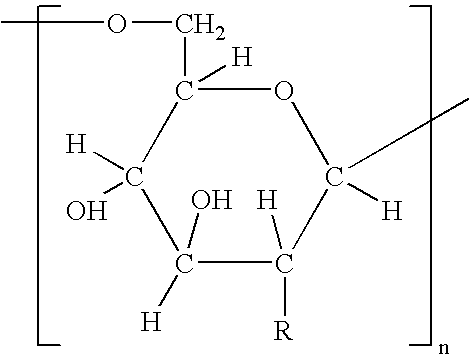
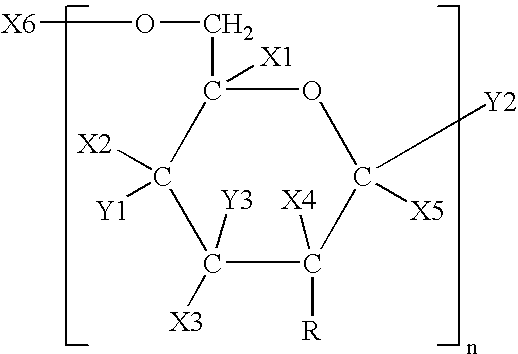
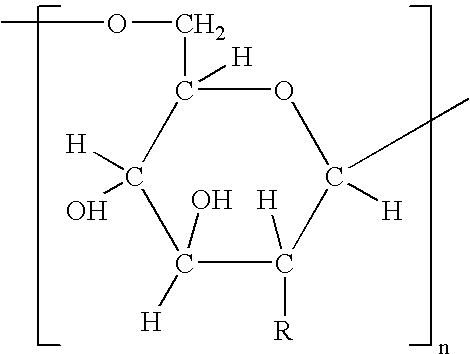
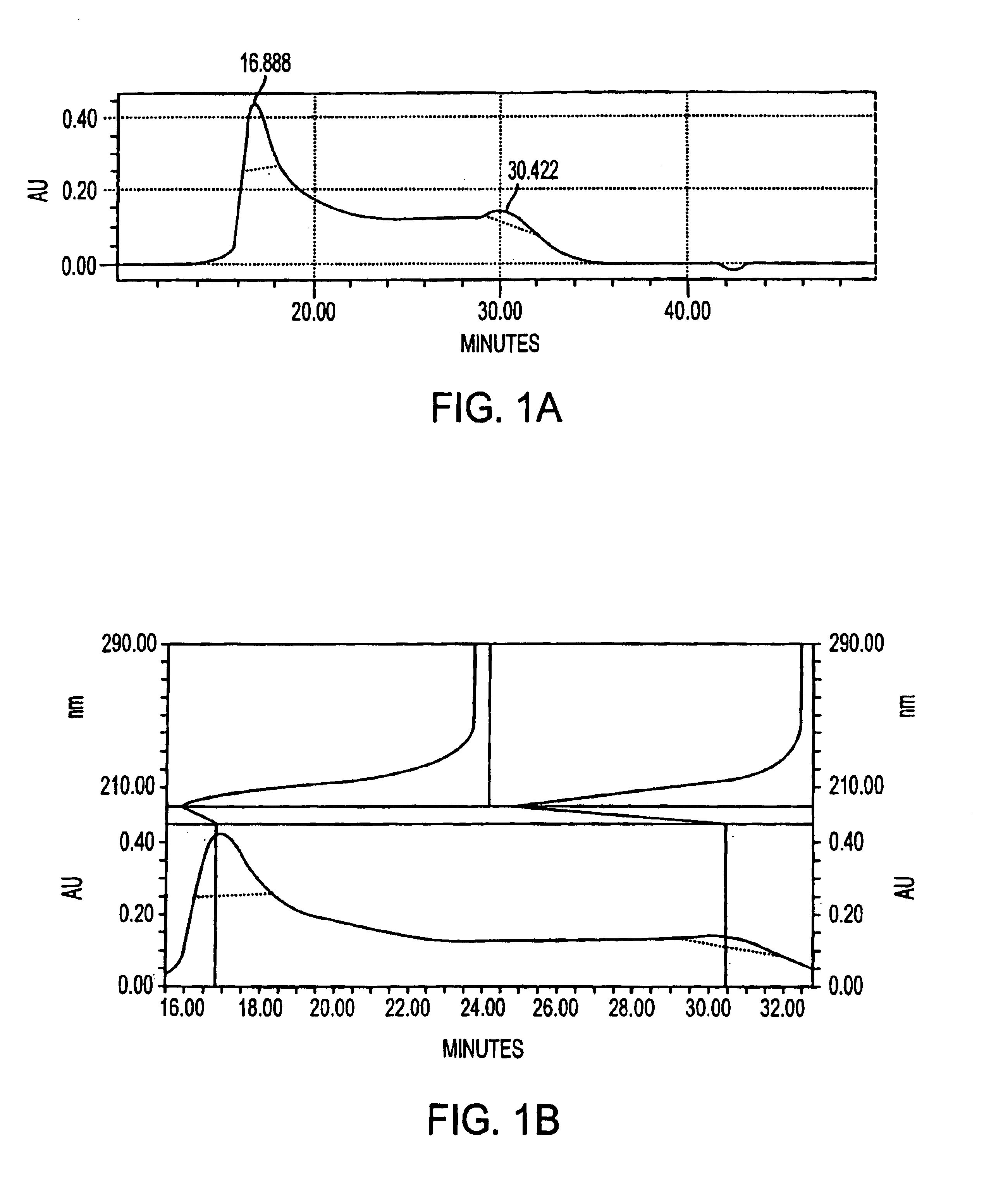
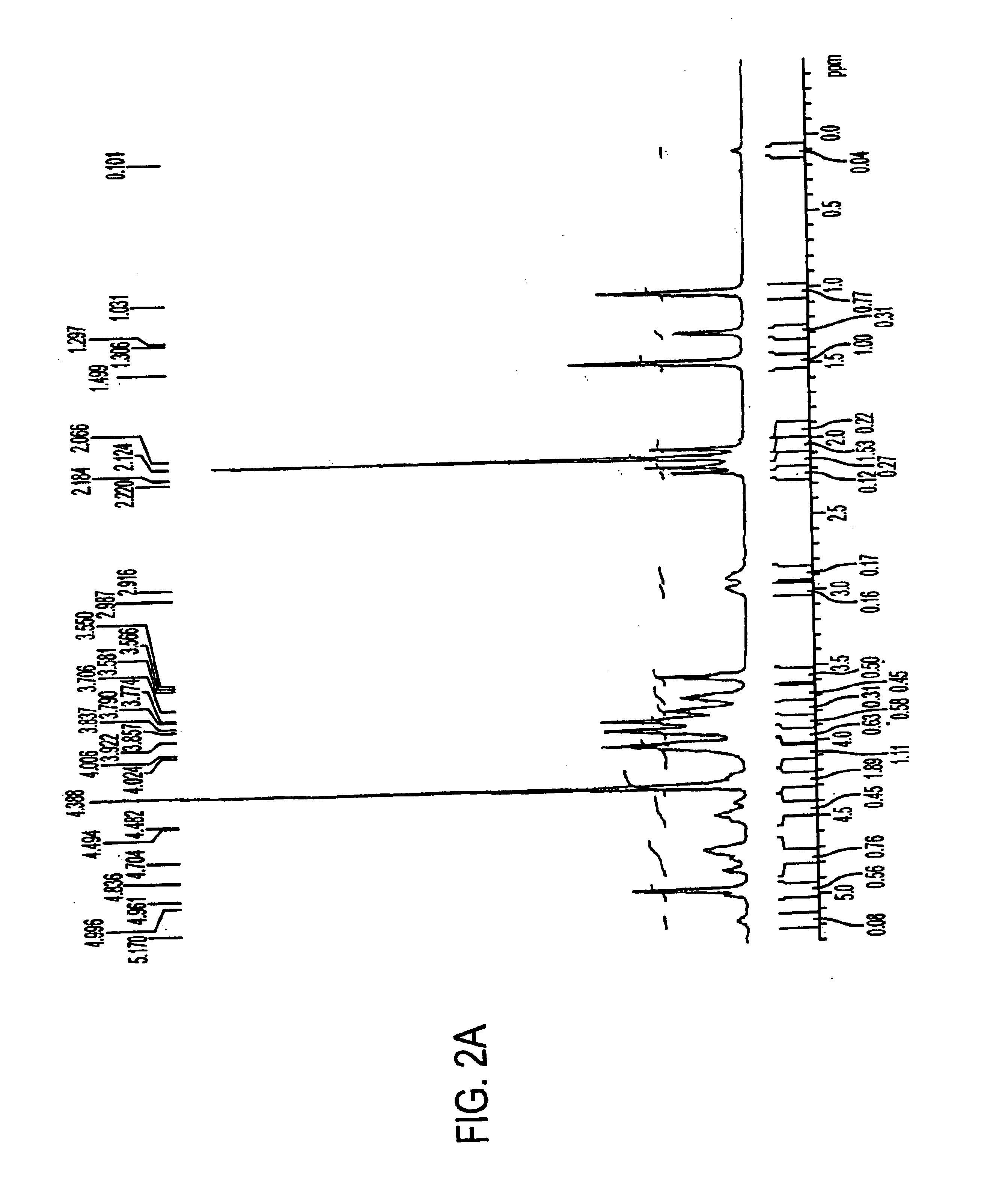
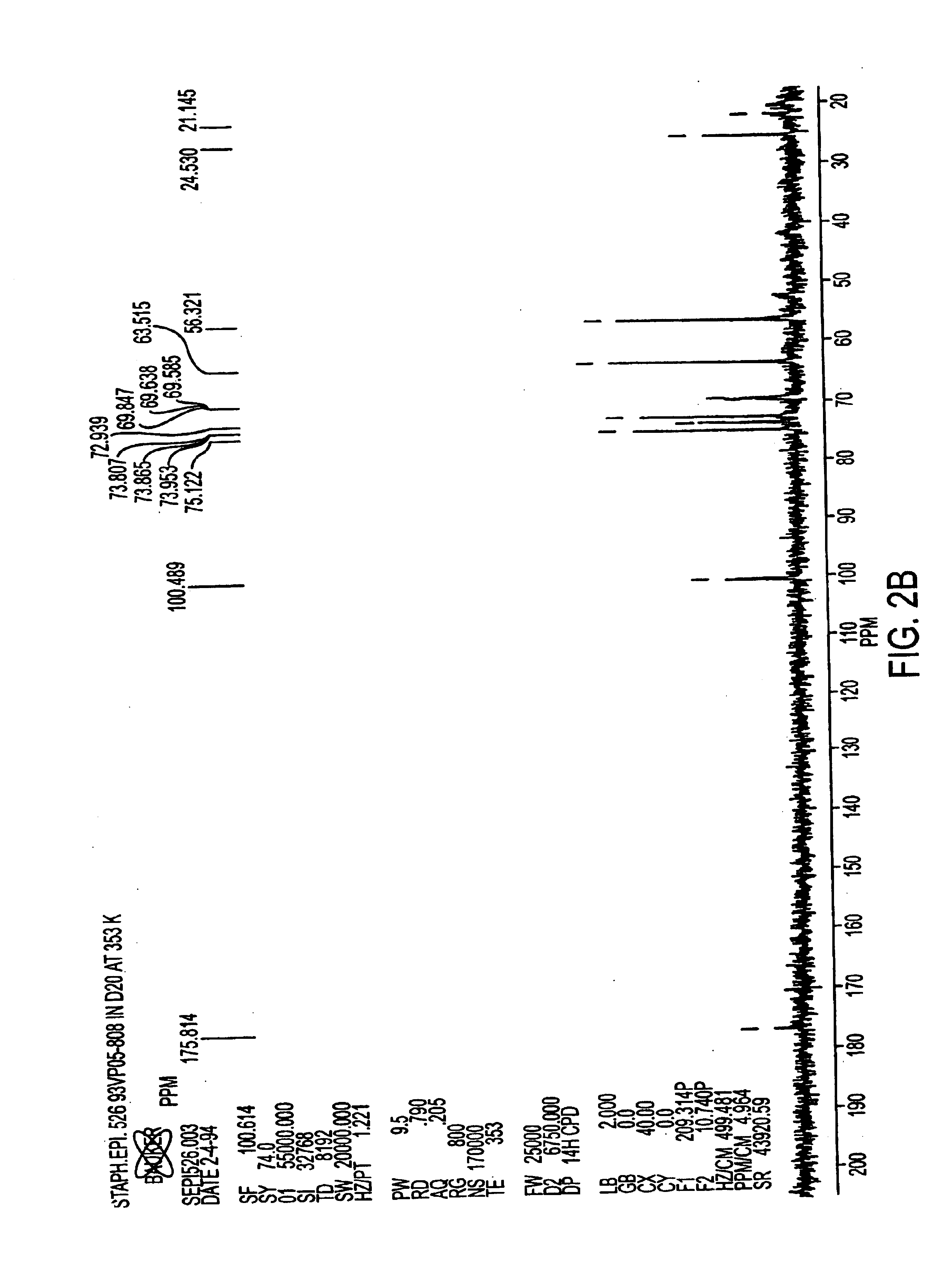
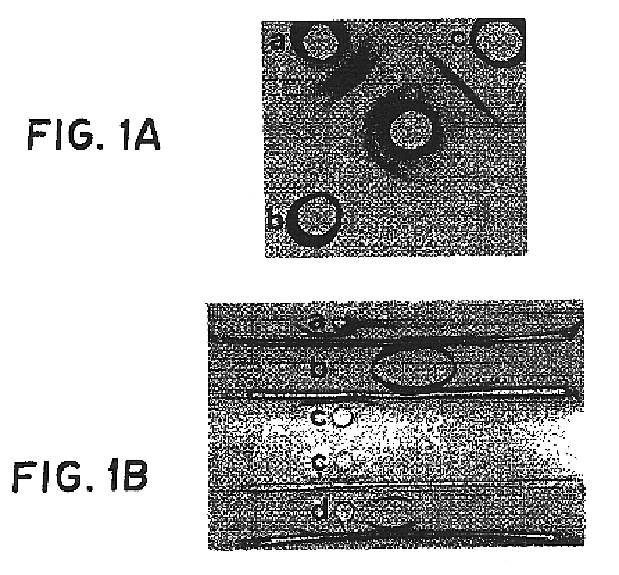
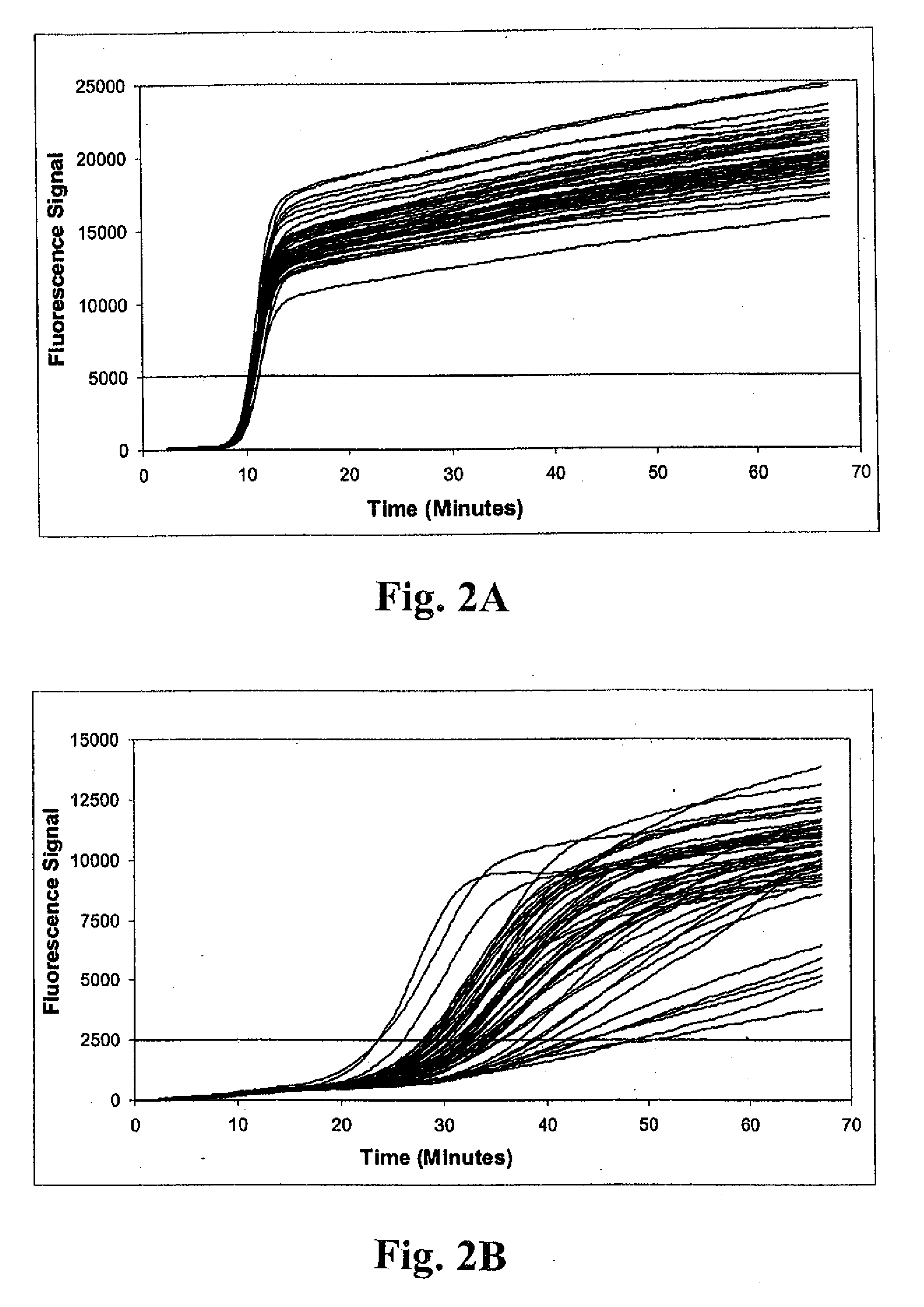
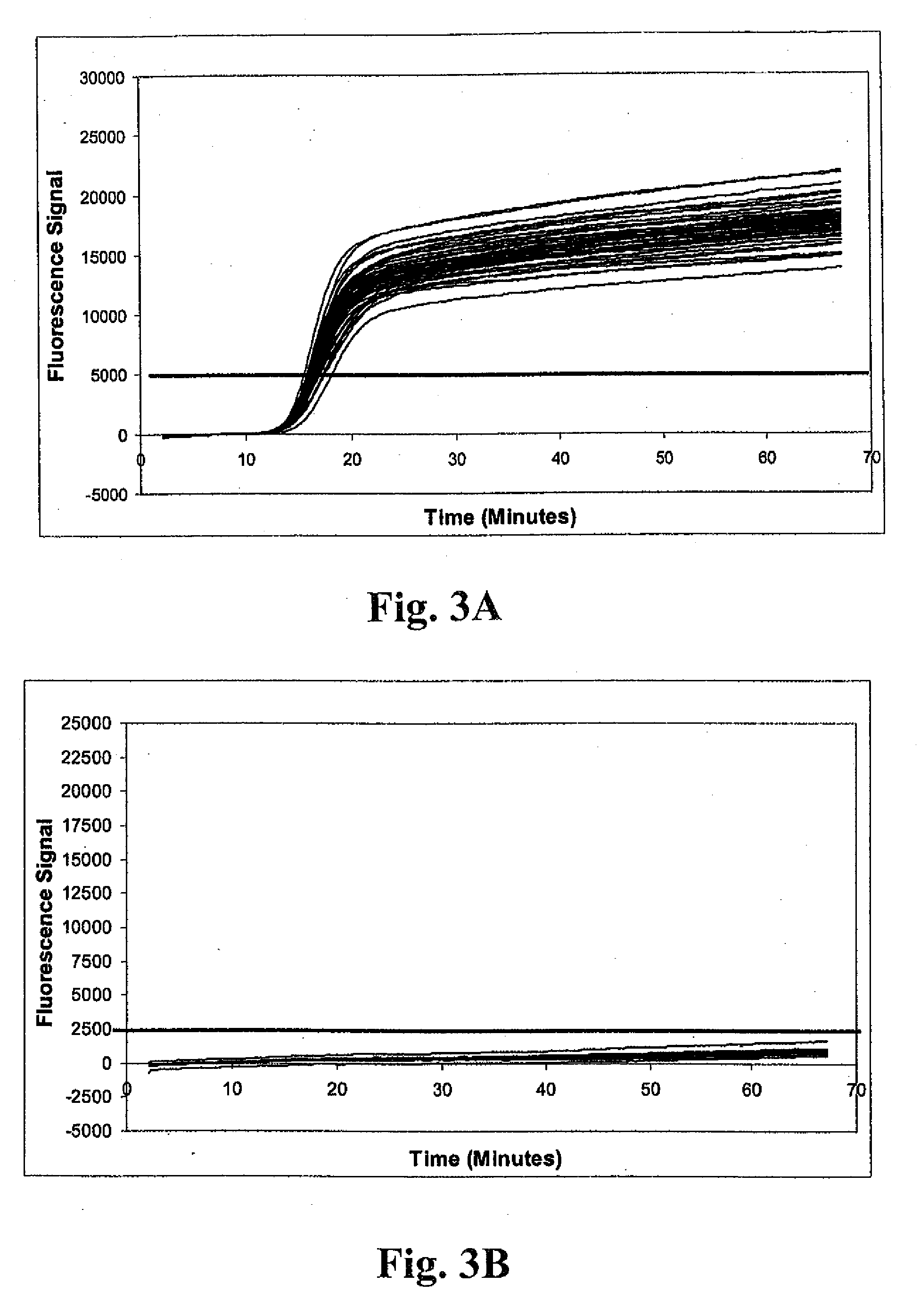
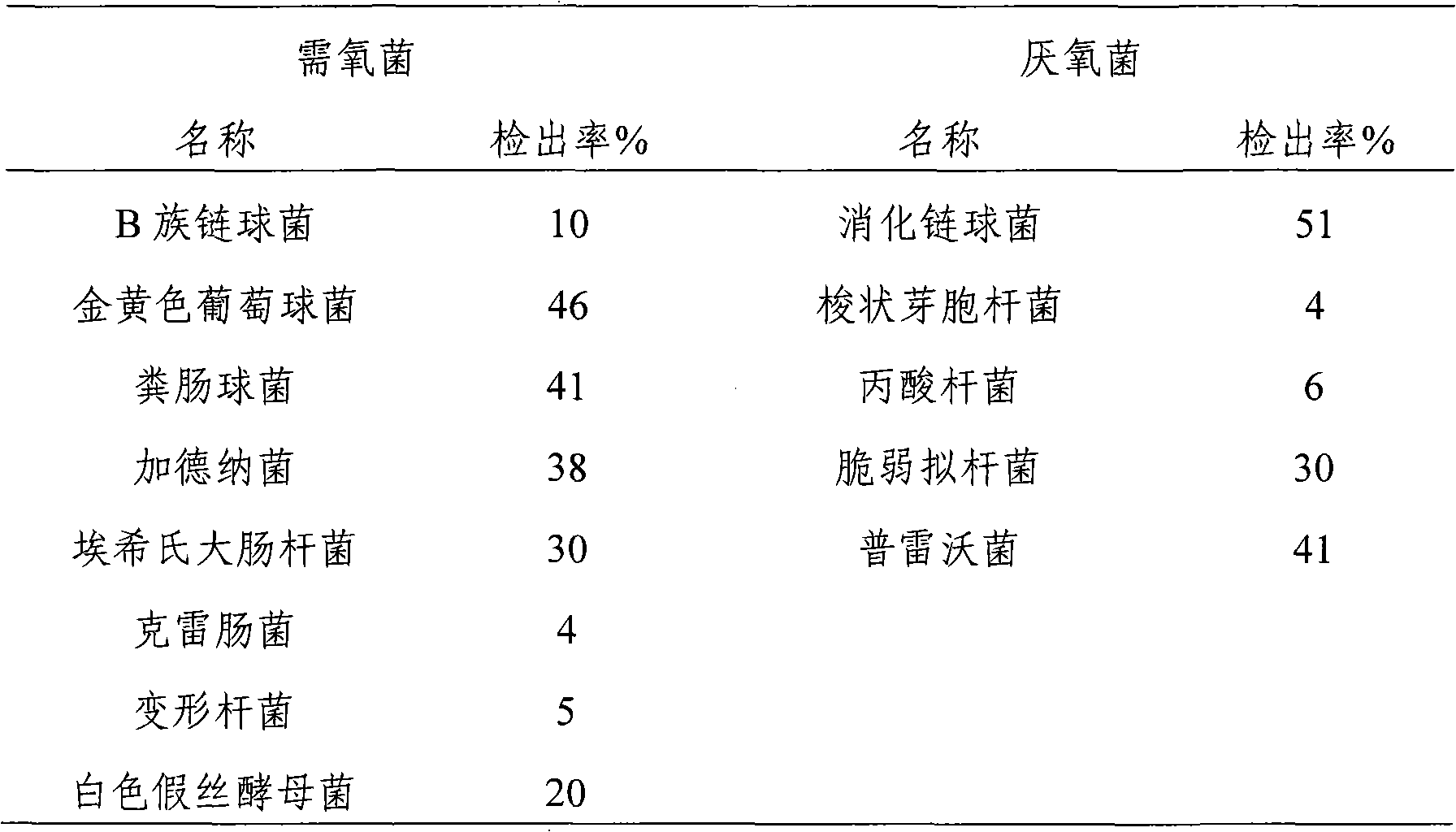
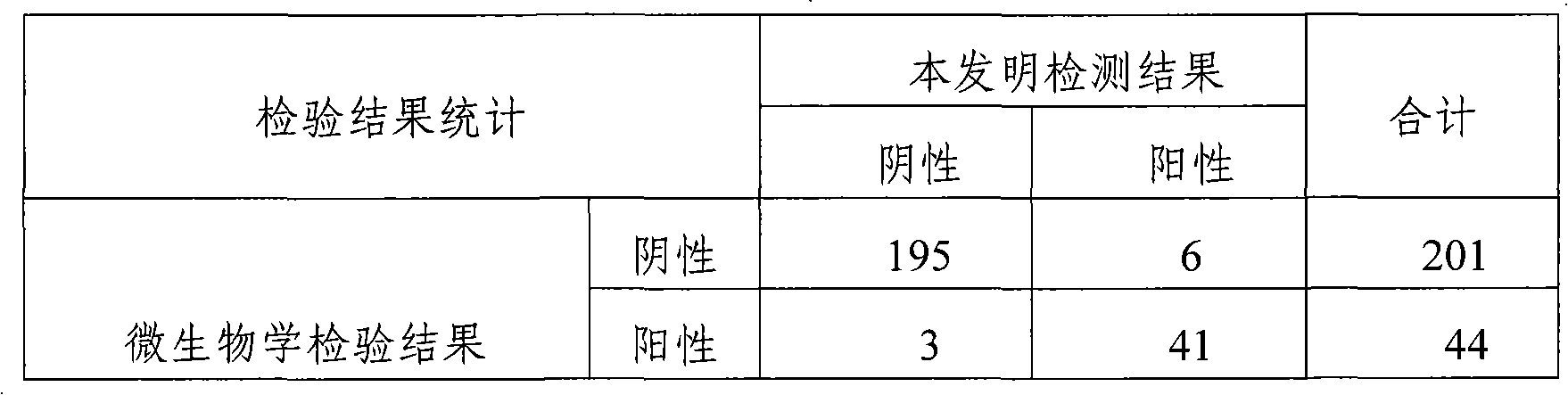
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Coagulase test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
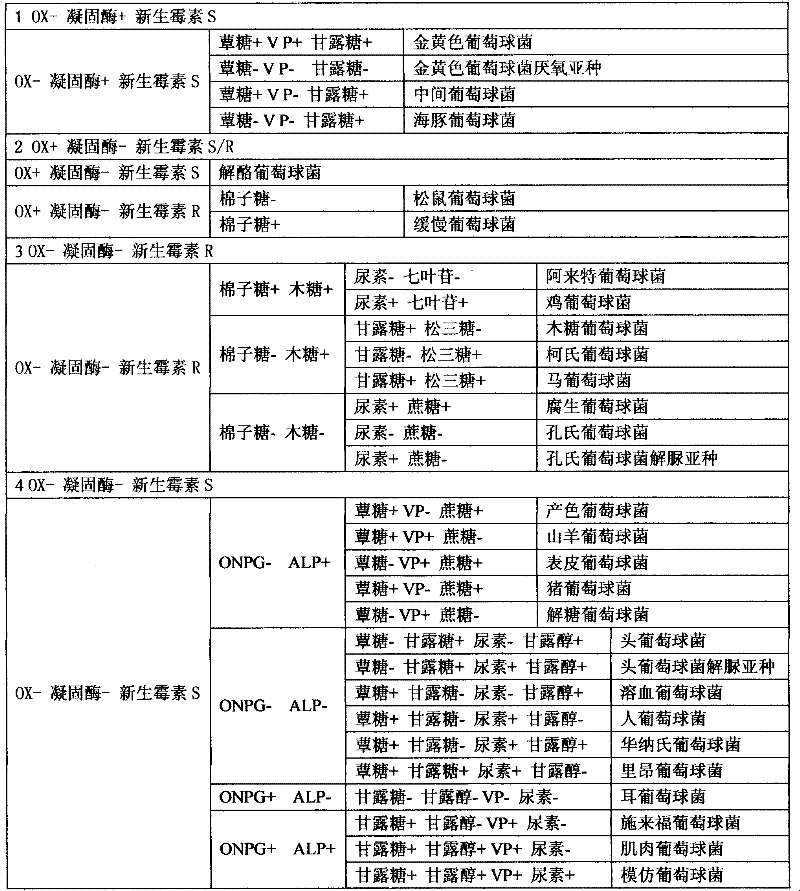
A slide coagulase test is run with a negative control to rule out autoagglutination. Two drops of saline are put onto the slide labeled with sample number, Test (T) and control (C).
Polysaccharide vaccine for staphylococcal infections
ActiveUS20050118198A1Improving immunogenicityAntibacterial agentsOrganic active ingredientsNatural sourceIntracellular
The invention relates to compositions of a deacetylated poly N-acetylated glucosamine (dPNAG) of Staphylococci. The dPNAG may be isolated from natural sources or synthesized de novo. The invention also relates to the use of dPNAG as a vaccine for inducing active immunity to infections caused by Staphylococcus aureus, S. epidermidis, other related coagulase-negative or coagulase-positive Staphylococci, and other organisms carrying the ica (intracellular adhesion) locus. The invention further provides methods of use for antibodies directed to dPNAG, particularly for inducing passive immunity to the same class of infections.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Staphylococcus antigen and vaccine
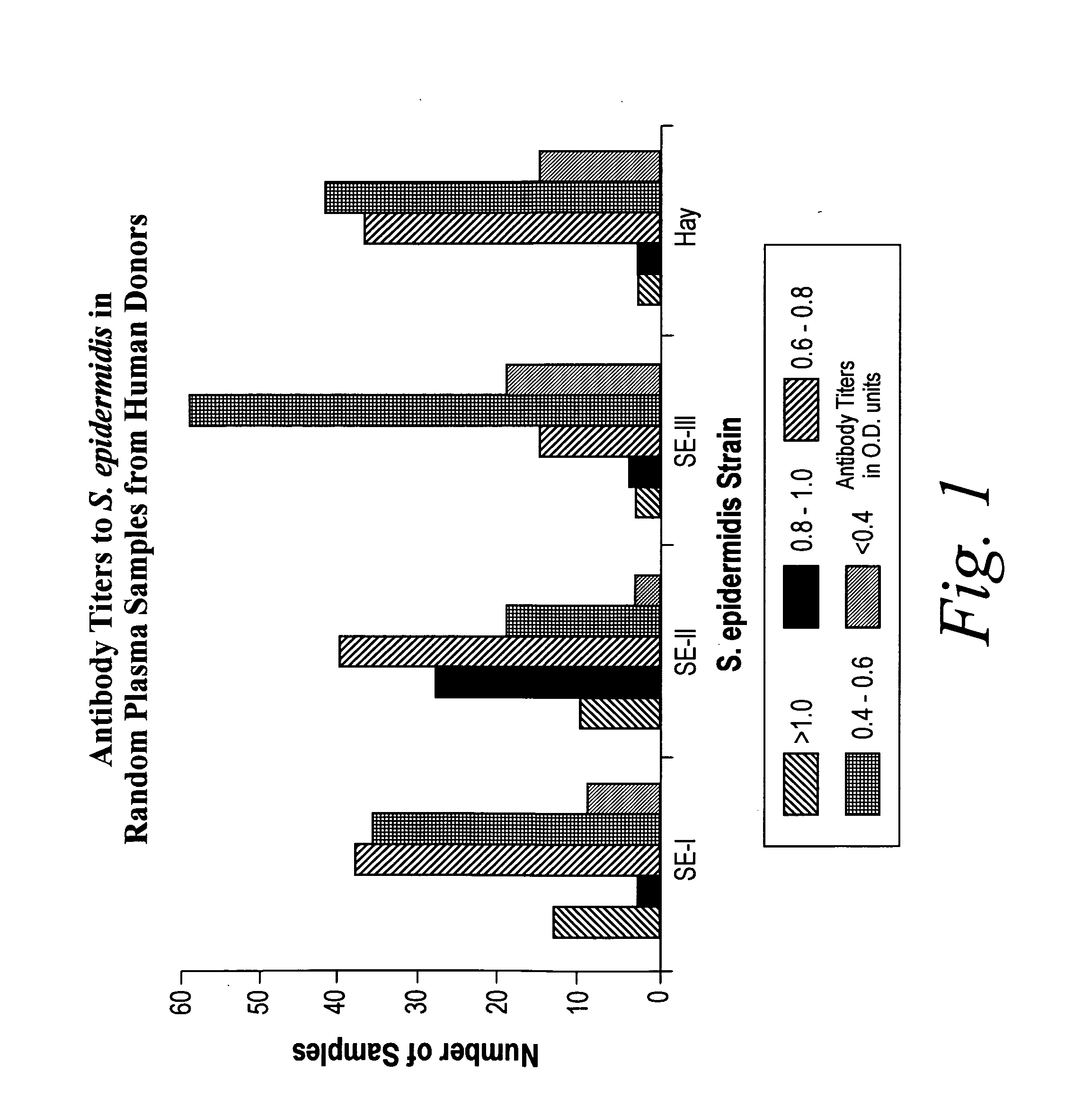
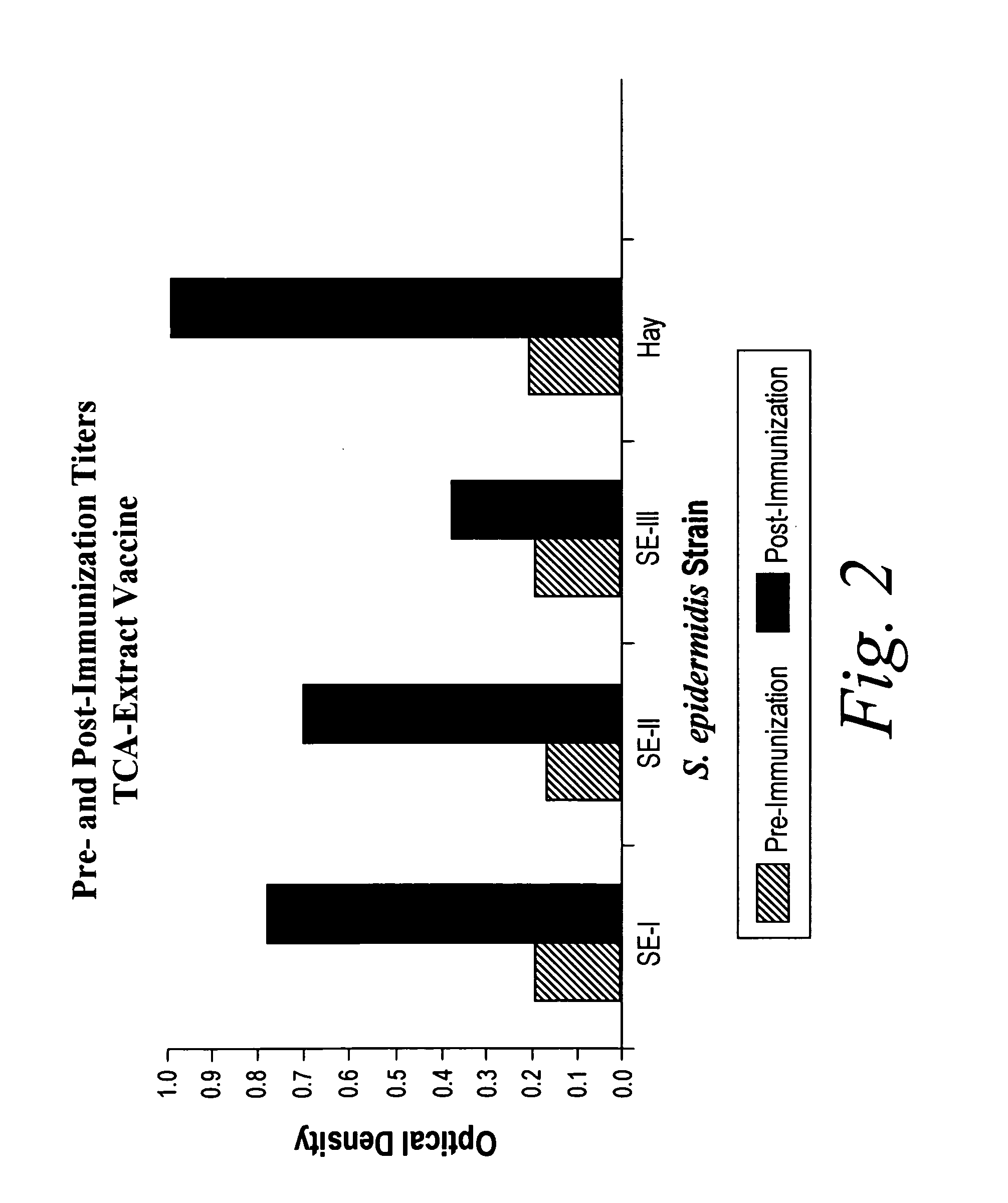
