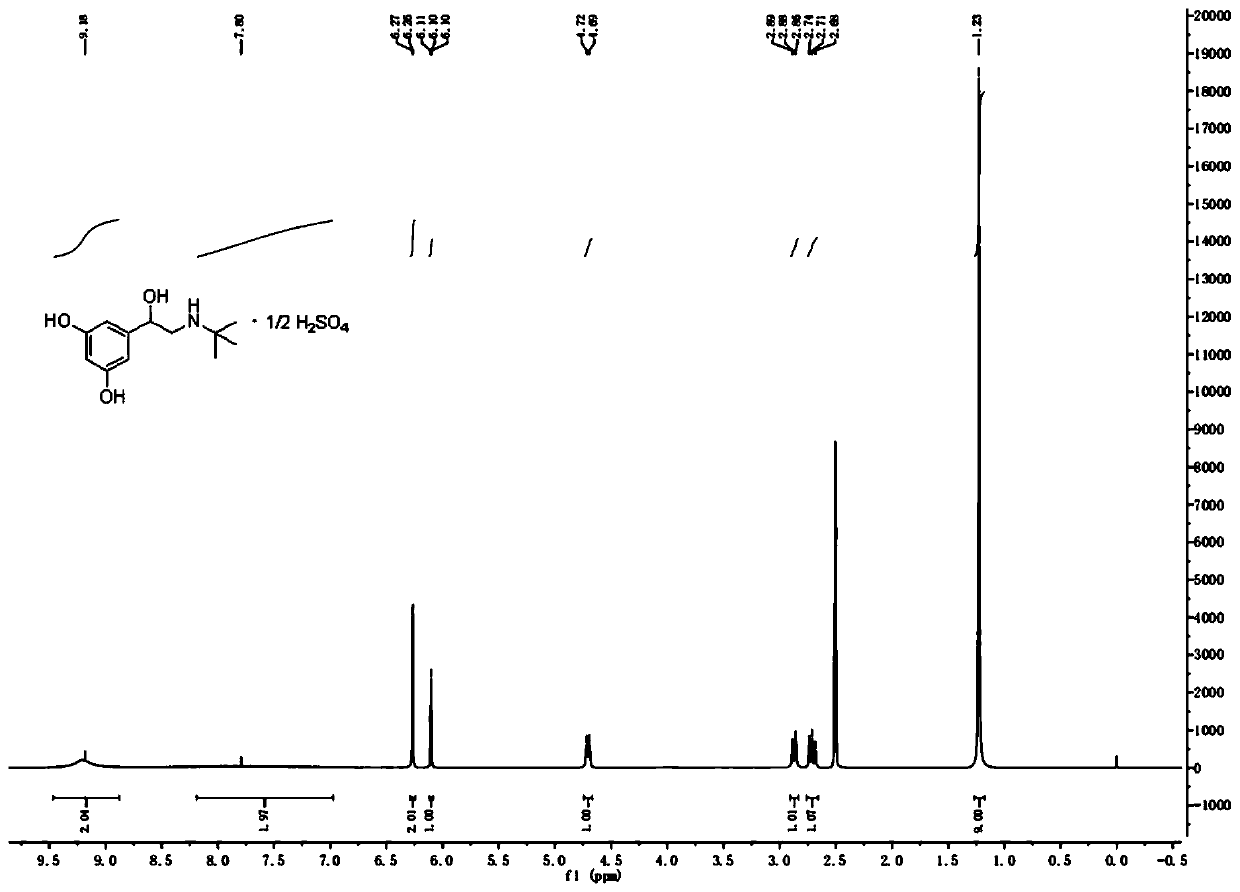
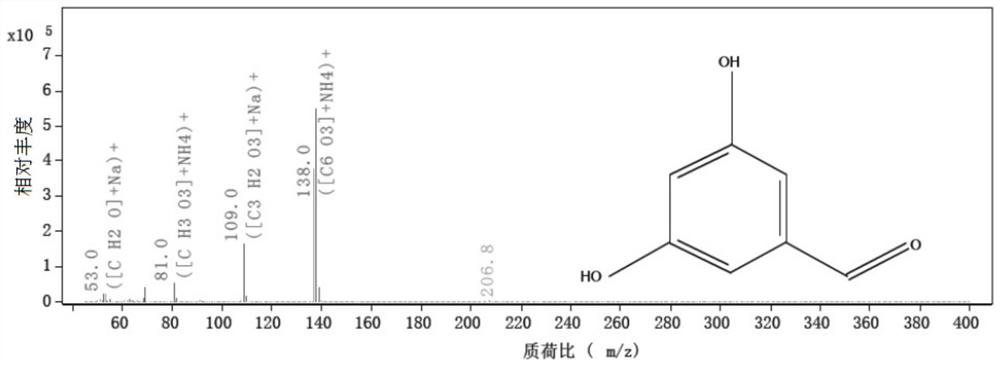
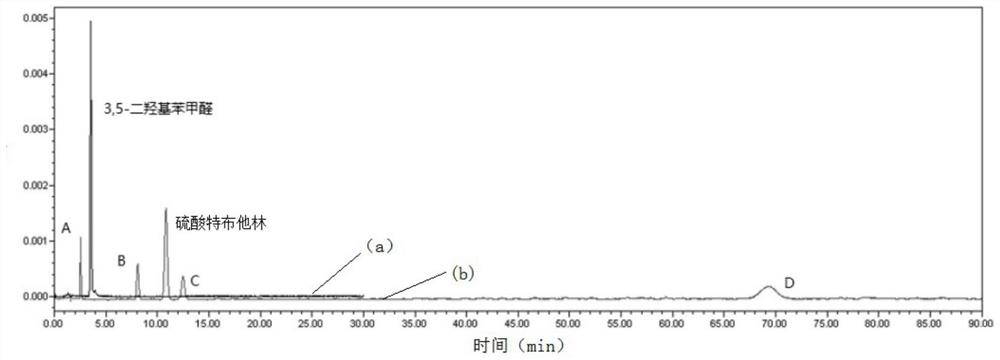
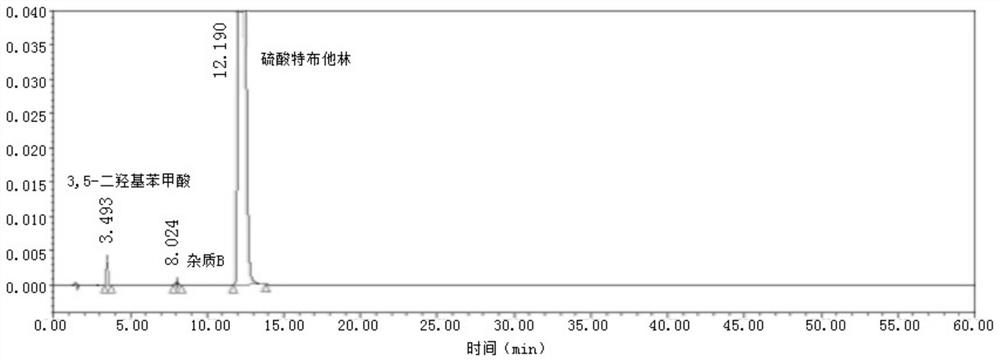
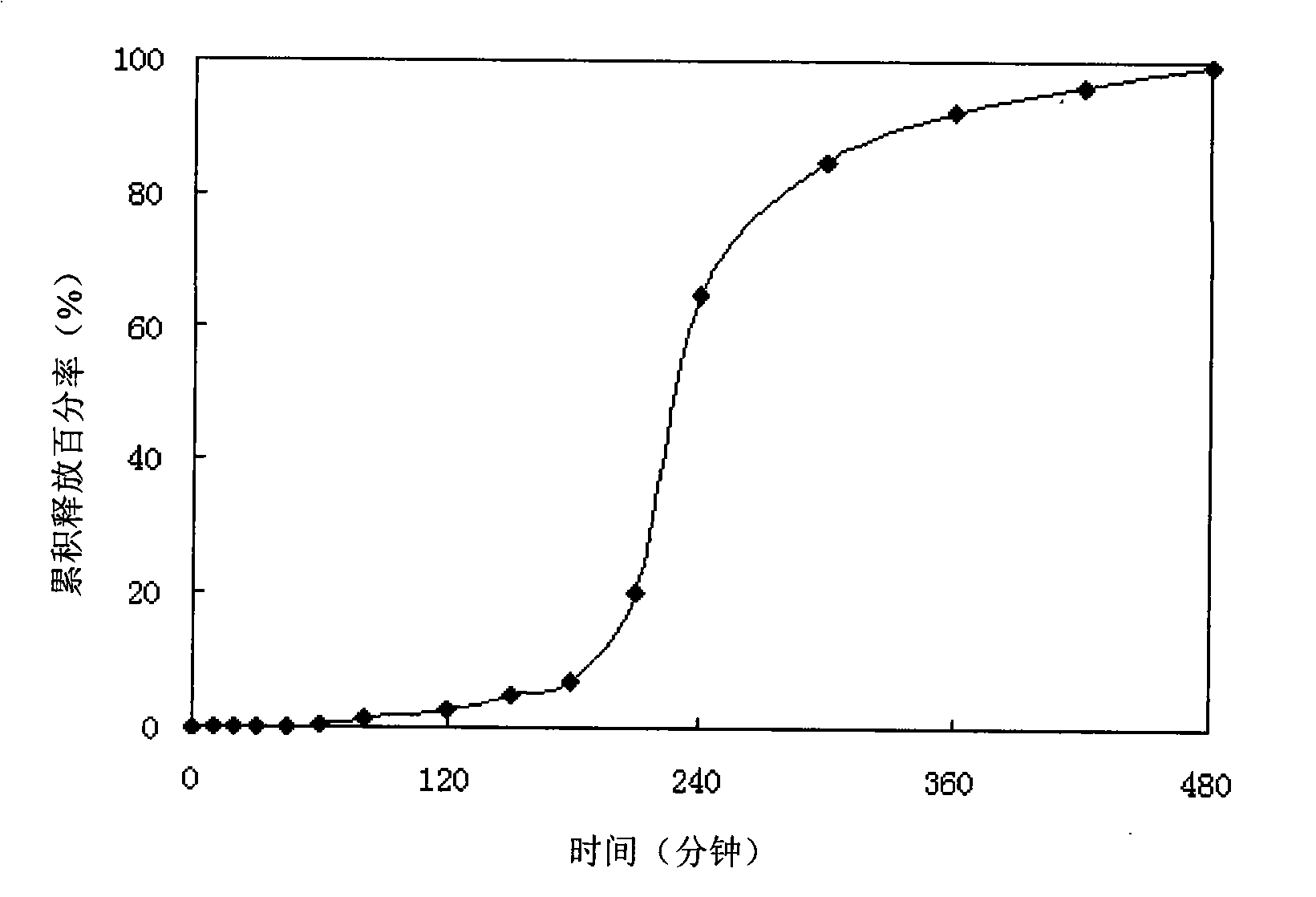
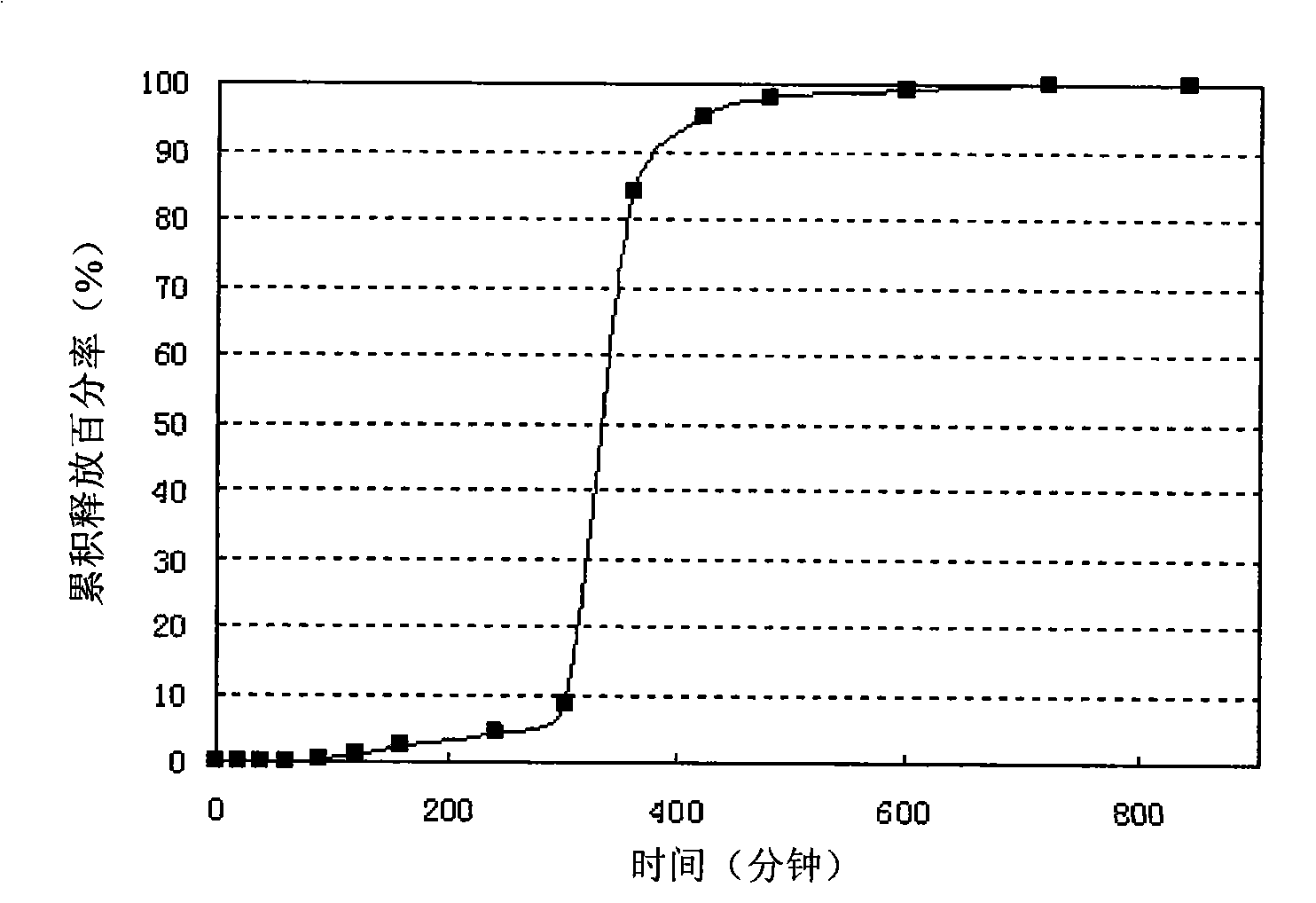
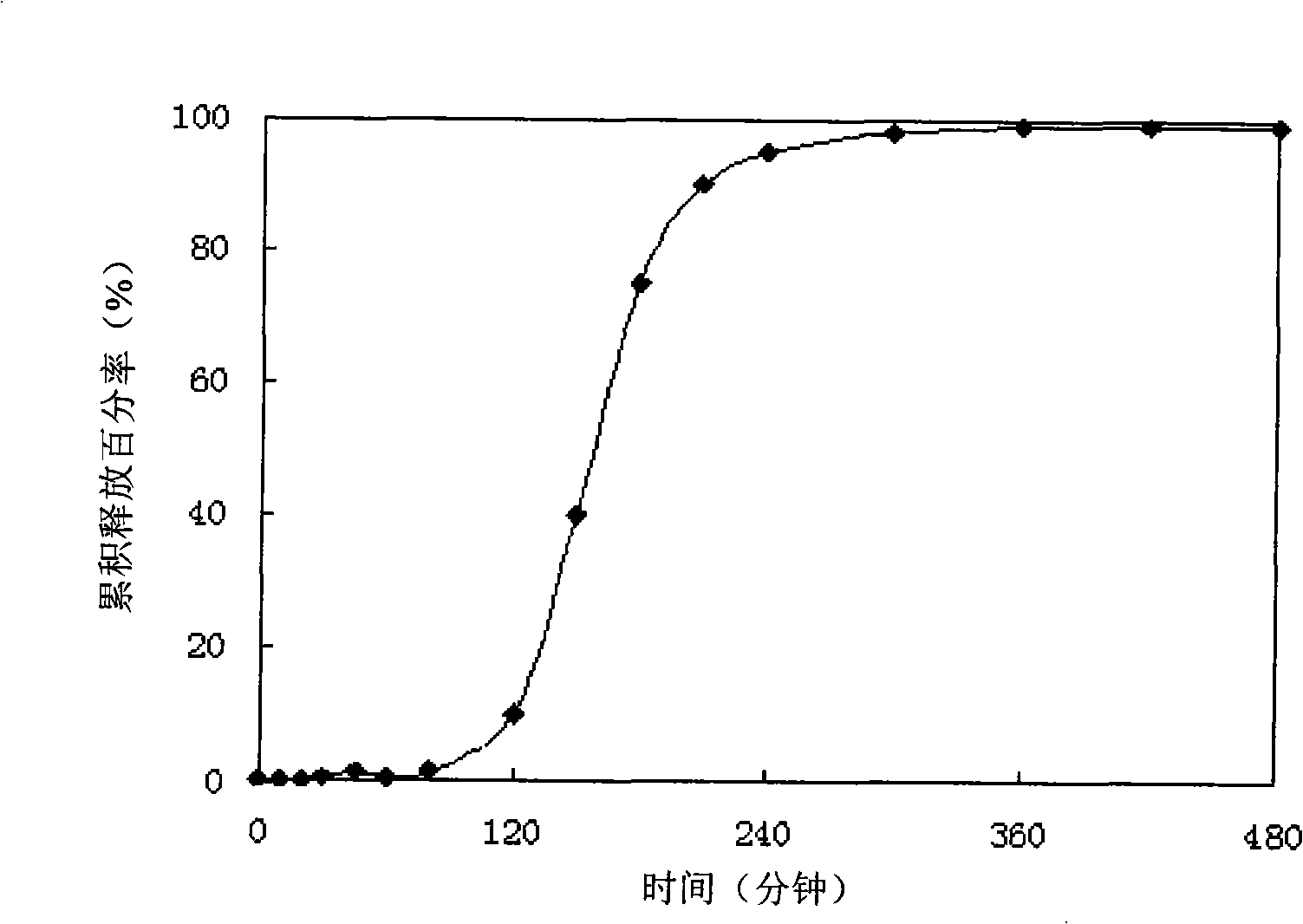
