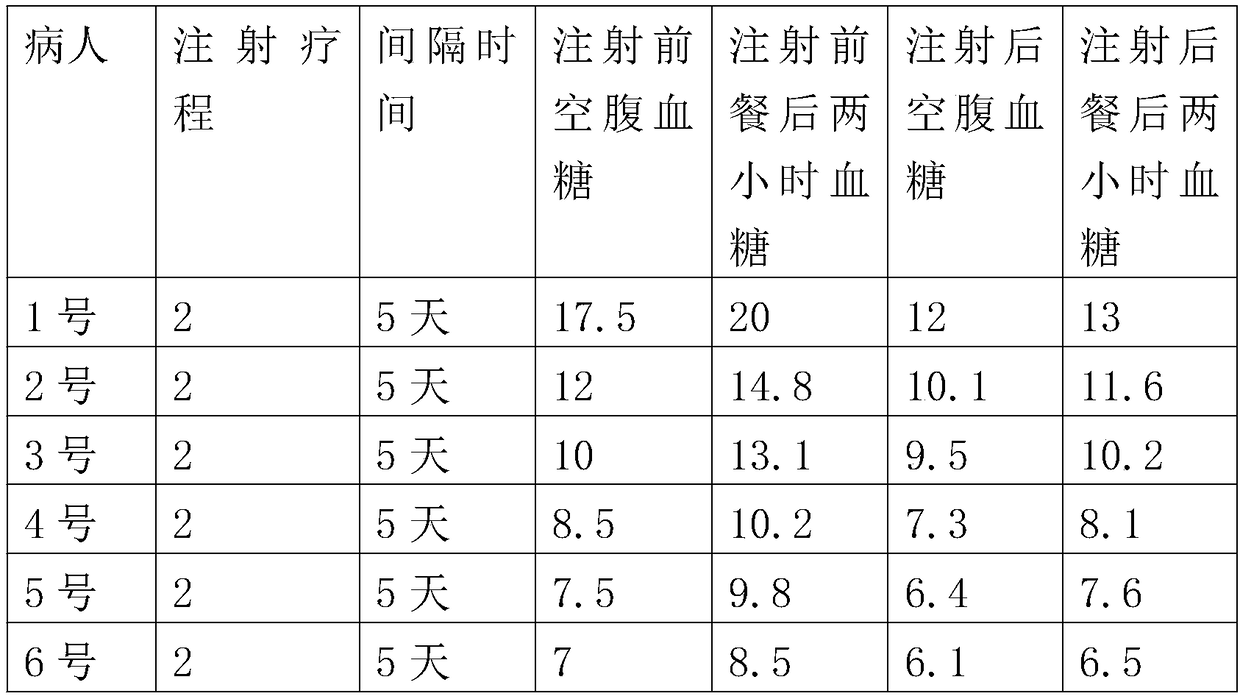
[0030] A method for preparing an autologous whole cell vaccine for treating chronic diseases, comprising the following steps:
[0031] Step 1: Collect, collect about 50ml of peripheral blood from the human body with chronic diseases; centrifuge the collected blood in a centrifuge at a speed of 1500rpm for 8 minutes, suck out the upper serum and transfer it to another centrifuge tube In the process, the plasma was placed in a 56°C water bath for 30 minutes of inactivation, and then centrifuged at a speed of 1000 rpm for 8 minutes. The processed supernatant was transferred to another centrifuge tube and placed. The ambient temperature is 4°C;
[0032] Step 2: Separation, separating peripheral blood and extracting autologous cells; adding the obtained plasma whole blood to normal saline to dilute 2 times, and slowly adding the above precipitated or diluted blood to the lymphocyte separation solution, and the lymphocyte separation solution according to 1: The ratio of 1 was added...