A negatively-charged Staphylococcus antigen contains amino acids and a N-acetylated hexosamine as a major carbohydrate component. The antigen is common to many coagulase-negative strains of Staphylococcus, including S. epidermidis, S. haemolyticus, and S. hominis. Staphylococcus strains that carry the antigen include many clinically significant strains of Staphylococcus. The antigen and antibodies to the antigen are useful in kits and assays for diagnosing Staphylococcus infection. Vaccines of the antigen and of whole cells that carry the antigen also are disclosed.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Capsular polysaccharide adhesin antigen, preparation, purification and use
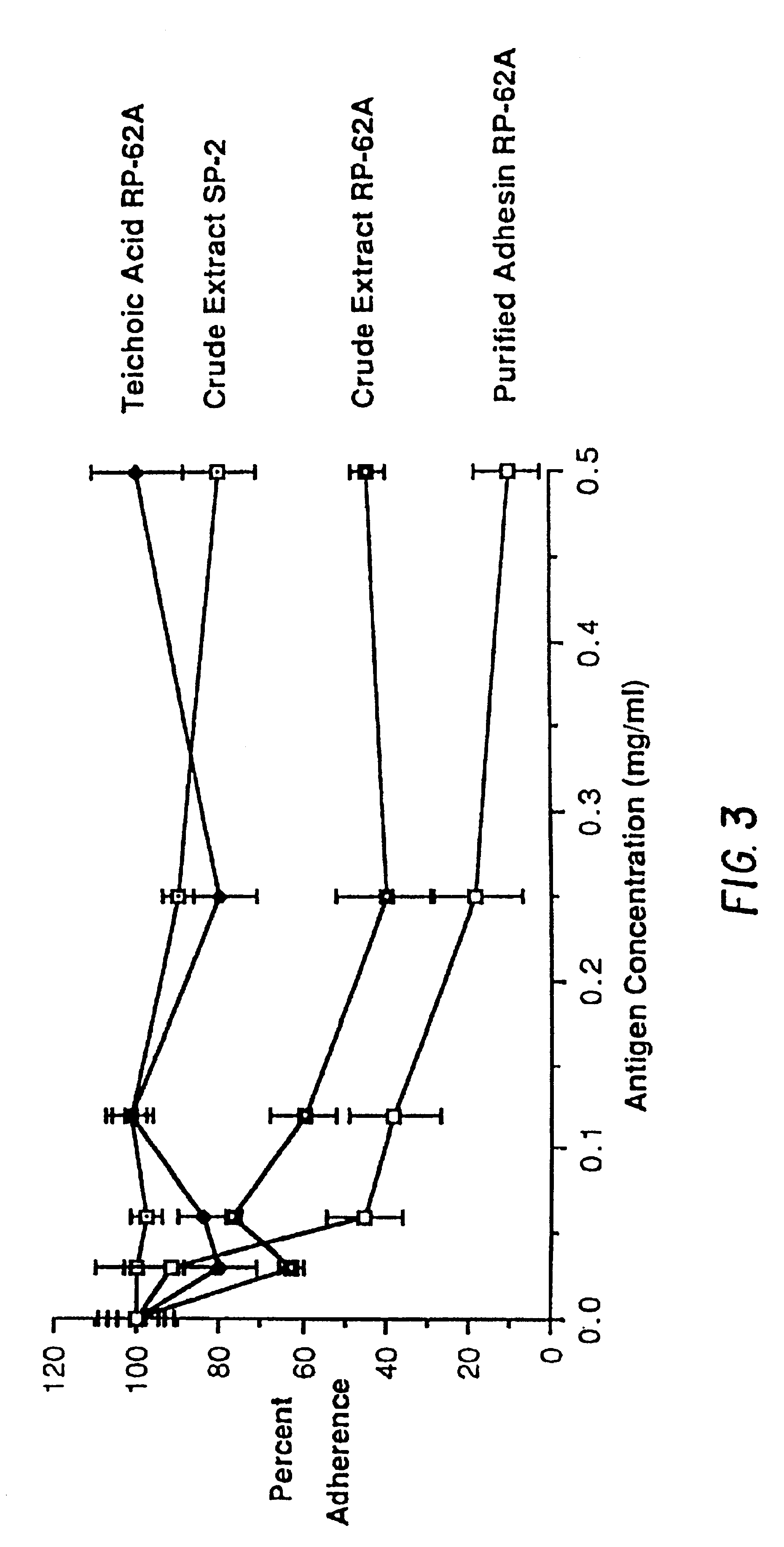
A substantially pure capsular exopolysaccharide adhesin of coagulaso-negative staphylococcal strains, and a general method to prepare such adhesins, are described. Vaccines composed of such adhesins, and uses of such adhesins to produce polyclonal and mono-clonal antibodies against such adhesins, are also disclosed. The adhesins are useful in coating polymeric medical materials to prevent colonization by coagulase-negative staphylococcal strains, and as a probe in selecting desirable polymeric medical materials. Such adhesin antibodies are useful in vivo to prevent infection by noso-comial coagulase-negative staphylococcal strains, in assays for the detection of such bacteria, in assays for the estimation of such adhesins in complex mixtures, and as an affinity chromatography matrix.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Isolated broadly reactive opsonic immunoglobulin for treating a pathogenic coagulase-negative staphylococcus infection
InactiveUS7279162B1Prevent CNSEffective treatmentDepsipeptidesAntibody ingredientsAntigenCoagulase test