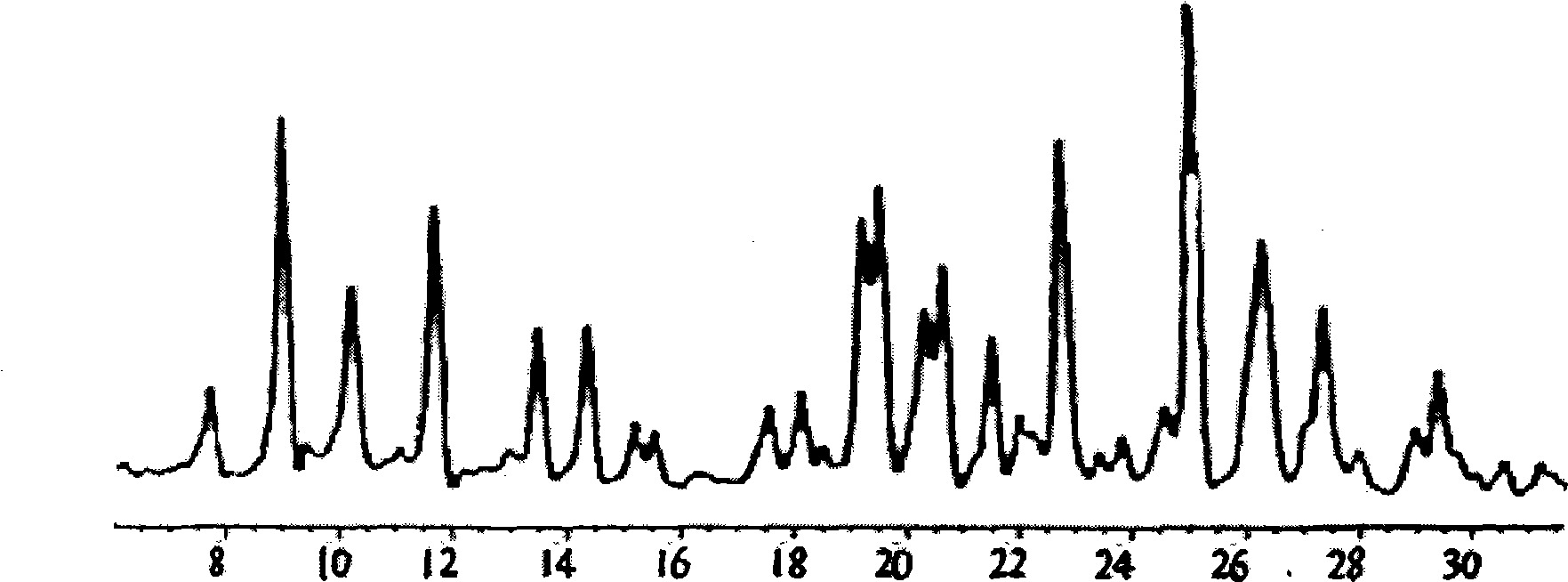
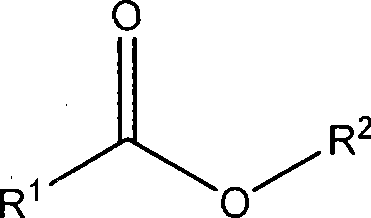
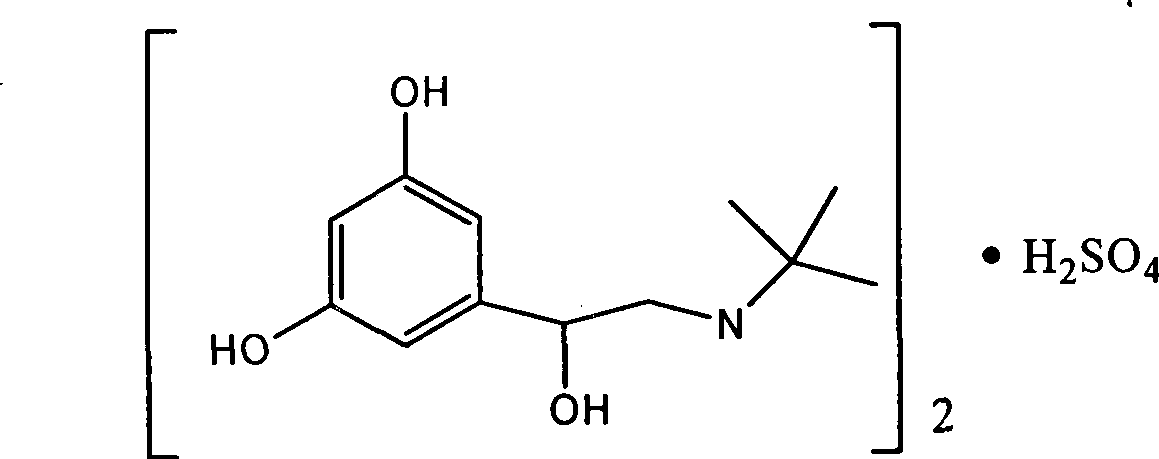
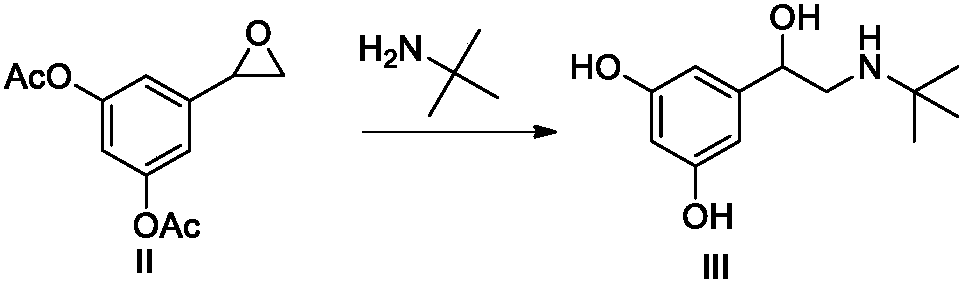
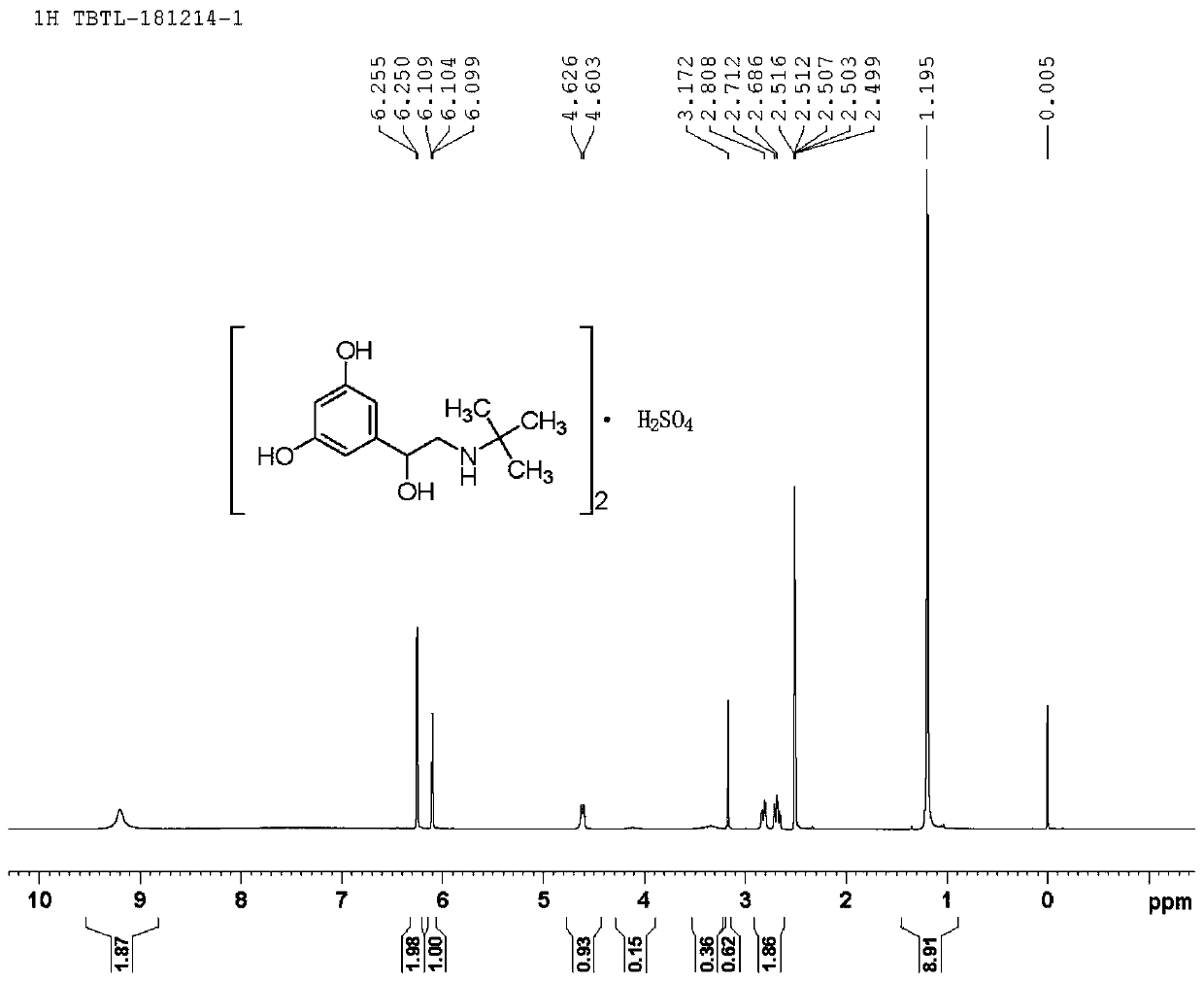
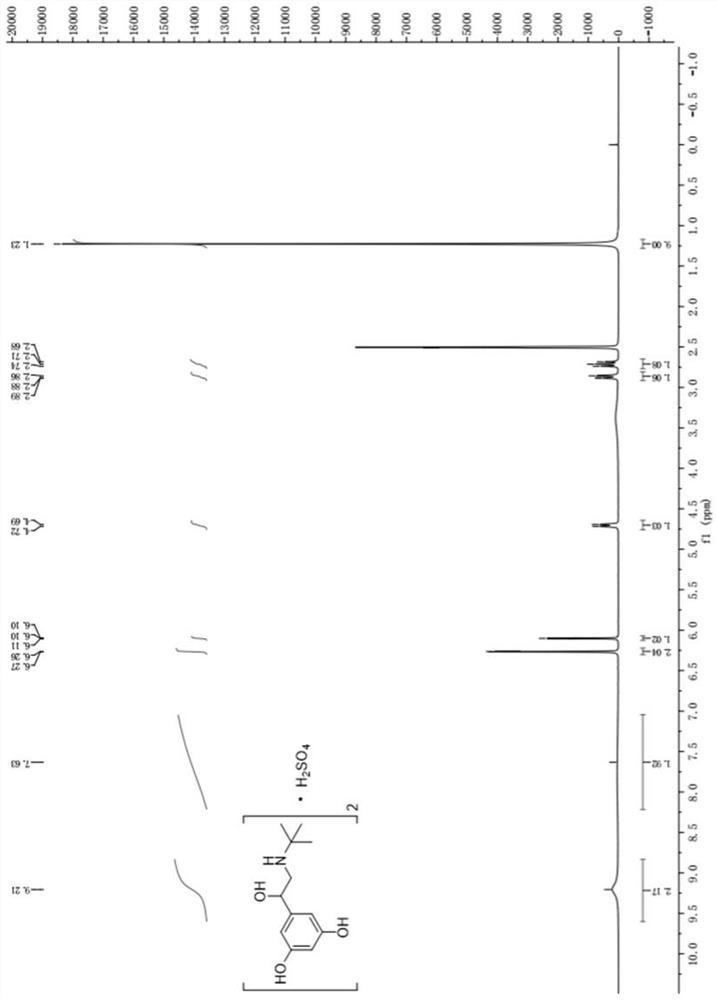
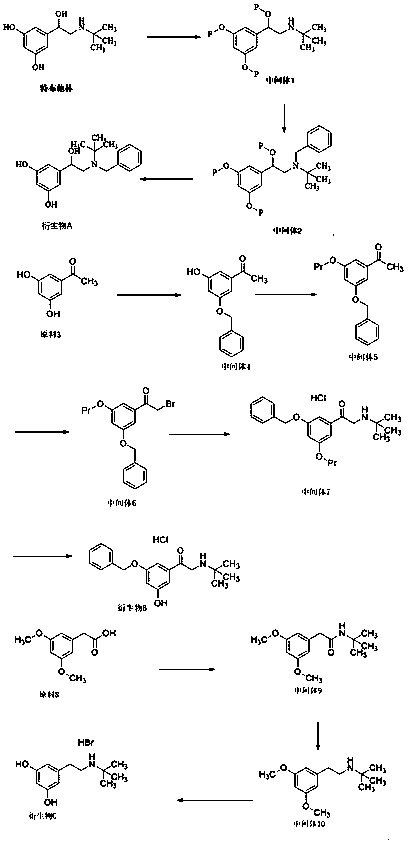