Embodiment 2
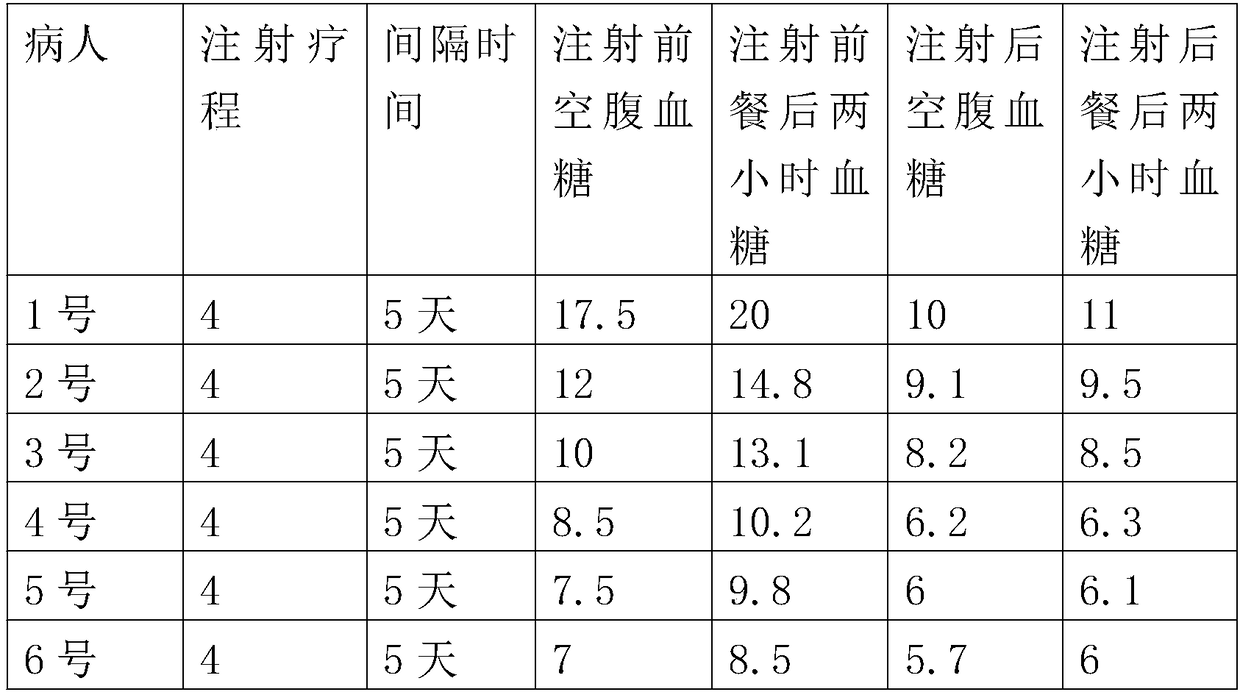
[0041] A method for preparing an autologous whole cell vaccine for treating chronic diseases, comprising the following steps:
[0042] Step 1: Collection, about 120ml of peripheral blood is collected from the human body with chronic diseases; the collected blood is centrifuged in a centrifuge at a speed of 3000rpm, and the centrifugation time is 8 minutes. The upper serum is sucked out and transferred to another centrifuge tube In the process, the plasma was placed in a 56°C water bath for 30 minutes of inactivation, and then centrifuged at a speed of 2800 rpm for 8 minutes. The treated supernatant was transferred to another centrifuge tube and placed The ambient temperature is 4°C;
[0043] Step 2: Separation, separating peripheral blood and extracting autologous cells; adding the obtained plasma whole blood to normal saline to dilute 2 times, and slowly adding the above precipitated or diluted blood to the lymphocyte separation solution, and the lymphocyte separation solutio...
Embodiment 3
[0052] A preparation method of autologous whole cell vaccine for treating chronic diseases, comprising the following steps:
[0053] Step 1: Collection, collect about 100ml of peripheral blood from people with chronic diseases; centrifuge the collected blood in a centrifuge, the speed of the centrifuge is 2300rpm, the centrifugation time is 8 minutes, the upper serum is sucked out and transferred to another centrifuge tube , the plasma was placed in a 56°C water bath for 30 minutes of inactivation, and then centrifuged at 1800 rpm for 8 minutes, and the treated supernatant was transferred to another centrifuge tube and placed. The ambient temperature is 4°C;
[0054] Step 2: separate, separate peripheral blood and extract autologous cells; add the gained plasma whole blood to normal saline to dilute 2 times, and slowly add the above precipitation or diluted blood dilution to contain the lymphocyte separation solution, and the lymphocyte separation solution is according to 1: 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com