The invention describes the identification, making, and isolation of immunoglobulin and antigen useful for preventing, diagnosing, and treating staphylococcal infections. The invention further describes an in vivo animal model useful for testing the efficacy of pharmaceutical compositions, including pharmaceutical compositions of immunoglobulin and isolated antigen.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Proteins and polypeptides from coagulase-negative staphylococci
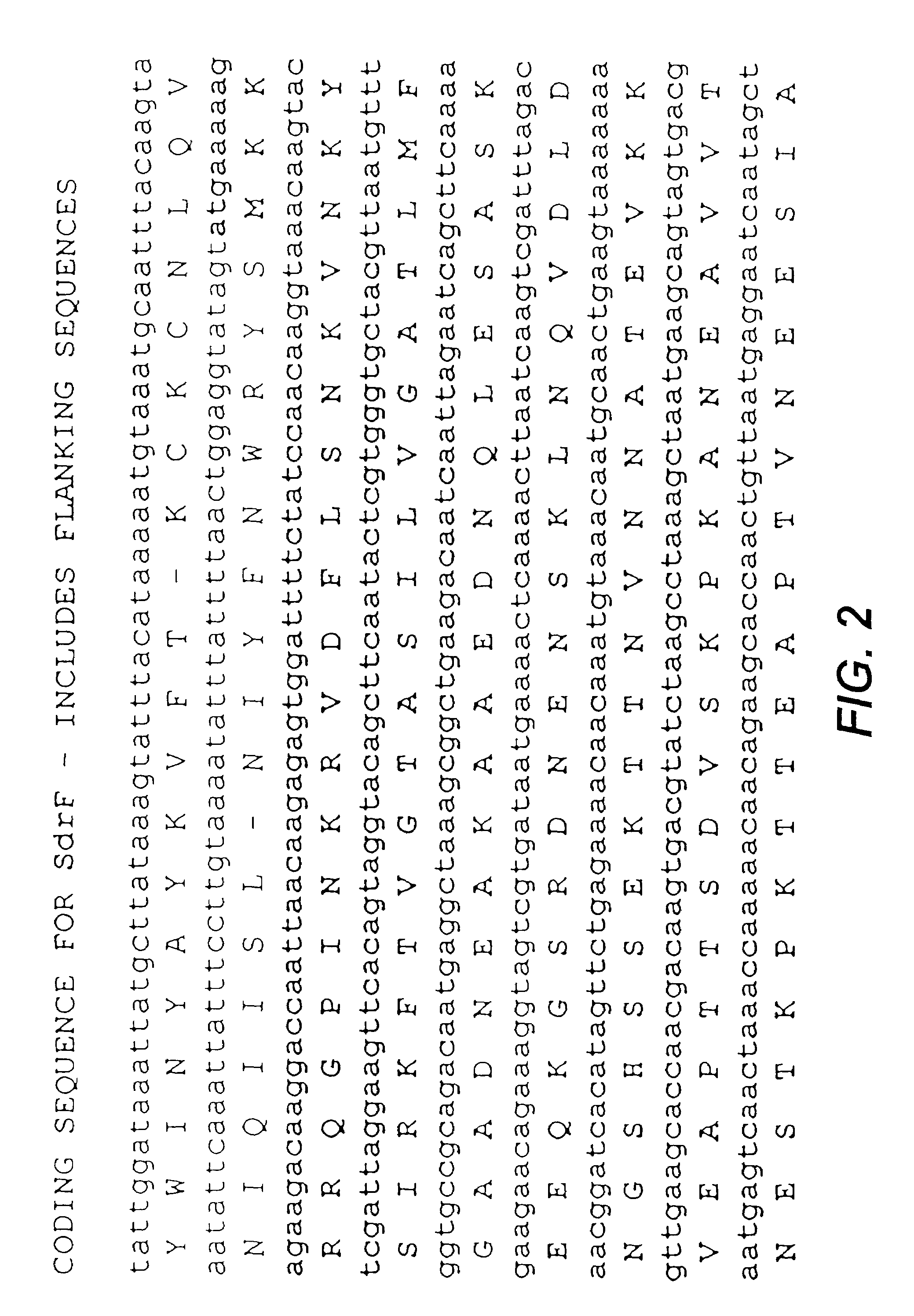
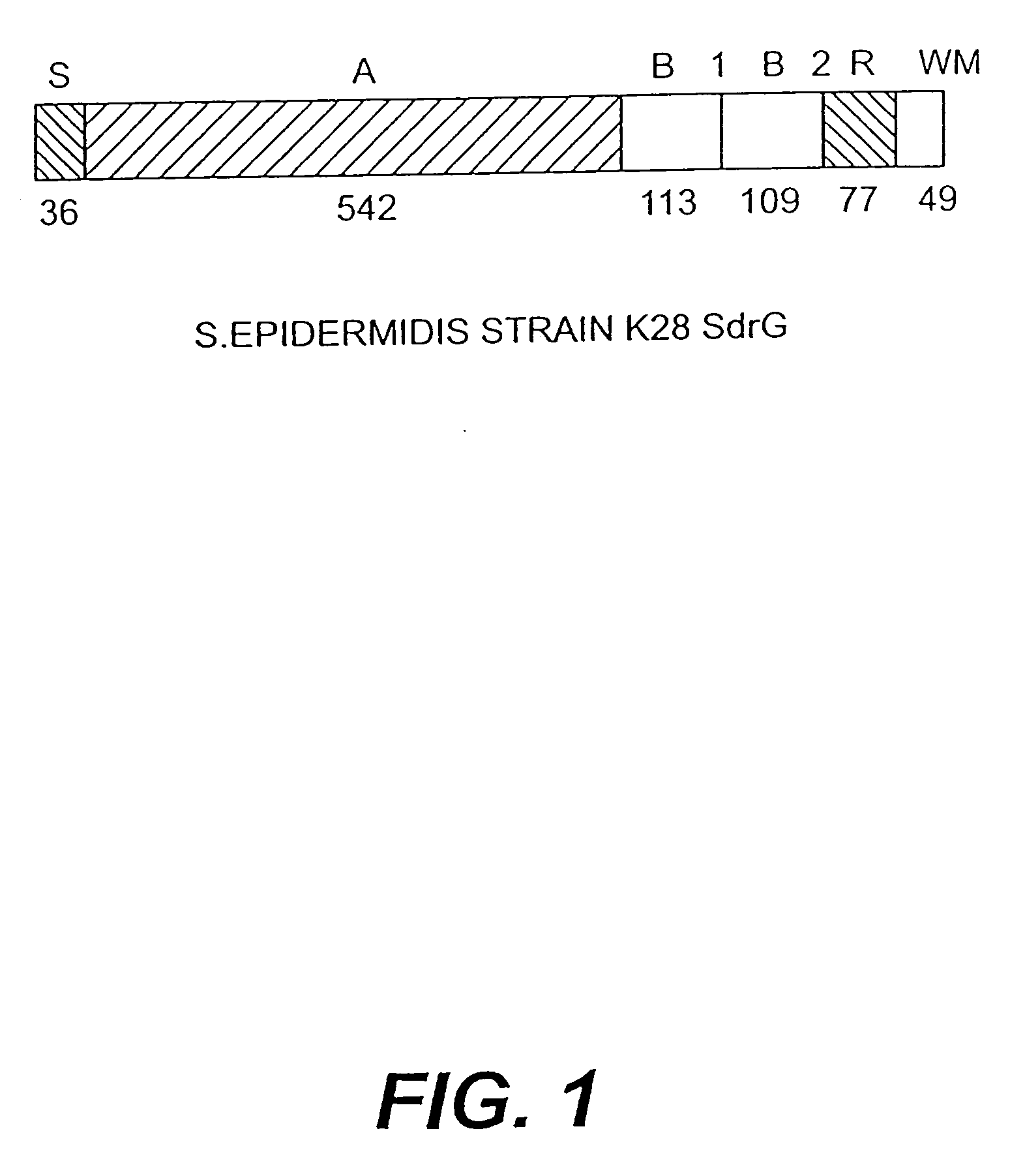
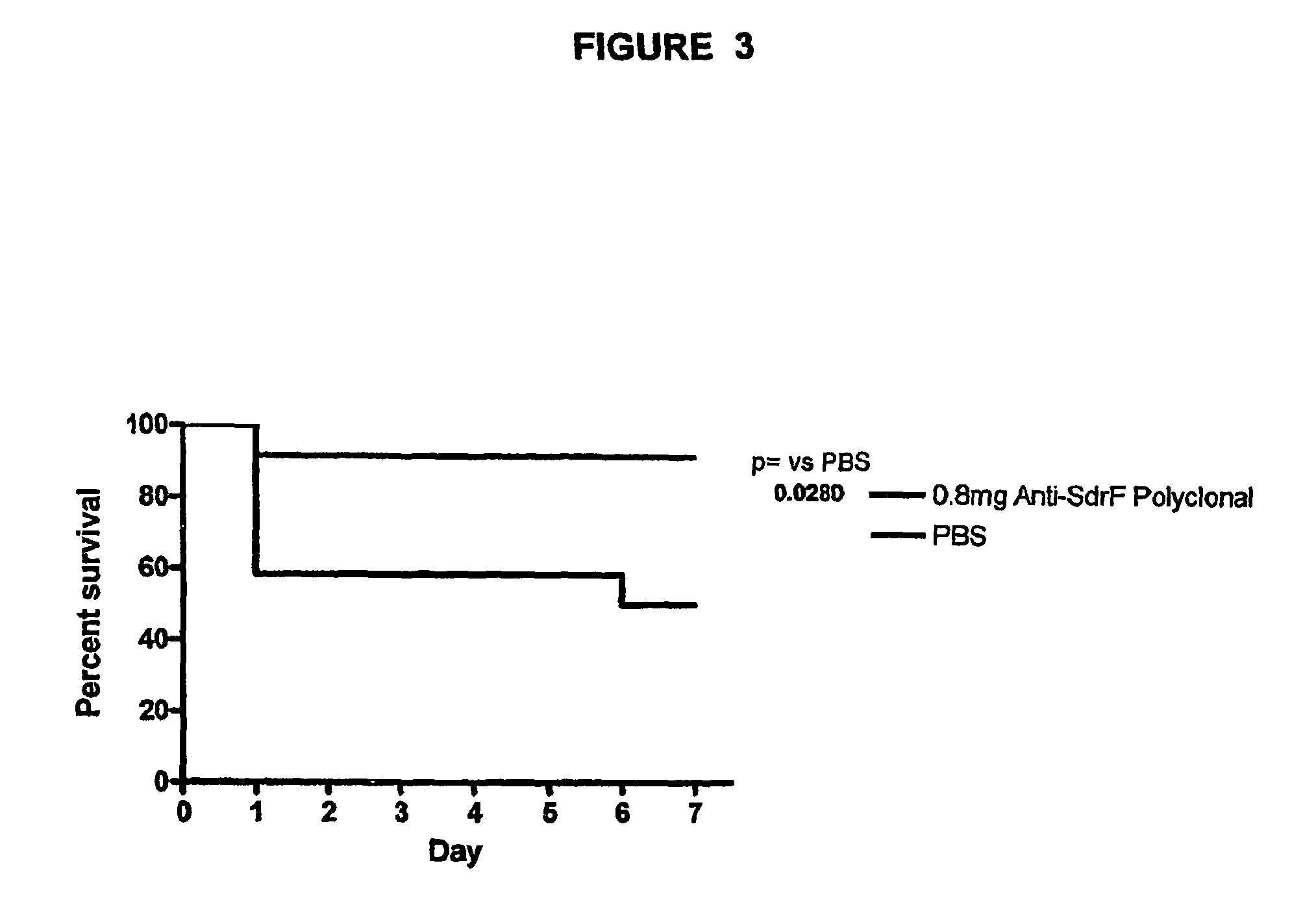
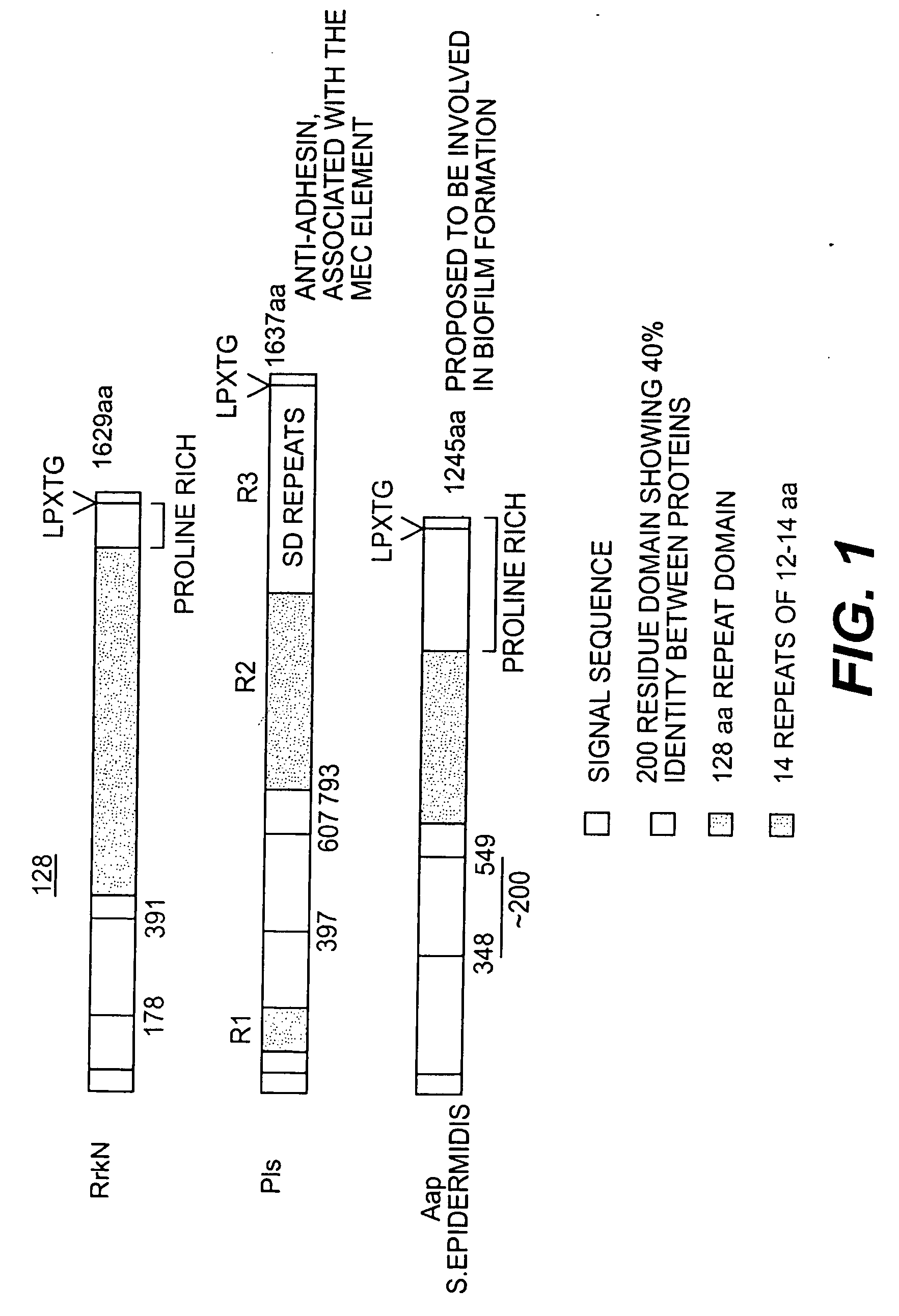
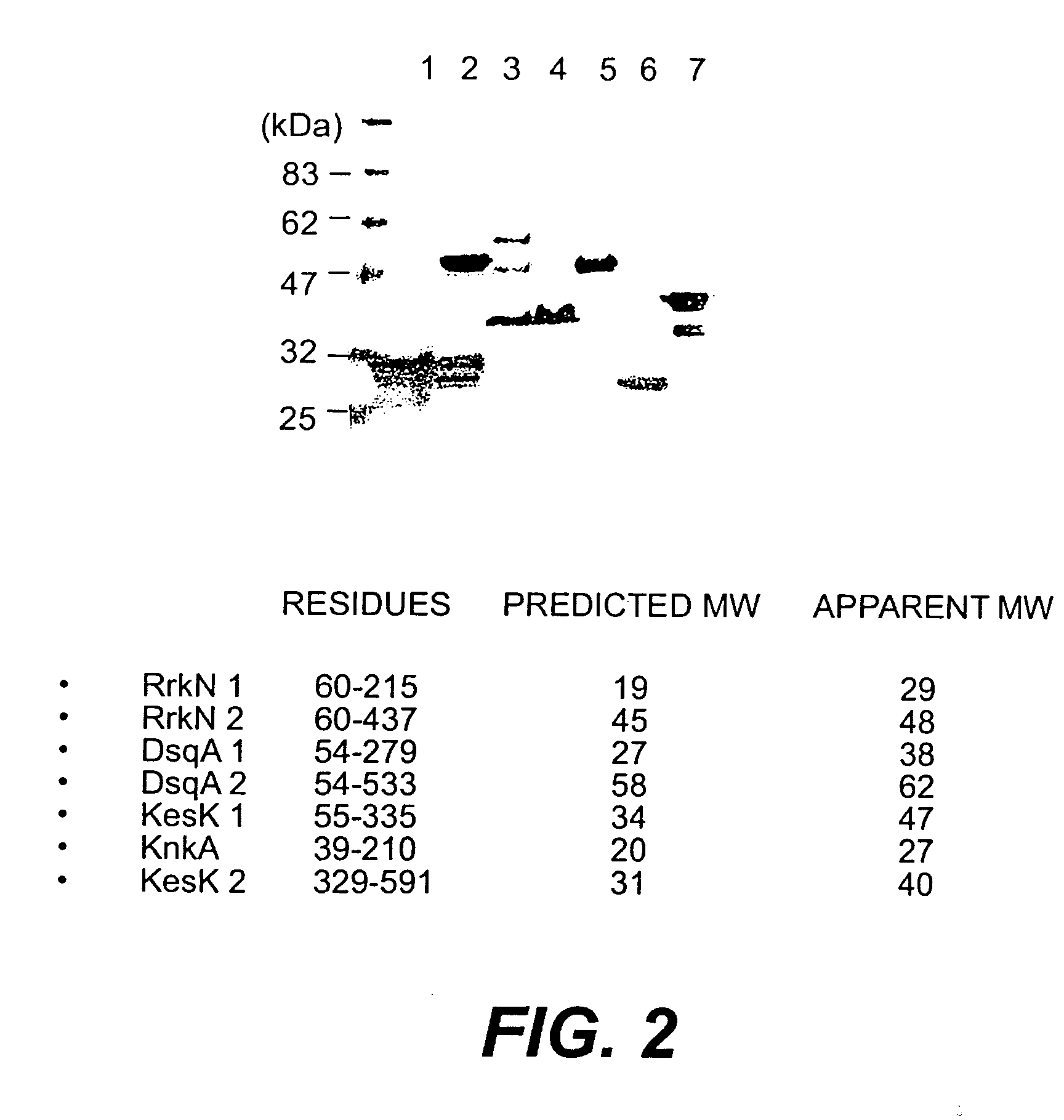
Proteins and polypeptides from coagulase-negative staphylococcal bacteria such as S. epidermidis, including proteins designated SdrF, SdrG and SdrH, and their effective fragments such as their respective A domains, are provided which are useful in the prevention and treatment of infection caused by coagulase-negative staphylococcal bacteria such as S. epidermidis. The SdrF, SdrG and SdrH proteins are cell-wall associated proteins that specifically bind host proteins and which each have a highly conserved motif of which the consensus sequence is TYTFTDYVD (SEQ ID NO: 16). The proteins and polypetides may be useful in generating antibodies for the diagnosis and treatment of coagulase-negative staphylococcal infections.
Owner:TRINITY COLLEGE DUBLIN +1
Isolated Broadly Reactive Opsonic Immunoglobulin for Treating a Pathogenic Coagulase-Negative Staphylococcus Infection
InactiveUS20080139789A1Immunoglobulins against animals/humansAntibody ingredientsAntigenImmunglobulin e