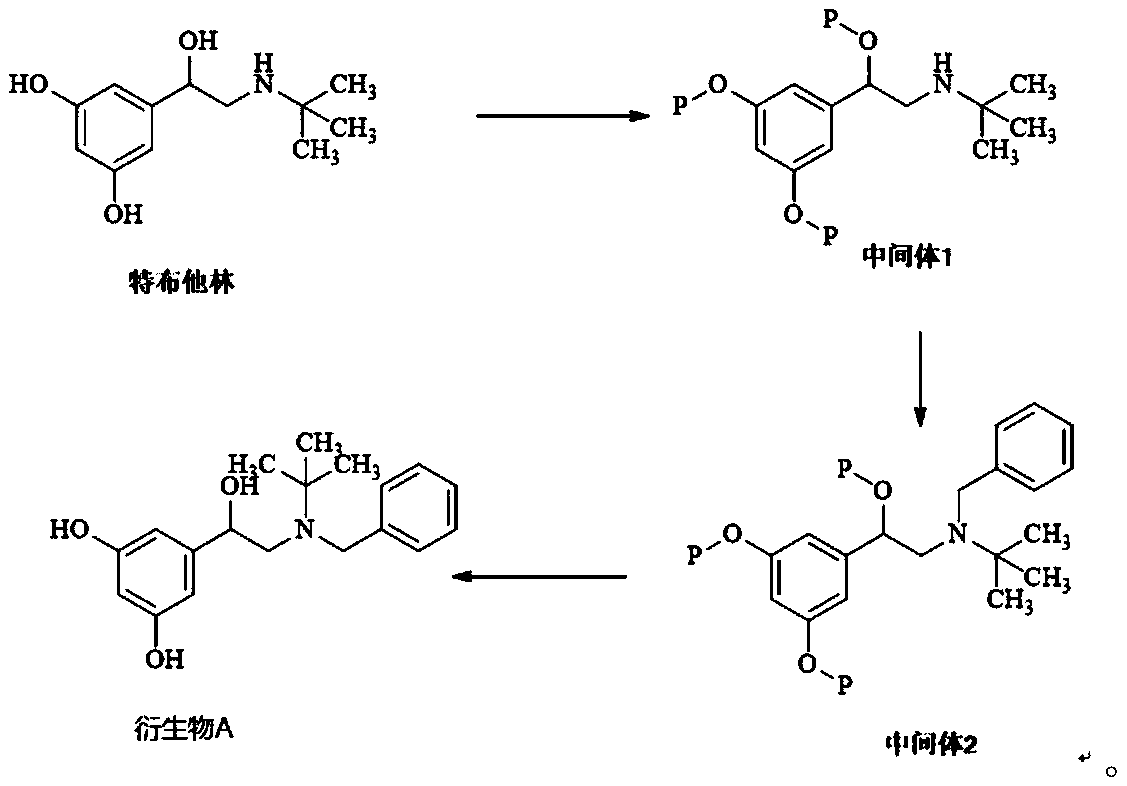
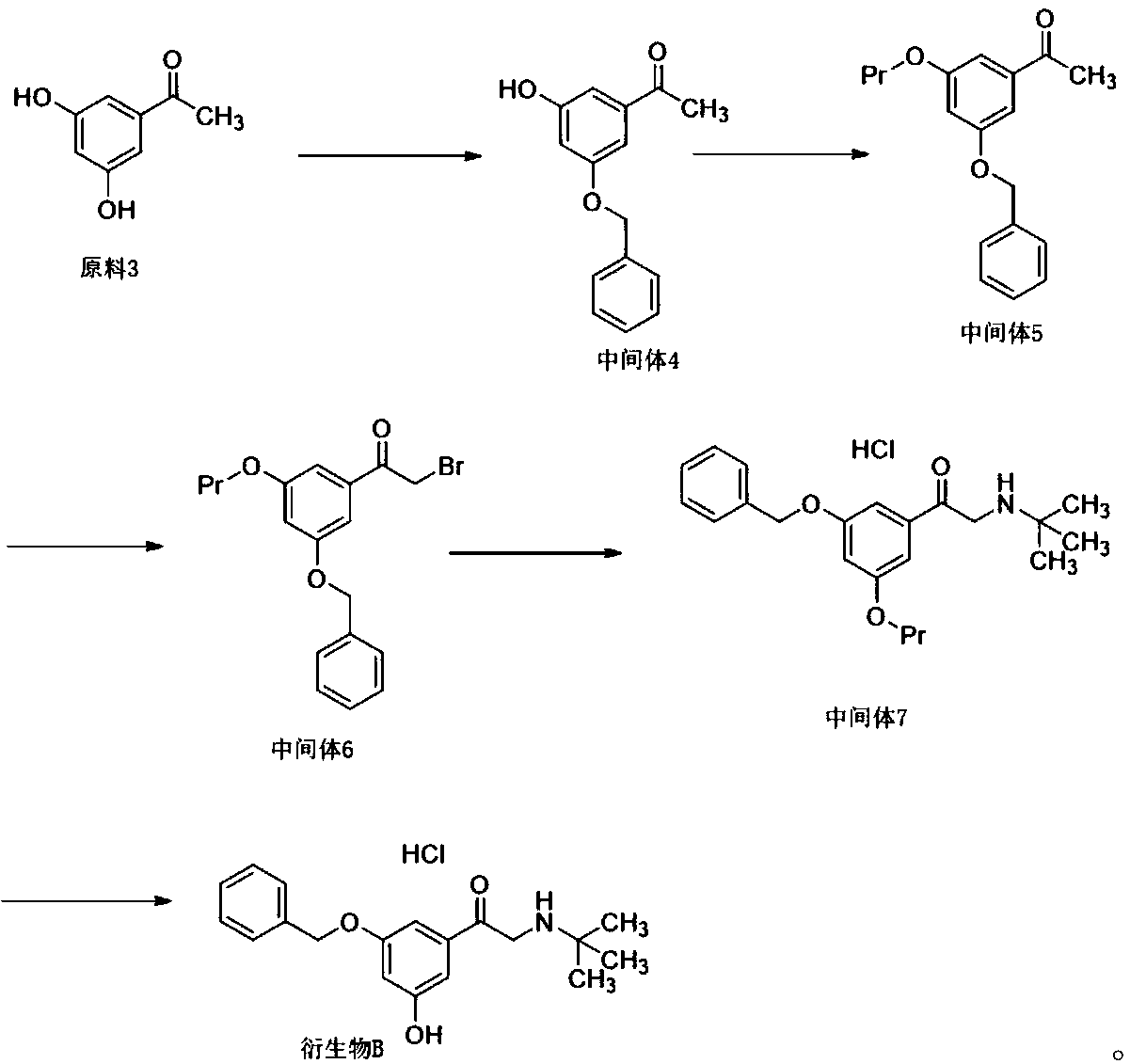
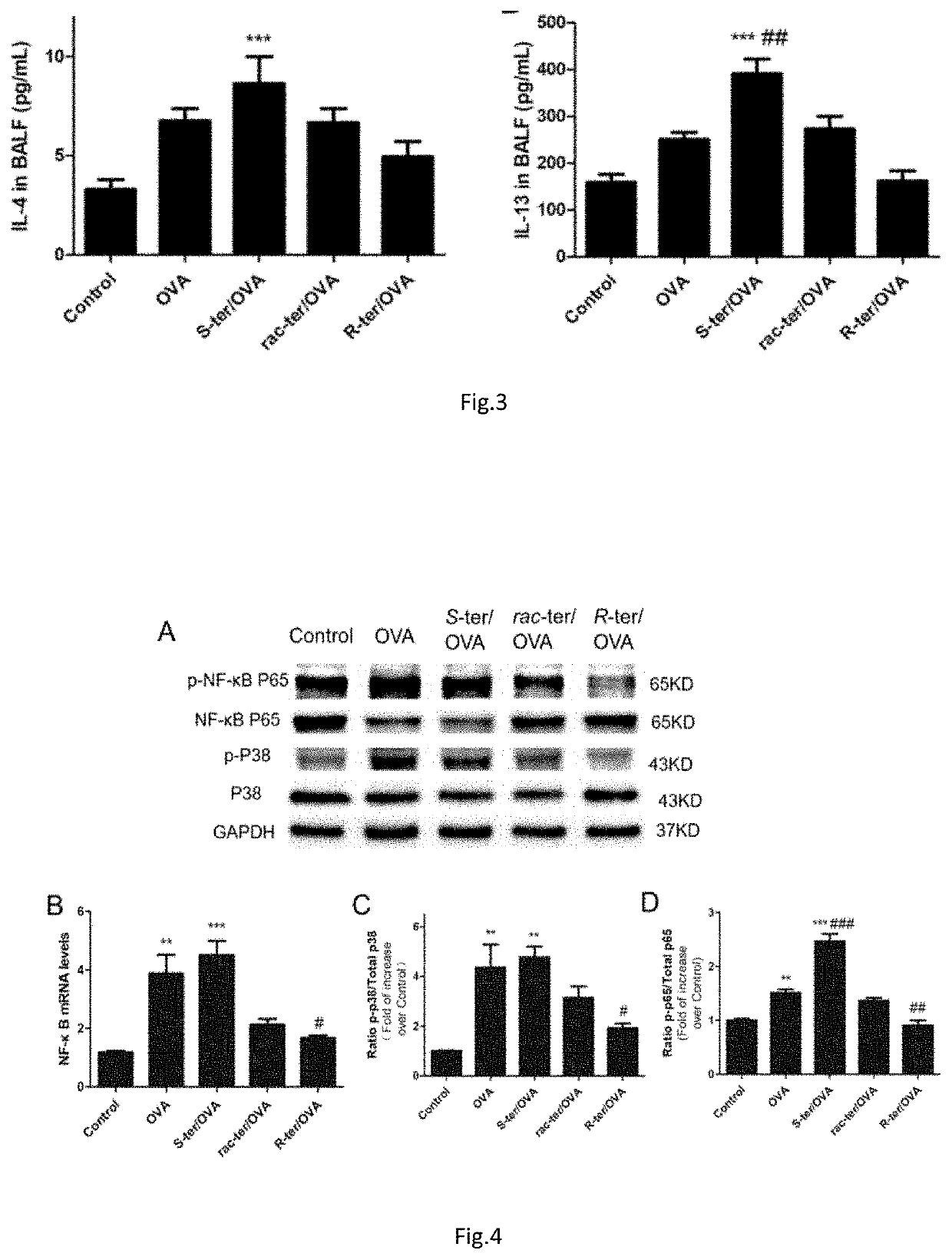
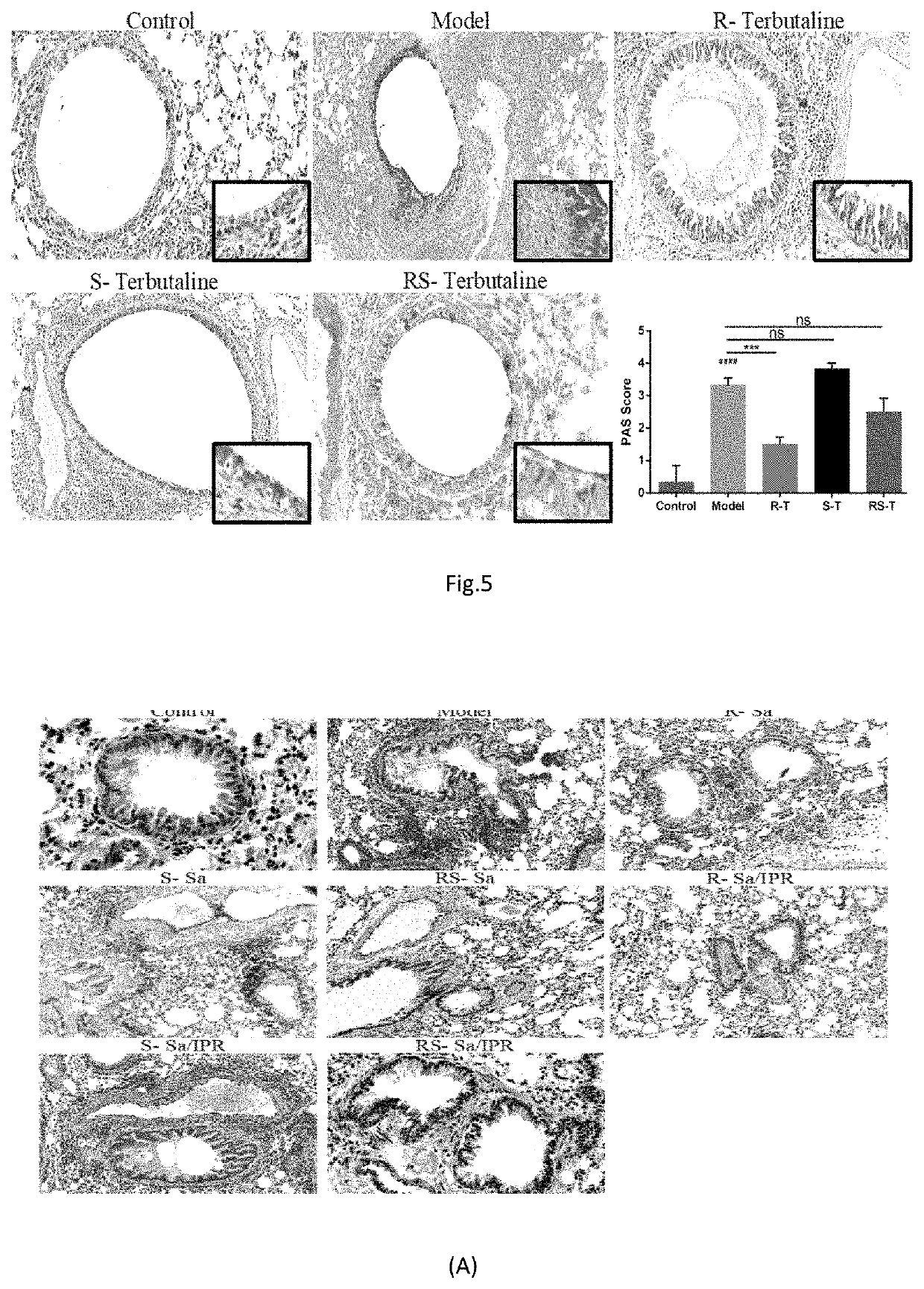
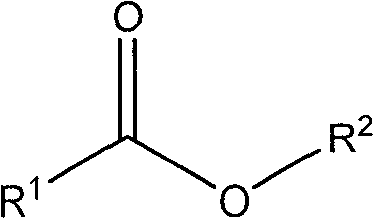
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Terbutaline sulphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
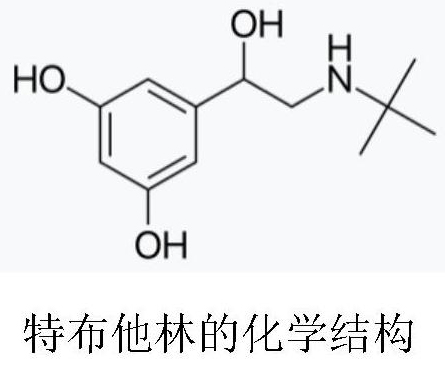
Terbutaline sulfate injection is indicated for the prevention and reversal of bronchospasm in patients 12 years of age and older with asthma and reversible bronchospasm associated with bronchitis and emphysema.