The invention describes the identification, making, and isolation of immunoglobulin and antigen useful for preventing, diagnosing, and treating staphylococcal infections. The invention further describes an in vivo animal model useful for testing the efficacy of pharmaceutical compositions, including pharmaceutical compositions of immunoglobulin and isolated antigen.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Quality control product of AV/BV combined test kit and preparation method thereof
ActiveCN102864208AEasy to prepareEasy to operateMicrobiological testing/measurementBULK ACTIVE INGREDIENTHydrogen peroxide
The invention provides a five-item quality control product of an AV / BV combined test kit and a preparation method thereof, belonging to quality control products for controlling the quality of kits used during female gynecological clinical detection. The quality control product comprises a vaginal discharge five-item positive quality control product and a vaginal discharge five-item negative quality control product, and the two types of quality control products are stored in the states of dry powder. The quality control product is used to carry out quality detection of a vaginitis test kit and can carry out quality control on five biochemical indexes including hydrogen peroxide, neuraminidase, leucocyte esterase, beta-glucuronidase and coagulase. By means of taking biological enzymes as active ingredients of the quality control product and preparing a dry powder quality control product by adopting a freezing-drying technology, the deactivation of a liquid quality control product in transportation and storage processes is avoided. The quality control product does not contain any human vaginal discharge, and does not cause infection to the environment and operators.
Owner:北京中生金域诊断技术股份有限公司
Staphylococcus aureus multiple PCR-MIX rapid detection kit and detection method thereof
ActiveCN101613745AStrong specificityQuick checkMicrobiological testing/measurementMicroorganism based processesSmaIStaphylococcus aureus
The invention discloses a staphylococcus aureus multiple PCR-MIX rapid detection kit and a detection method thereof; the detection kit comprises PCR MIX; wherein, the PCR MIX contains 2*PCR buffer, 1.0-3.0mmol / L of MgCl2, 180-220mu mol / L of dNTP each, heat-resisting deoxyribonuclease nuc gene primer of which the base sequence is SEQ NO.1 and the concentration of SEQ NO.1 is 100-600nmol / L, plasma-coagulase clfA gene primer of which the base sequence is SEQ NO.3 and the concentration of SEQ NO.4 is 100-600nmol / L, SmaI restriction fragment specific sequence primer of which the base sequence is SEQ NO.5 and the concentration of SEQ NO.6 is 100-600nmol / L, 20-60U of Taq enzyme and bromophenyl blue dye. The multiple PCR-MIX rapid detection kit and the detection method provided by the invention have simple configuration, the detection kit and the detection method can be used in industrialized production; the detection method is sensitive and has short detection cycle and strong maneuverability, so that the method can be widely applied in the fields of food hygiene, environmental monitoring, etc.
Owner:GUANGDONG INST OF MICROORGANISM +1
Polypeptides and polynucleotides from coagulase-negative staphylococci
InactiveUS20060171964A1Readily apparentAntibacterial agentsBacteriaNucleotideStaphylococcal infections