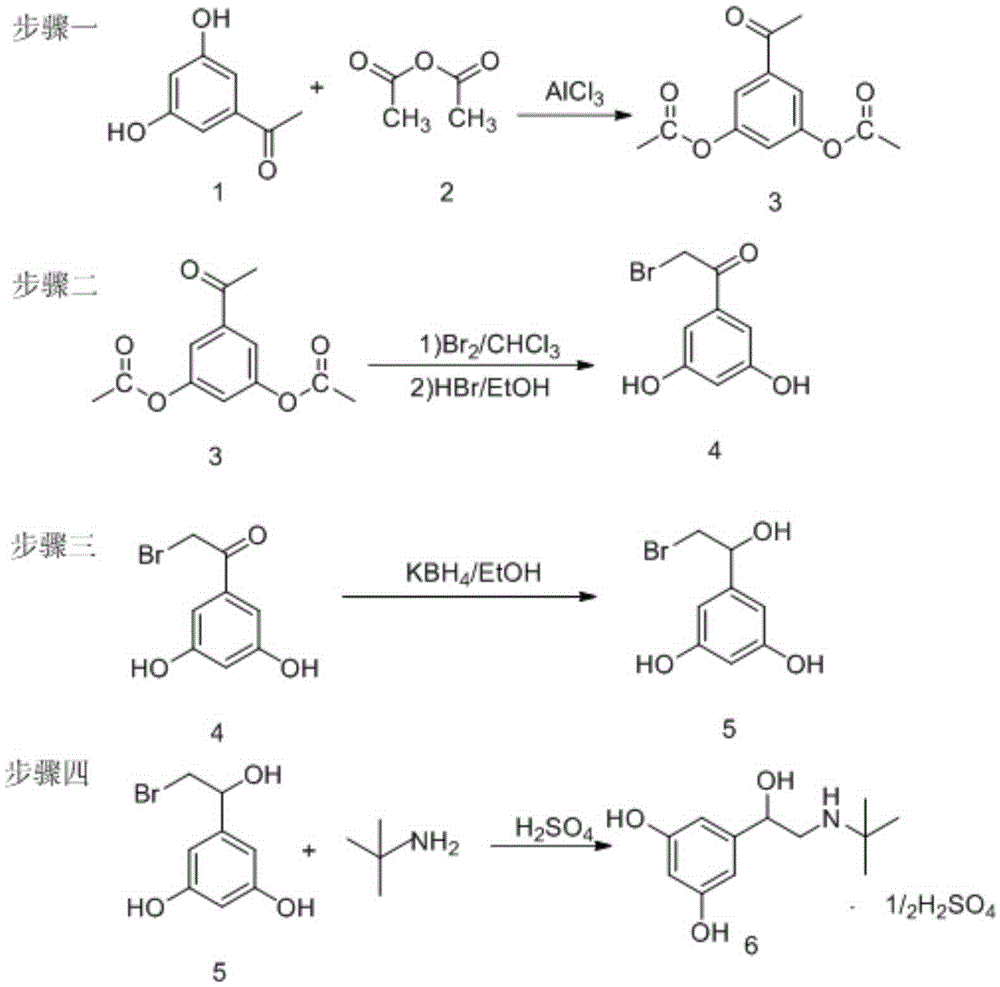
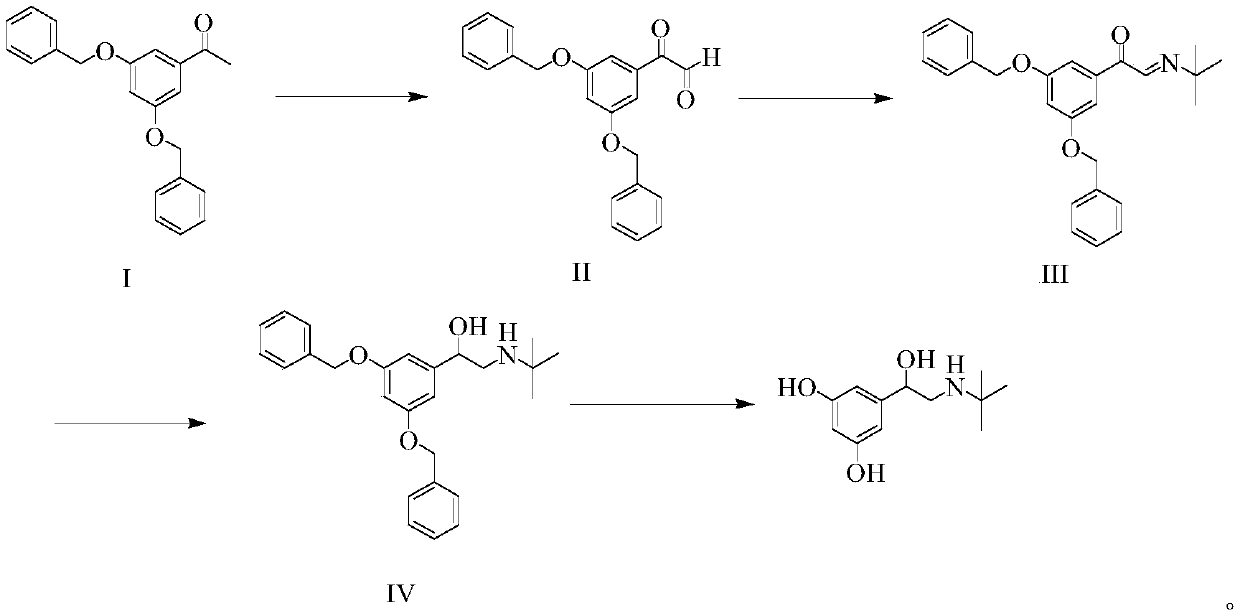
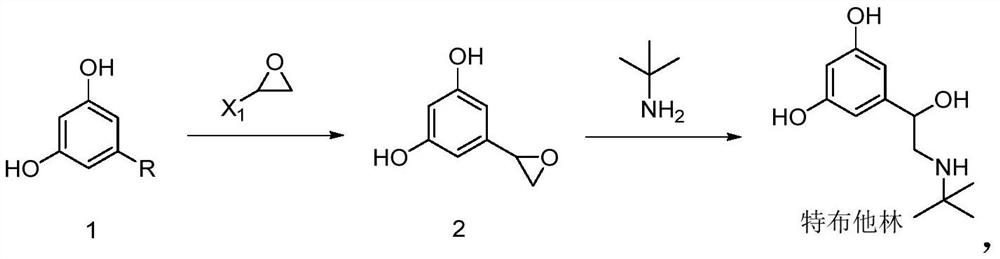
Preparation method of terbutaline sulphate
ActiveCN105254512AMild responseImprove securityAmino preparation from aminesBulk chemical productionLithiumHigh pressure
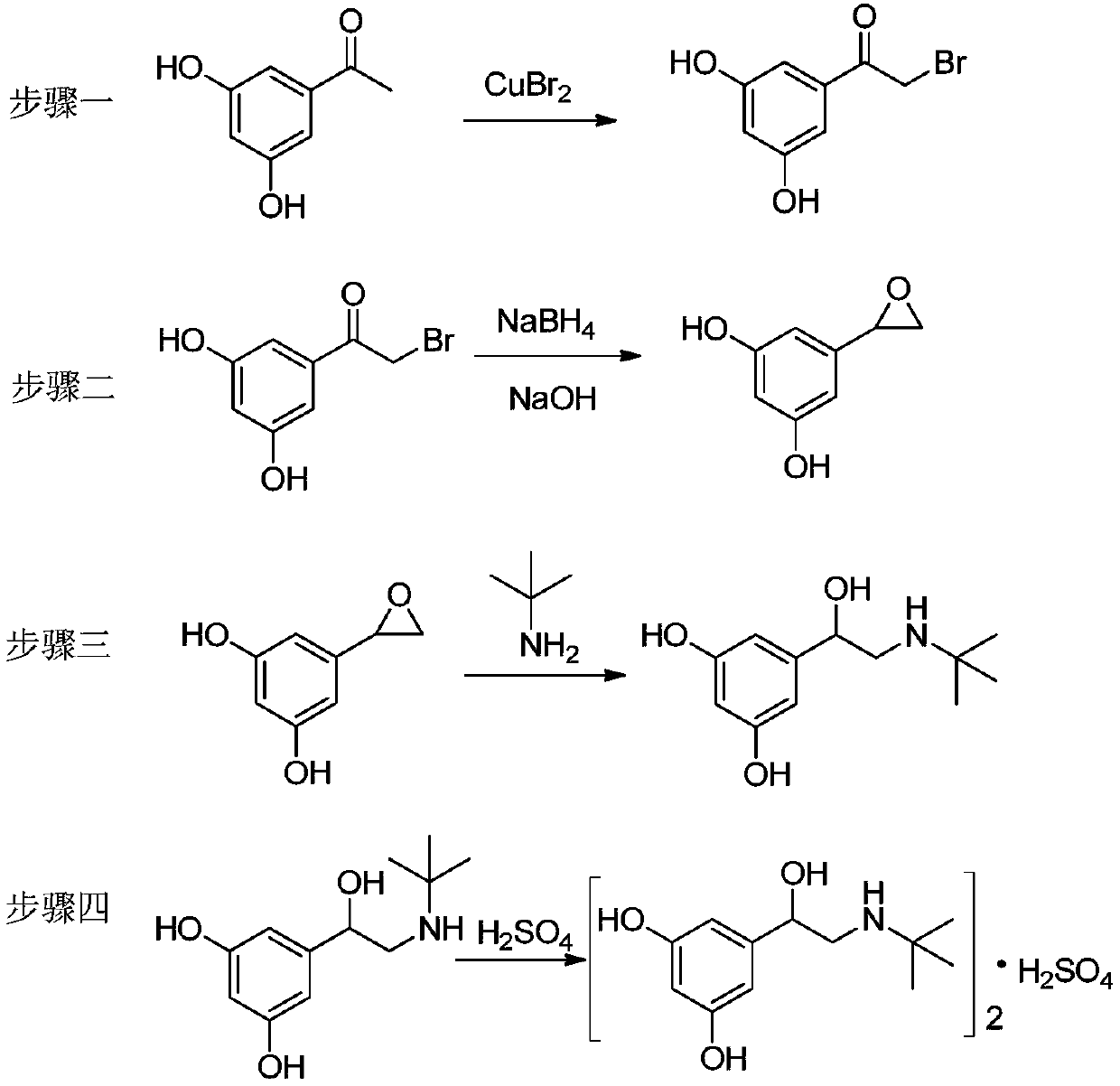
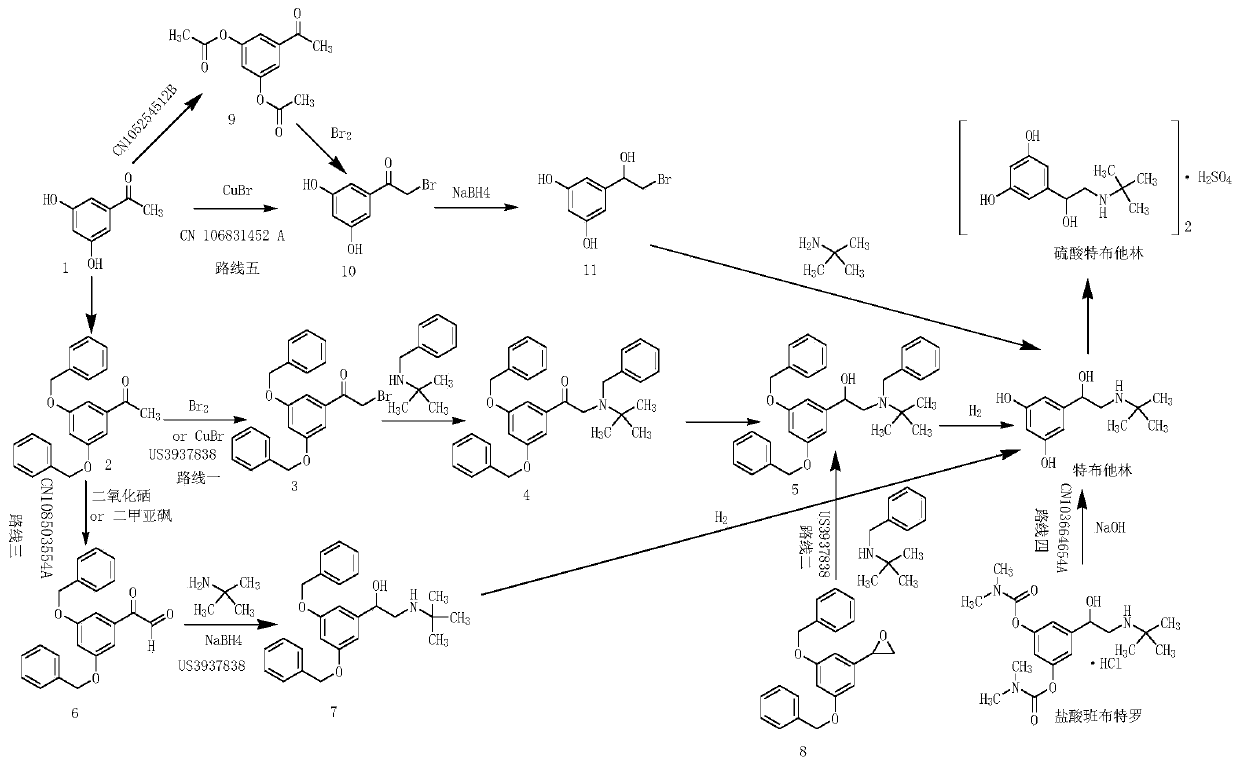
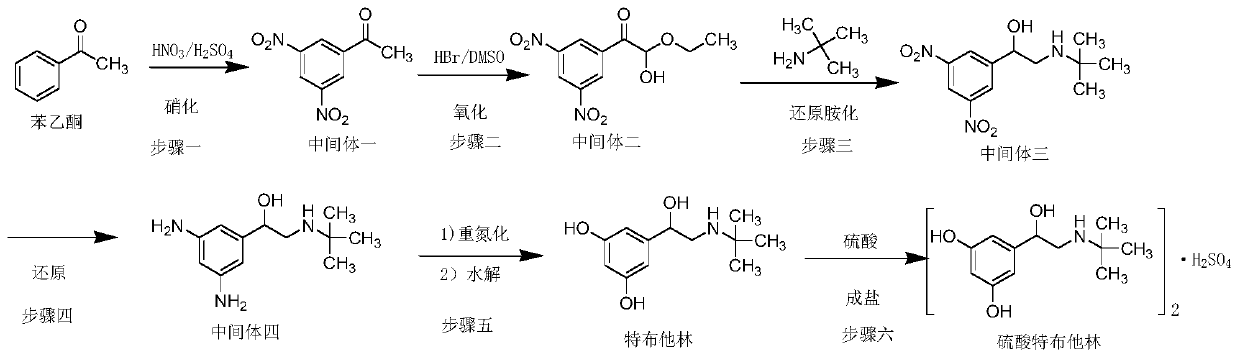
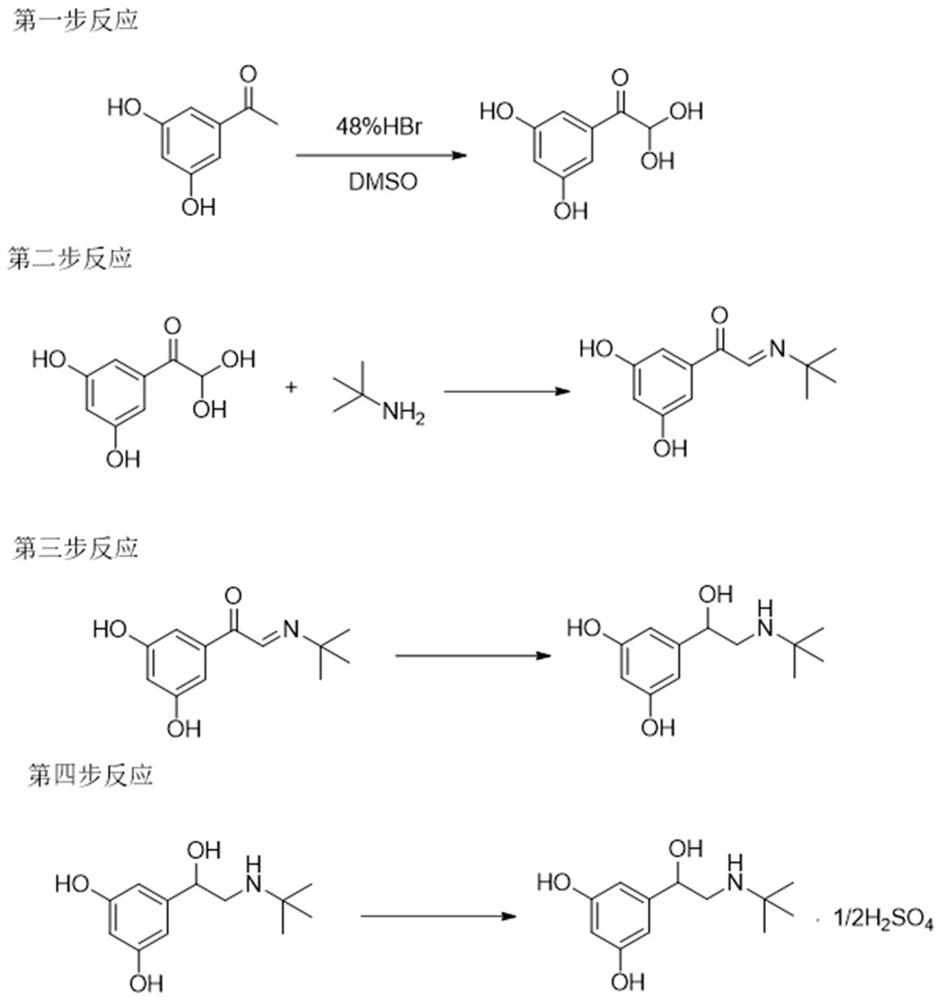
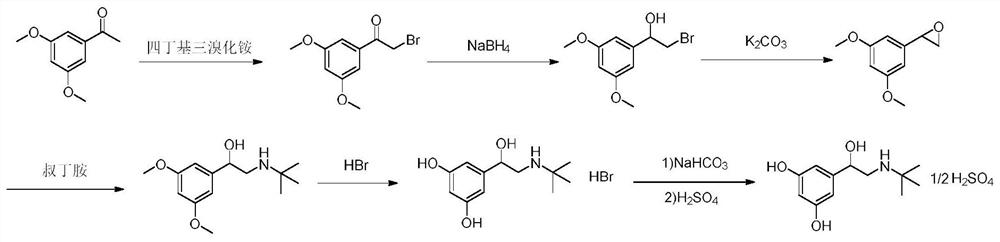
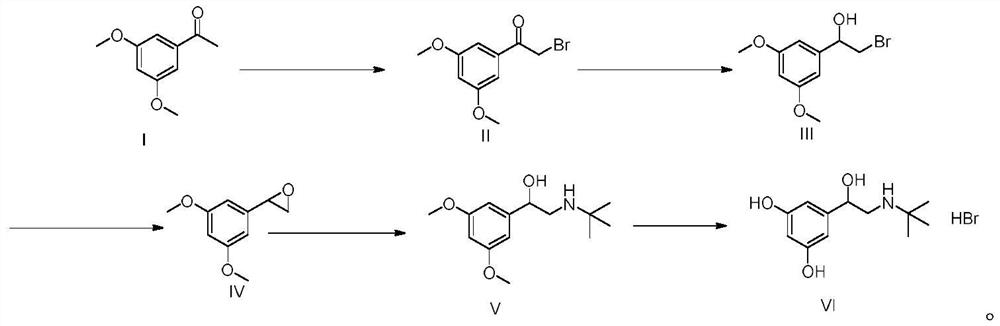
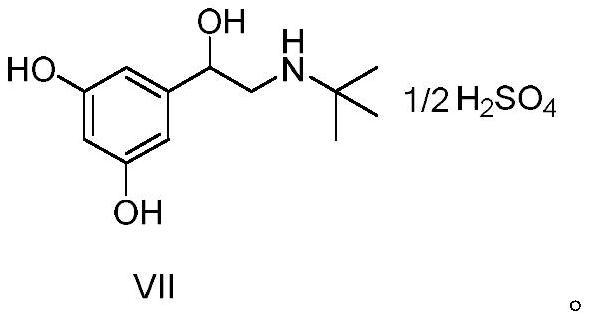
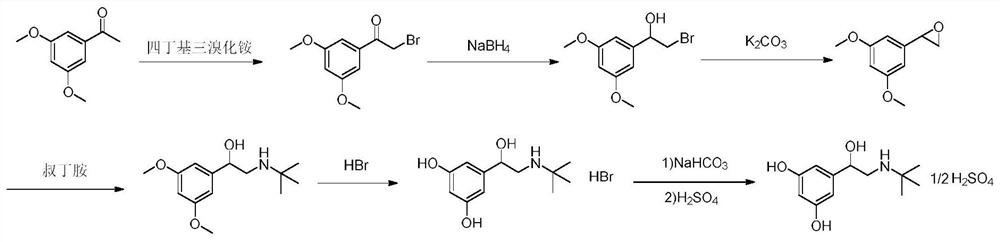
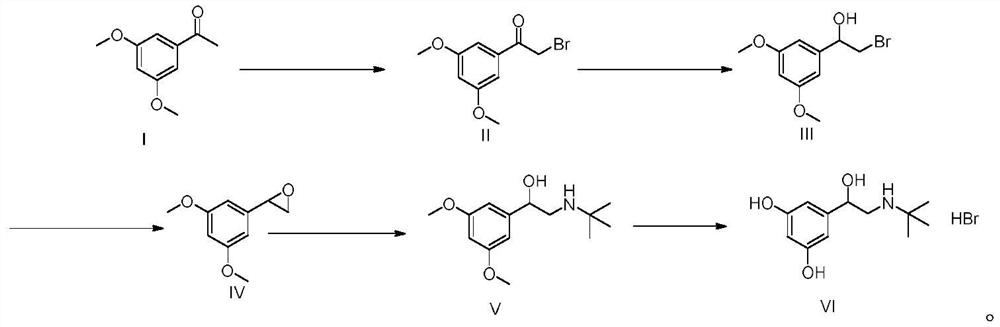
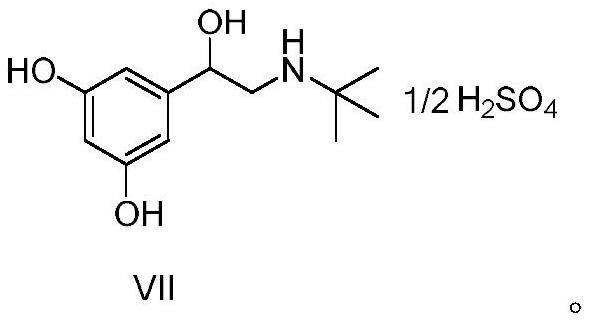
The invention relates to a preparation method of terbutaline sulphate. The technical problems that according to an existing preparation method of terbutaline sulphate, high-pressure hydrogenation and other high-risk operations, and lithium methide, azomethane and other high-risk reagents exist, and cost is high are solved through the preparation method. 3,5-resacetophenone serves as a raw material, and terbutaline sulphate is obtained through hydroxyl protection, the bromination reaction, carbonyl reduction, the condensation reaction and sulphating. The preparation method can be suitable for industrially-produced terbutaline sulphate.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