Methods for treating or preventing infections from coagulase-negative staphylococci using proteins and polypeptides from coagulase-negative staphylococcal bacteria such as S. epidermidis, including proteins designated SdrF, SdrG and SdrH, and their effective fragments such as their respective A domains, are provided. Methods are also provided wherein antibodies that recognize the SdrG protein or its ligand binding A region are used to treat or prevent staphylococcal infection, and these methods can also be utilized to prevent the formation of infections on indwelling medical devices.
Owner:TRINITY COLLEGE DUBLIN +1
Monoclonal antibodies recognizing a coagulase-negative staphylococcal protein
ActiveUS8475798B2High affinityMethod can be usedAntibacterial agentsAntibody ingredientsADAMTS ProteinsMonoclonal antibody
Owner:INHIBITEX INC +1
Cross-reactive monoclonal and polyclonal antibodies which recognize surface proteins from coagulase-negative staphylococci and staphylococcus aureus
InactiveUS20050106648A1Preventing and treating staphylococcal infectionWide rangeAntibacterial agentsImmunoglobulins against bacteriaStaphylococcus cohniiStaphylococcal infections
Polyclonal and monoclonal antibodies which are cross-reactive to both coagulase-positive staphylococcus bacteria, such as S. aureus and to coagulase-negative bacteria, such as S. epidermidis and S. hemolyticus, are provided which can recognize surface proteins from both coagulase-positive and coagulase negative staph bacteria. The antibodies may be generated from surface proteins that have been isolated on the basis of characteristics that may be common between S. aureus and coagulase-negative staphylococci, and these recombinant surface proteins are used to generate the antibodies of the invention. There is also provided vaccines and methods which utilize these proteins and antibodies for the treatment or protection against a wide variety of staphylococcal infections.