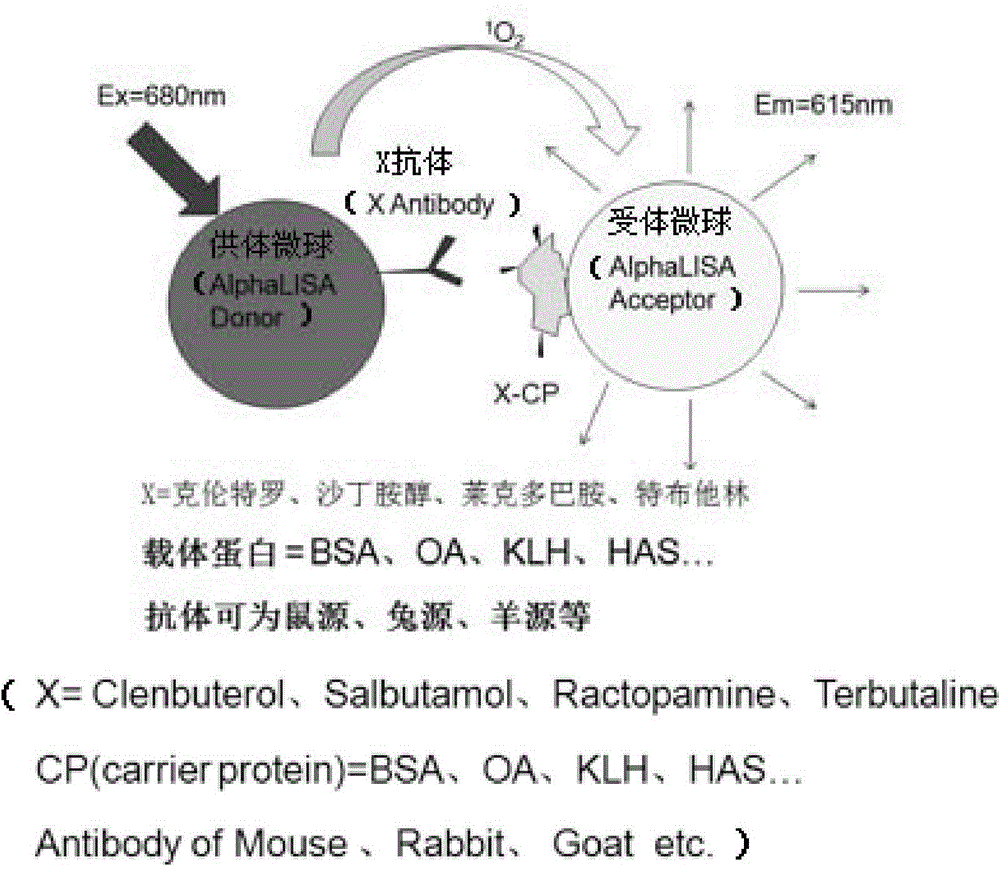
One-step homogeneous chemiluminescent detection method for micromolecule and particle used therein
InactiveCN104897652ALow costLong storage timeChemiluminescene/bioluminescenceSalbutamolCarrier protein
The invention discloses a particle used for detection of a veterinary drug micromolecule. The particle comprises a receptor particle and a donor particle and is prepared through the following steps: marking carrier protein with a veterinary drug molecule; coupling the veterinary drug molecule-marked veterinary drug with a homogeneous chemiluminescent receptor ball; and preparing a homogeneous chemiluminescent receptor ball coupled with a veterinary drug molecule antibody. The invention further discloses a one-step homogeneous chemiluminescent method for detection of the micromolecule with the particle. The one-step homogeneous chemiluminescent method is used for homogeneous, rapid and high-sensitivity detection of veterinary drug residue and for testing of the contents of frequently used veterinary drug components in feeds, e.g., clenbuterol, ractopamine, salbutamol and terbutaline.