Owner:FOSTER TIMOTHY +5
Detection of antibiotic-resistant microorganisms
ActiveUS20090181395A1Easy to separateReduce morbidityMicrobiological testing/measurementFermentationCoagulase testMethicillin sensitive
Method of detecting methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) in a nucleic acid coamplification assay. The invention advantageously reduces the incidence of false-positive MRSA determinations in real-time assays by requiring satisfaction of a threshold criterion that excludes certain co-infections from the MRSA determination. The invention further provides for determination of MSSA, even when the MSSA is present in combination with methicillin-resistant coagulase-negative (MR-CoNS) bacteria at high or low levels.
Owner:GEN PROBE INC
Kit for detecting aerobic bacteria in vaginal discharge and preparation method thereof
ActiveCN101792792ANo training requiredSimple and fast operationMicrobiological testing/measurementMicroorganism based processesEscherichia coliBacteroides
The invention provides a kit for detecting aerobic bacteria in vaginal discharge, a dried kit comprises a reactor for detecting the enzyme activity of beta-glucuronidase and coagulase and color-developing agent, and a humidified kit comprises beta-glucuronidase substrate solution, coagulase substrate solution and color-developing agent. The invention also provides a preparation method of the kit. The kit in the invention directly identifies the aerobic flora, in particular to the aerobic floras such as staphylococcus aureus, Enterococcus faecalis, streptococcus group B, Escherichia coli and the like which can cause aerobic bacteria vaginalitis in vaginal discharge by detecting the activity of beta-glucuronidase and coagulase. The method has simple, convenient and rapid operation and high accuracy, and is suitable for convention application in clinics especially in hospital outpatient.
Owner:北京中生金域诊断技术股份有限公司
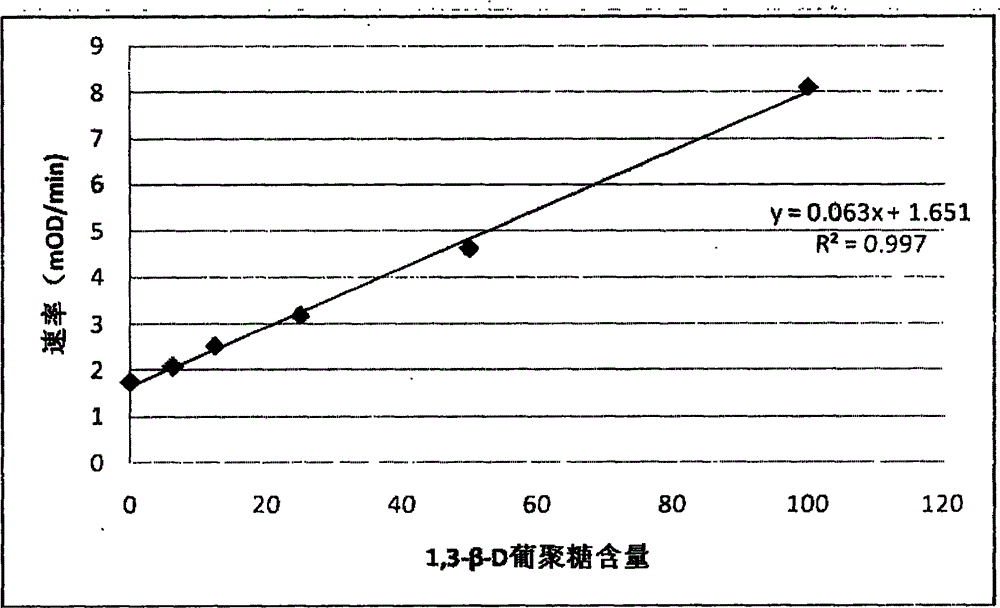
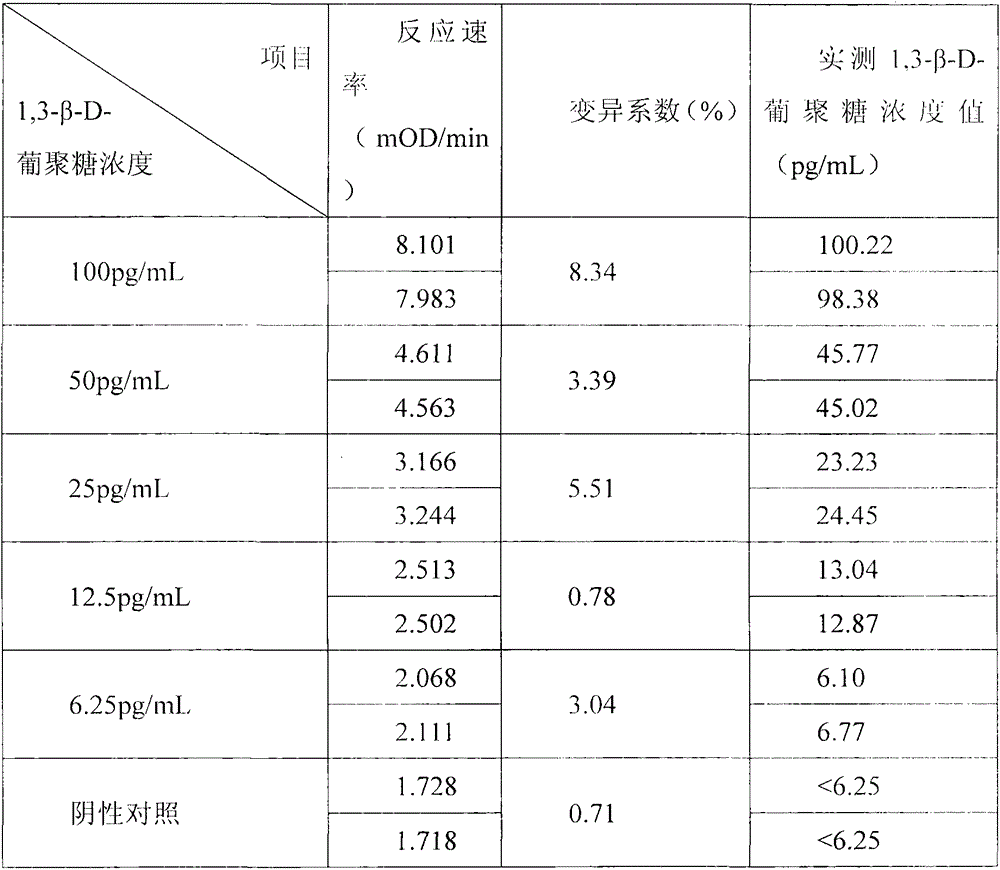
Developing-process fungus 1,3-beta-D-glucan detection kit for human body fluid
ActiveCN105021817AReduce false positive rateLess susceptible to interferenceColor/spectral properties measurementsBiological testingZymogenEnzyme digestion
The invention relates to a developing-process fungus 1,3-beta-D-glucan detection kit for human body fluid. The developing-process fungus 1,3-beta-D-glucan detection kit comprises a reaction main agent, a main agent compound solution, a sample treatment solution, heat-source-free water, a standard product and a quality control product, wherein the reaction main agent takes horseshoe crab blood cells as a main raw material and contains G factors, coagulase, coagulase zymogen and a polypeptide developing substrate; the polypeptide developing substrate is synthesized tripeptide or tetrapeptide with a Gly-Arg tail end connected with a PNA; the polypeptide developing substrate is subjected to enzyme digestion by adopting the coagulase; after the free paranitroaniline (PNA) is generated, a microplate reader is used for directly detecting so that a detection route is shortened and the cost is reduced; the microplate reader is used for carrying out a velocity-method enzyme kinetics detection method so that the sensitivity is relatively high when being compared with a nephelometry detection method; and the reaction main agent is not easily interfered by protein in a body fluid sample and medicines to generate non-specific turbidity, so that the probability of a false positive detection result is reduced and the detection accuracy is relatively high.
Owner:DYNAMIKER BIOTECH TIANJIN
52 kda protein from coagulase negative staphylococci and fragments thereof
InactiveUS20030082200A1Inhibit bindingAntibacterial agentsBacteriaPassive ImmunizationsProtein isolate
A protein isolated from Staphylococcus epidermidis having an approximate MW of 52 kD determined by SDS-PAGE and an N-terminal amino acid sequence (SEQ ID NO:1), and antigenic determinant-containing fragments of the protein, optionally coupled to an inert carrier or matrix, are disclosed. Disclosed are also a recombinant DNA molecule coding for the protein or the protein fragments; a vector comprising the DNA molecule or the corresponding RNA molecule; antibodies or antigen-binding peptides recognizing and specifically binding to the protein or protein fragment; use of the protein or protein fragment, or the vector, for the production of vaccines against Staphylococcal infections; use of the antibodies or antigen-binding peptides for the production of a medicament for passive immunization; a vaccine against Staphylococcal infections comprising the protein or protein fragment, or the vector, a medicament for passive immunization comprising the antibodies or antigen-binding peptides; and a method of prophylactic and / or therapeutic treatment of Staphylococcal infections.
Owner:BIOSTAPRO
Antibodies to a fibrinogen binding protein of staphylococcus epidermidis
A new fibrinogen binding protein or polypeptide originating from coagulase negative staphylococci, biotechnological methods for producing the protein or polypeptide having fibrinogen binding activity and a recombinant DNA molecule coding for the protein (or fragments thereof), and micro-organisms (including viruses) containing this recombinant DNA molecule. The present invention further comprises the therapeutic and diagnostic use of the protein and / or DNA, e.g., a diagnostic kit for determining the presence and / or type of coagulase negative staphylococci and a vaccine composition, comprising the protein or DNA.
Owner:BIOSTAPRO
Aerobic and bacterial vaginosis combined determination kit
The invention discloses an aerobic and bacterial vaginosis combined determination kit and a preparation method thereof. The kit comprises a color-developing agent and a reaction device, wherein the reaction device comprises a hydrogen oxide reaction pad, a neuraminidase reaction pad, a leucocyte esterase reaction pad, a beta-glucuronidase reaction pad and a coagulase reaction pad. Sample liquid is sucked by using straw, one drop of the sample liquid is dropwise added on each of the hydrogen oxide reaction pad, the neuraminidase reaction pad, the leucocyte esterase reaction pad, the beta-glucuronidase reaction pad and the coagulase reaction pad of the reaction device, the volume of each drop of the sample liquid is 30 mu l, after the reaction device is put into a water bath at a temperature of 36-38 DEG C or a dry bath at a temperature of 48 DEG C to carry out color developing for 15 minutes, one drop of color-developing liquid A is added on the neuraminidase reaction pad, one drop of color-developing liquid B is added on the coagulase reaction pad, and 30 seconds later, a result is shown. Thus, an effect of simultaneously detecting hydrogen oxide, neuraminidase, leucocyte esterase, beta-glucuronidase and coagulase to diagnose aerobic and bacterial vaginosis can be achieved.
Owner:浙江亚培生物技术有限公司
Method for detecting the presence or absence of methicillin resistant staphylococcus aureus (MRSA) in a test sample
InactiveUS8524468B2Facilitates handling and packaging and storingFavorable source of coagulase substrateMicrobiological testing/measurementBiological material analysisThroatLesion swab
A dry mixture, a liquid menstrum, and a method, are described for use in detecting the presence or absence of Methicillin Resistant Staphylococcus aureus (“MRSA”) in a first generation biological or environmental specimen sample. First generation specimen samples that can be analyzed include nasal swabs, lesion swabs, skin swabs, throat swabs, food swabs, tanning salon swabs, gym swabs, restaurant swabs, hotel swabs, and the like. The menstrum and method include an anti-ribosomal antibiotic component that will selectively prevent Methicillin Susceptible Staphylococcus aureus (“MSSA”) from growing in the menstrum, while allowing MRSA to grow in the menstrum. The menstrum also includes components which will stimulate growth of MRSA, plus coagulase reacting factors which will cause the menstrum to clot in the event that MRSA is present in the sample. The menstrum also includes components which will produce a detectable signal in the clot, which signal indicates the presence of MRSA in the sample.
Owner:PILOTS POINT LLC
Detection of methicillin-resistant staphylococcus aureus
This invention relates to the use of genetic probes for detection of the presence of the SCCmec cassette in Staphylococcus aureus. In one aspect, the invention allows specific detection and identification of methicillin-resistant S. aureus (MRSA) in a clinical sample without interference from the presence of other non-S. aureus methicillin-resistant staphylococci. In another aspect, the invention allows specific detection and identification of methicillin-resistant coagulase negative staphylococci (MRCNS) originating from a clinical sample without interference from the presence of methicillin-resistant S. aureus.
Owner:ADVANDX
Reagent for detecting Yersinia pestis and method for carrying out fluorescence quantitative PCR (Polymerase Chain Reaction) detection on Yersinia pestis
InactiveCN102146467AAmplifyEasy to detectMicrobiological testing/measurementFluorescence/phosphorescenceYersinia pestisFluorescence
The invention provides a reagent for detecting Yersinia pestis, which comprises an upstream primer, a downstream primer, a fluorescent probe and a quenching probe. The invention also provides a method for carrying out fluorescence quantitative PCR (Polymerase Chain Reaction) detection on Yersinia pestis by utilizing the reagent. The primer and the probe are designed according a specific pathogenic gene-plasma coagulase and plasmin original activating factor pla gene on a Yersinia pestis 6MD plasmid; and by carrying out BLAST retrieval with the whole genebank database, the reagent is specific only on a pla gene sequence on Yersinia pestis, has no isogeny with nucleotide sequences of other species and ensures the specificity of the detecting method. The method can realize the amplification and synchronous detection of DNA in the same pipe without carrying out gel electrophoresis analysis after PCR, can finish quantitative detection on a sample in 2 hours or so and has the advantages of simple and convenient operation, high efficiency, high speed and specificity.
Owner:浙江国际旅行卫生保健中心
Antibody specific to staphylococcus aureus, therapeutic method and detection method using same
ActiveUS20150210775A1Inhibition of activationInhibition is effectiveImmunoglobulins against blood coagulation factorsSugar derivativesMonoclonal antibodyStaphylococcus aureus
We provide new monoclonal antibody inhibitors of coagulases for treatment of S. aureus. The monoclonal antibodies are useful in targeting the SC N-terminus and inhibiting prothrombin activation. The monoclonal antibodies are able to bind to and interfere with, modulate, and / or inhibit the binding interactions between the staphylocoagulase protein and its ligand protein prothrombin in blood and tissues. The antibodies are effective in inhibiting the activation of prothrombin.
Owner:CHURCH WILLIAM R
Method for rapidly detecting staphylococcus aureus in diet
InactiveCN108410945AShorten detection timeReduce the difficulty of detectionMicrobiological testing/measurementBiological material analysisMicroorganismStaphylococcus cohnii
The invention provides a method for rapidly detecting staphylococcus aureus in diet. The method comprises the following steps: 1) culture enrichment: weighing a sample to sodium chloride broth, and carrying out anaerobic culture after homogenization; 2) separation: carrying out streak inoculation on a culture after culture enrichment to a staphylococcus aureus selective flat plate, and carrying out anaerobic culture; 3) preliminary identification: preliminarily determining a staphylococcus aureus bacterial colony according to morphology; and 4) confirmation identification: inoculating suspicious bacteria on the staphylococcus aureus selective flat plate on a blood plate, culturing, observing whether the suspicious bacteria are dissolved in blood, selecting bacterial colonies which are dissolved in blood and carrying out biochemical identification by using a VITEK 2 COMPACT full-automatic microorganism analyzing system to obtain the result. Compared with the prior art, the method has the advantages that by the characteristics that the staphylococcus aureus is aerobic and anaerobic bacteria, and the metabolites of the staphylococcus aureus can produce plasma-coagulase, the suspiciousbacteria which are not dissolved in blood do not need to be identified, the staphylococcus aureus detecting time is shortened, and the detecting difficulty is reduced.
Owner:芜湖市食品药品检验中心
Polysaccharide vaccine for staphylococcal infections
InactiveCN1708506AAntibacterial agentsOrganic active ingredientsNatural resourceStaphylococcal infections
The present invention relates to compositions of staphylococcal deacetylated poly-N-acetylglucosamine (dPNAG). Deacetylated poly-N-acetylglucosamine can be isolated from natural sources or synthesized de novo. The present invention also relates to the use of deacetylated poly-N-acetylglucosamine as a vaccine to induce resistance to Staphylococcus aureus, Staphylococcus epidermidis, other related coagulase-negative or coagulase-positive staphylococci, and other carrier intracellular Active immunity to infection by organisms attached to loci. The present invention also provides a method for using the antibody targeting deacetylated poly-N-acetylglucosamine, especially a method for inducing passive immunity against similar infections.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Method for quantitatively detecting staphylococcus aureus in food
PendingCN111961704AThe result is accurateImprove accuracyMicrobiological testing/measurementBiological material analysisBiotechnologyPetri dish
The invention discloses a method for quantitatively detecting staphylococcus aureus in food. The method comprises four steps of preparation, sample preparation, culture and counting. After a sample isprepared, each dilution sample sucks 1mL of sample homogenate into a culture dish, then adding 15-20mL of a Baird-Parker culture medium solution added with potassium tellurite egg yolk enrichment broth into each culture dish, performing uniform mixing, then performing culturing, counting suspicious colonies after culturing, and carrying out confirmation and identification on the suspicious colonies by virtue of a plasma coagulase test. Different from existing coating methods, the method provided by the invention can be used for rapidly, simply, conveniently and accurately determining staphylococcus aureus in food.
Owner:郑州中检科测试技术有限公司
Detection of methicillin-resistant Staphylococcus aureus
This invention relates to the use of genetic probes for detection of the presence of the SCCmec cassette in Staphylococcus aureus. In one aspect, the invention allows specific detection and identification of methicillin-resistant S. aureus (MRSA) in a clinical sample without interference from the presence of other non-S. aureus methicillin-resistant staphylococci. In another aspect, the invention allows specific detection and identification of methicillin-resistant coagulase negative staphylococci (MRCNS) originating from a clinical sample without interference from the presence of methicillin-resistant S. aureus.
Owner:ADVANDX
Primer, kit and method for detecting staphylococcus aureus through PCR(Polymerase Chain Reaction)-pyrophosphate method
ActiveCN102816844AGuaranteed specificityStrong specificityMicrobiological testing/measurementMicroorganism based processesStaphylococcus cohniiDesign software
The invention discloses a primer, kit and method for detecting staphylococcus aureus through a PCR (Polymerase Chain Reaction)-pyrophosphate method. In the invention, a PCR primer and a sequencing primer are designed via PyroMark Assay Design software according to a conserved region in a staphylococcus aureus plasma-coagulase DNA base sequence; and then a method for detecting staphylococcus aureus from the level of the DNA base sequence is established through a PCR-pyrophosphate sequencing method. In the invention, PCR augmentation is firstly carried out on a target segment under the condition of ensuring the specificity of the augmented segment; then a single-chain template is prepared from the augmented product; and then pyrophosphate sequencing is carried out under the guide of the sequencing primer. The base sequences obtained behind the plasma-coagulase DNA sequencing primer are discovered to have good specificity through repeated comparison by using a BLAST function of an NCBI (National Center of Biotechnology Information) website; and the staphylococcus aureus can be identified when 29 DNA base sequences behind the sequencing primer are CAACCGACGACACCGAACCCTATTTTAGA.
Owner:许龙岩 +4
Preparation method for coagulase test paper
InactiveCN109991218AGood dispersionImprove uniformityMaterial analysis by observing effect on chemical indicatorCombined useCoagulase test
The invention relates to a preparation method for coagulase test paper. According to employed impregnation liquid, through utilization of a buffer system, a good neutral-weak alkaline reaction environment for reaction is provided; through independent or combined use of dispersing agents, dispersing capability of the test paper can be improved, and uniform distribution of colors is facilitated; andthrough utilization of a stabilizer, enzyme substances in a sample can be protected, and detection accuracy is improved. According to employed color development liquid, through utilization of the stabilizer and the dispersing agents, a good reaction environment for improvement of uniformity and stability of the test paper is provided. According to the method, the test paper is prepared through adoption of an impregnation drier automatic drying method. An impregnation drying mode has the advantages of convenience, saving time and automation. Influence resulting from artificial errors in a testpaper preparation process is effectively controlled. According to an impregnation drier, through main and auxiliary heating, a temperature is monitored comprehensively, the temperature is set in a stated range, errors between test paper batches can be greatly reduced, and test paper preparation accuracy, stability and uniformity are ensured.
Owner:DIRUI MEDICAL TECH CO LTD
Drug for repairing tunica mucosa bronchiorum
InactiveCN104840950APromote regenerationOrganic active ingredientsPeptide/protein ingredientsUse medicationBronchial mucosa
The invention discloses a drug for repairing tunica mucosa bronchiorum. The drug is prepared by extracting lecithin, amino acid, pseudo-ginseng, saffron crocus and free-ion coagulase in an extraction tank so as to obtain medicinal liquid, filtering, evaporating and concentrating the medicinal liquid, and drying in vacuum to obtain dry medicinal powder. The drug disclosed by the invention is capable of effectively repairing injured tunica mucosa bronchiorum and promoting healing of bronchus.
Owner:李杰
Coagulase detection reagent, reaction pad, preparation method thereof and kit
ActiveCN106645721ASimple and convenient conditions of useImprove efficiencyMaterial analysisSucroseMorpholine
The invention discloses a coagulase detection reagent, a reaction pad, a preparation method thereof and a kit. The coagulase detection reagent comprises reaction agents and a color developing agent, the reaction agents include morpholine ethane sulfonic acid, cane sugar, polyvinylpyrrolidone and glycyl-protamine tetramethoxy-beta-naphthylamine, the reaction pad comprises a carrier and ingredients of the reaction agents, and the ingredients of the reaction agents are coated on the carrier. The preparation method of the reaction pad includes: 1), dissolving the cane sugar, polyvinylpyrrolidone and glycyl-protamine tetramethoxy-beta-naphthylamine in morpholine ethane sulfonic acid to obtain soaking liquid; 2), putting the carrier in the soaking liquid, taking out after sufficient soaking, and drying to obtain the reaction pad. The reaction pad obtained by the preparation method is combined with ingredients of the detection agent to be applied in preparing the kit which is high in sensitivity and accuracy.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com