Owner:HANGZHOU JINXI BIOLOGICAL TECH
Preparation method of terbutaline sulfate
ActiveCN110950765AReduce usageAvoid high temperature and high pressure reactionsOrganic compound preparationCarbonyl compound preparationAcetophenonePhenyl group
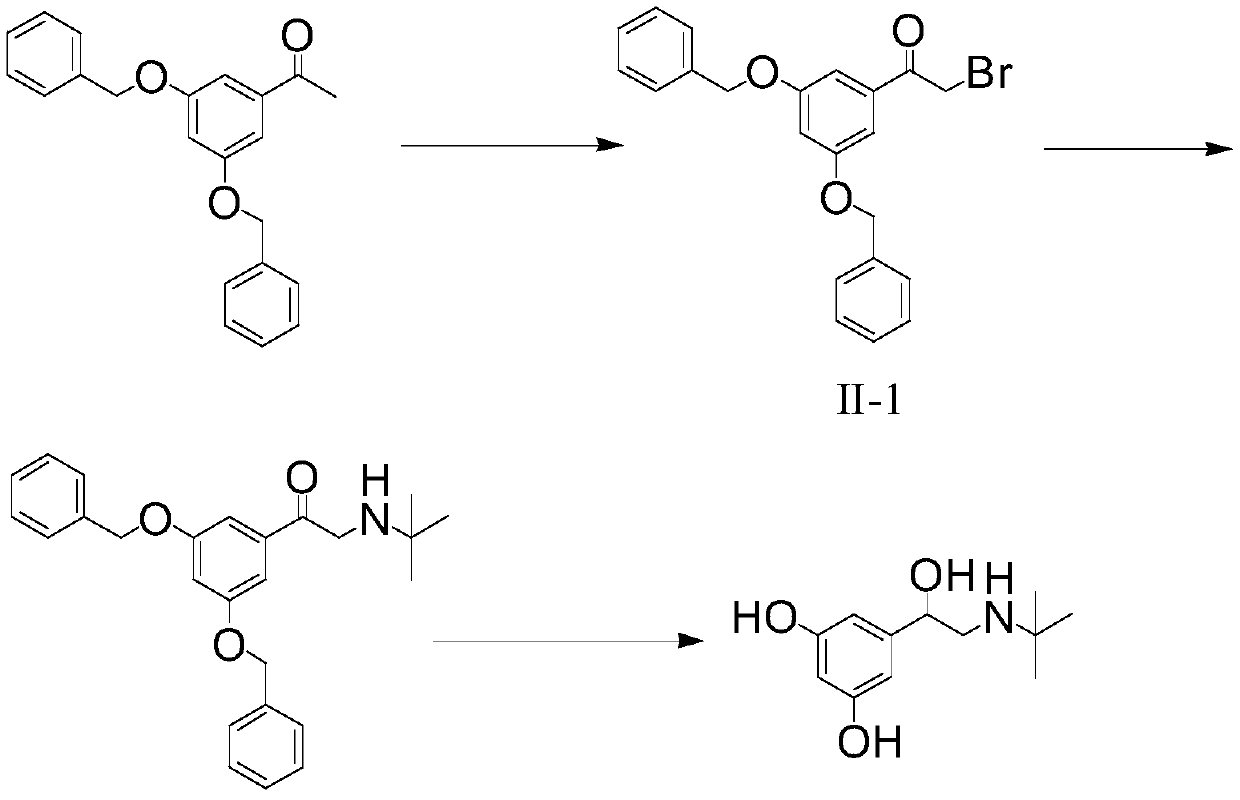
The invention discloses a preparation method of terbutaline sulfate. The method comprises the steps: taking 3,5-dihydroxyacetophenone as an initial raw material, firstly, carrying out benzyl protection to obtain 3,4-dibenzyloxyacetophenone, carrying out copper bromide bromination and DMSO oxidation on 3,5-dibenzyloxyacetophenone to obtain 3,5-dibenzyloxyacetophenone aldehyde, carrying out reductive amination on 3,5-dibenzyloxyacetophenone aldehyde and tert-butylamine to generate 1-[3,5-di(benzyloxy)phenyl]-2-(tert-butylamino)ethanol, and finally carrying out hydrogenation debenzylation and sulfuric acid salification, to obtain terbutaline sulfate. Compared with the prior art, the method has the advantages that the used raw materials and auxiliary materials are cheap and easily available, the use of high-risk and highly-toxic reagents is avoided, high-temperature and high-pressure reaction is avoided, the operation is simple and convenient, the reaction conditions are mild, and the defects of long steps, low yield, potential safety hazards and the like in the prior art are overcome.
Owner:ZHEJIANG PHARMA COLLEGE
Terbutaline sulfate injection and preparation method thereof
PendingCN112826793AAvoid influenceImprove stabilityOrganic active ingredientsInorganic non-active ingredientsInjection solutionAmpoule
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses a terbutaline sulfate injection and a preparation method thereof. The preparation tank is subjected to complexing treatment by using a sodium ethylene diamine tetracetate solution, and after complexing is completed, the preparation tank is flushed by using water for injection until the pH value is 5.0-7.0 and the electrical resistivity is less than 0.5, so that the preparation tank can be used for preparing the injection; and in the preparation process, the water for injection is filled with nitrogen, the terbutaline sulfate is added after dissolved oxygen in the water is controlled to be lower than 100 ppb, and the headspace residual oxygen content in an ampoule bottle is controlled to be smaller than 2% in cooperation with nitrogen introduction in the filling process, so that the influence of oxygen and metal ions on the terbutaline sulfate is effectively avoided. The terbutaline sulfate injection provided by the invention is simple in prescription, and the stability of terbutaline sulfate is remarkably improved on the premise of not introducing other auxiliary materials such as an antioxidant or a complexing agent, a stabilizer and the like, so that the clinical application safety is improved.
Owner:SHIJIAZHUANG NO 4 PHARMA
Five-conjugate testing card for detecting beta-stimulant drugs and preparation method thereof
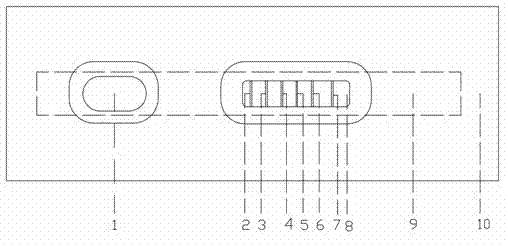
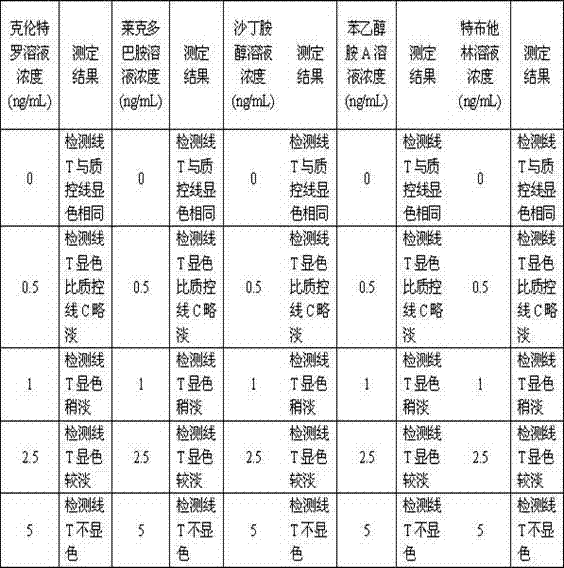
The invention relates to a five-conjugate testing card for detecting beta-stimulant drugs and a preparation method thereof and belongs to the technical field of beta-adrenergic receptor stimulant detection. A test strip is arranged in a casing of the testing card which is composed of a polyvinyl chloride (PVC) glue board, a sample pad, a colloidal gold combination pad, a coated film and absorbent cotton. A colloidal gold film is a glass cellulose film which contains colloidal gold markers of a clenbuterol monoclonal antibody, a ractopamine monoclonal antibody, a salbutamol monoclonal antibody, a phenylethanolamine A monoclonal antibody and a terbutaline monoclonal antibody. The coated film is a nitrocellulose film, wherein five detection lines T and a quality control line C are arranged on the coated film, and the quality control line C is coated with a rabbit anti-mouse immunoglobulin G (IgG) antibody. The five-conjugate testing card can simultaneously detect out clenbuterol, ractopamine, salbutamol, phenylethanolamine A and terbutaline, and is simple, convenient and fast in method, and accurate in result.
Owner:JIANGSU WISE SCI & TECH DEV
Preparation method of terbutaline sulfate
ActiveCN110981739ASolve the shortcomings of large pollutionOrganic compound preparationCarbonyl compound preparationTerbutaline sulphateAcetophenone
The invention discloses a preparation method of terbutaline sulfate, wherein the method comprises the following steps: carrying out a bromination reaction on 3,5-dihydroxyacetophenone as a raw material by using a bromination reagent without hydroxyl protection, carrying out reduction and ring closing, carrying out a ring-opening reaction with tert-butylamine, and finally forming a salt with sulfuric acid to obtain terbutaline sulfate. According to the method, the defects of requirement of deprotection after hydroxyl protection, use of various high-risk highly toxic reagents, long reaction stepand low yield in the prior art are overcome.
Owner:浙江赛默制药有限公司 +1
Terbutaline impulse formulation and preparation method thereof
The invention discloses a terbutaline pulse preparation used for relieving asthma. The terbutaline pulse preparation comprises a tablet core containing the active ingredient of the terbutaline or the salt thereof and one or two coating layers. The tablet core is made by tabletting. Rollover coating in a coating pan, dry pressure coating or fluidized-bed coating is adopted for preparing the terbutaline pulse tablet, and the terbutaline pulse tablet can be further used for filling to prepare a terbutaline capsule. The time lag of the terbutaline preparation is controlled according to the weight increment and the proportion of the ingredients of the coating layer during the process of coating. The invention is designed by being based on the principles of chrono-pharmacology and chrono-therapeutics. If taken at bed-time, the ingredient of the terbutaline or the salt thereof can be released quickly at the preset time lag, thus preventing and relieving the clinical symptoms of asthma attacks quickly in a wee hour.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Method for preparing terbutaline sulphate crystal form B meeting medicinal requirements
InactiveCN101475497AChemically stableStable physical propertiesOrganic compound preparationRespiratory disorderDissolutionSolvent
The invention provides a method for preparing terbutaline sulphate B crystal form. The method comprises: selecting a solvent, dissolving 1-(3,5-dibenzyloxy phenyl)-2-n-tert-butyl aminoethanol into the solvent, removing benzyl groups through hydrogenolysis by a general method, obtaining terbutaline free alkali, and adding sulfuric acid into the terbutaline free alkali to form terbutaline sulphate of crystal form B; or dissolving terbutaline sulphate of non-crystal form B into the solvent, stirring the terbutaline sulphate of the non-crystal form B in the solvent at a certain temperature until the terbutaline sulphate of the non-crystal form B is converted into the terbutaline sulphate B crystal form through dissolution and lixiviation, and reclaiming the leached terbutaline sulphate B crystal form. The method has the advantage of obtaining the terbutaline sulphate B crystal form which has stable chemical and physical properties and meets the requirements of medical preparation.
Owner:李勤耕
Novel preparation method of terbutaline sulfate
InactiveCN110835306AMild reaction conditionsSuitable for industrial scale-upOrganic compound preparationCarboxylic acid esters preparationBronchial SpasmDisease
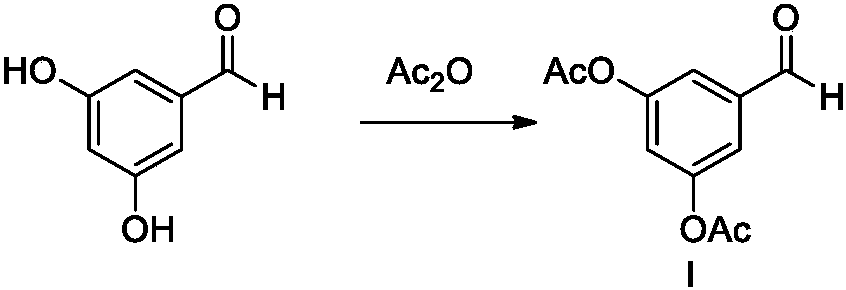
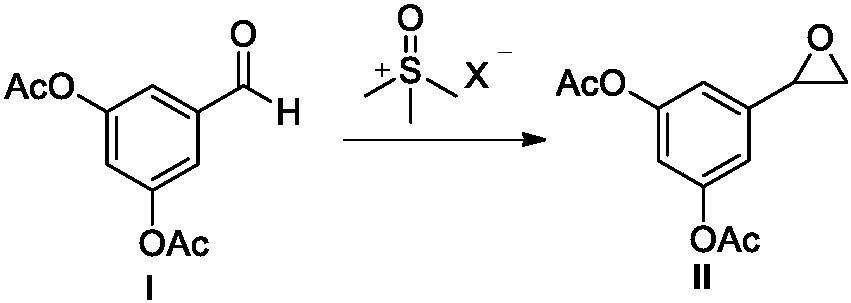
The invention belongs to the field of organic synthesis of medicines, and concretely relates to a novel preparation method of a medicine terbutaline sulfate for treating bronchial spasm caused by bronchial asthma, chronic bronchitis, emphysema and other lung diseases. The synthesis route of the preparation method comprises the following steps: reacting 3,5-dihydroxybenzaldehyde with acetic anhydride to generate a compound I; reacting the compound I with trimethyloxosulfonium halide to generate a compound II; reacting the compound II with tert-butylamine to generate terbutaline; and salifying the terbutaline to generate the terbutaline sulfate. The method has the advantages of avoiding of dangerous chemical reagents, low price of adopted reagents, mild reaction conditions, and suitablenessfor industrial amplification.
Owner:HARVEST PHARMA HUNAN CO LTD
Solution preparation for terbutaline sulphate aerosol inhalation and preparation method of solution preparation
InactiveCN110693861AAvoid first pass effectAvoid destructionOrganic active ingredientsPowder deliveryMedicinal chemistryPharmacology
The invention provides a solution preparation for terbutaline sulphate aerosol inhalation and a preparation method of the solution preparation. The solution preparation for terbutaline sulphate aerosol inhalation comprises terbutaline sulphate and / or a hydrate thereof, an osmotic pressure regulator, a pH regulator and a solvent. The solution preparation for terbutaline sulphate aerosol inhalationdisclosed by the invention has the characteristics of being efficient, low in toxicity, good in stability and high in safety degree.
Owner:BEIJING INCREASE INNOVATIVE DRUG RESEARCH CO LTD
Compound 1-(2-benzyl-3,5-bis(benzyloxy)phenyl)ethanone, preparation method and application thereof
PendingCN111217691AGood effectAmino preparation from aminesComponent separationChemical compoundDrugs synthesis
The invention belongs to the field of medical chemistry, particularly relates to 1-(2-benzyl-3,5-bis(benzyloxy)phenyl)ethanone, a preparation method and application thereof, and provides a new compound, which has a chemical name of 1-(2-benzyl-3,5-bis(benzyloxy)phenyl)ethanone, wherein the new compound can be used as a reference substance for impurity detection in a terbutaline raw material or intermediate drug synthesis process, and the content percentage of the compound in an intermediate is not more than 1.0%.
Owner:INCREASEPHARM TIANJIN INST CO LTD
Terbutaline sulfate intermediate, preparation method thereof, and method for preparing terbutaline sulfate from terbutaline sulfate intermediate
ActiveCN111499528AStable in natureReduce usageOrganic compound preparationCarboxylic acid esters preparationHydrobromideCombinatorial chemistry
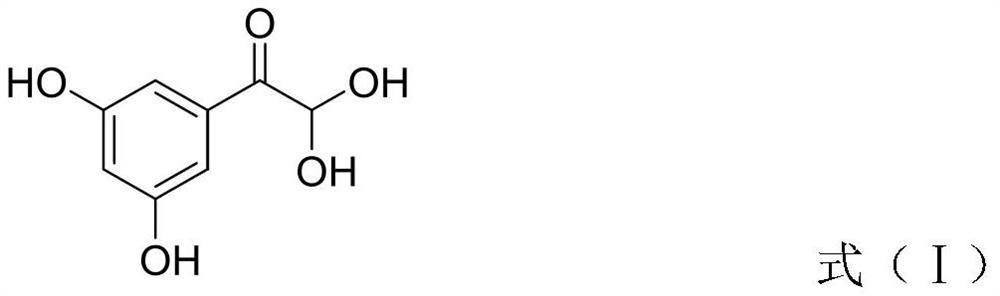
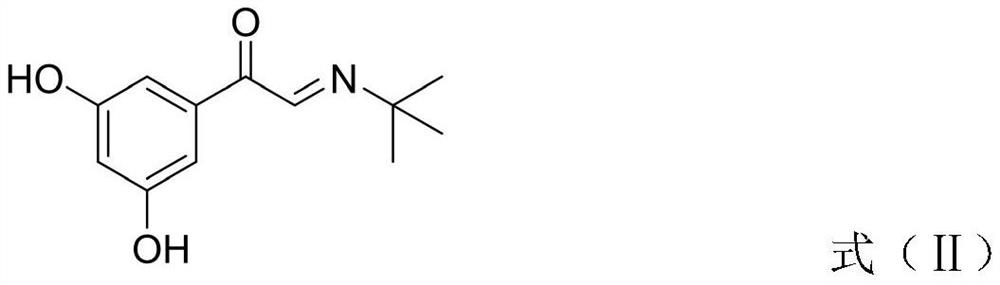
The invention discloses a terbutaline sulfate intermediate, a preparation method thereof, and a method for preparing terbutaline sulfate from the terbutaline sulfate intermediate, and belongs to the technical field of medical chemistry. The novel method for preparing terbutaline sulfate is mild in reaction condition, high in yield and environment-friendly, and comprises the following steps: firstly, providing the terbutaline sulfate intermediate represented by formula I; and then carrying out hydrogenation reduction by taking 10% Pd / C as a catalyst and an alcohol-water solution as a solvent toobtain the terbutaline sulfate represented by formula II. The free alkali hydrochloride or hydrobromide in a formula I is converted into the compound of the formula I before hydrogenation reduction,and the terbutaline sulfate is directly obtained after hydrogenation reduction, so extremely unstable terbutaline free alkali is prevented from appearing independently, and the method has the advantages of mild reaction conditions, high product yield, high purity, low production cost and the like, and is beneficial to industrial production of the terbutaline sulfate.
Owner:成都瑞特恩科技有限公司
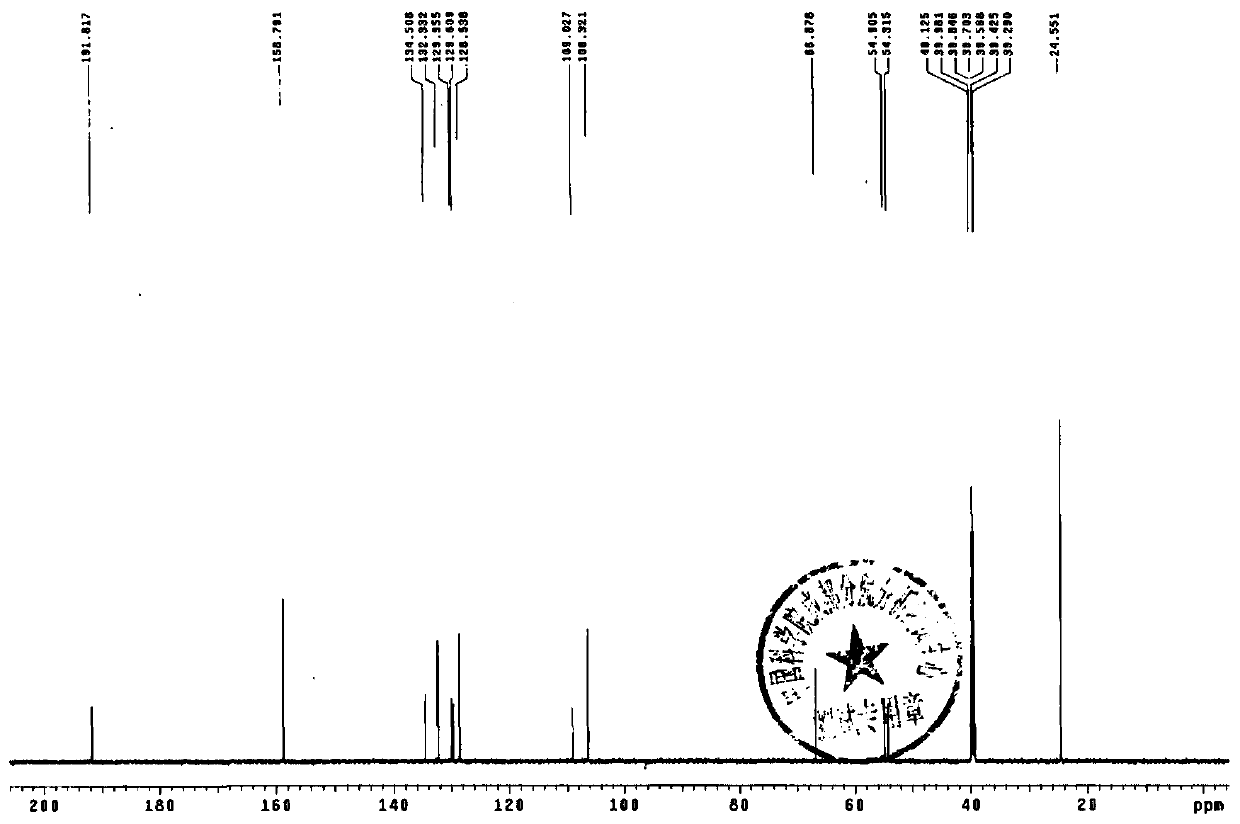
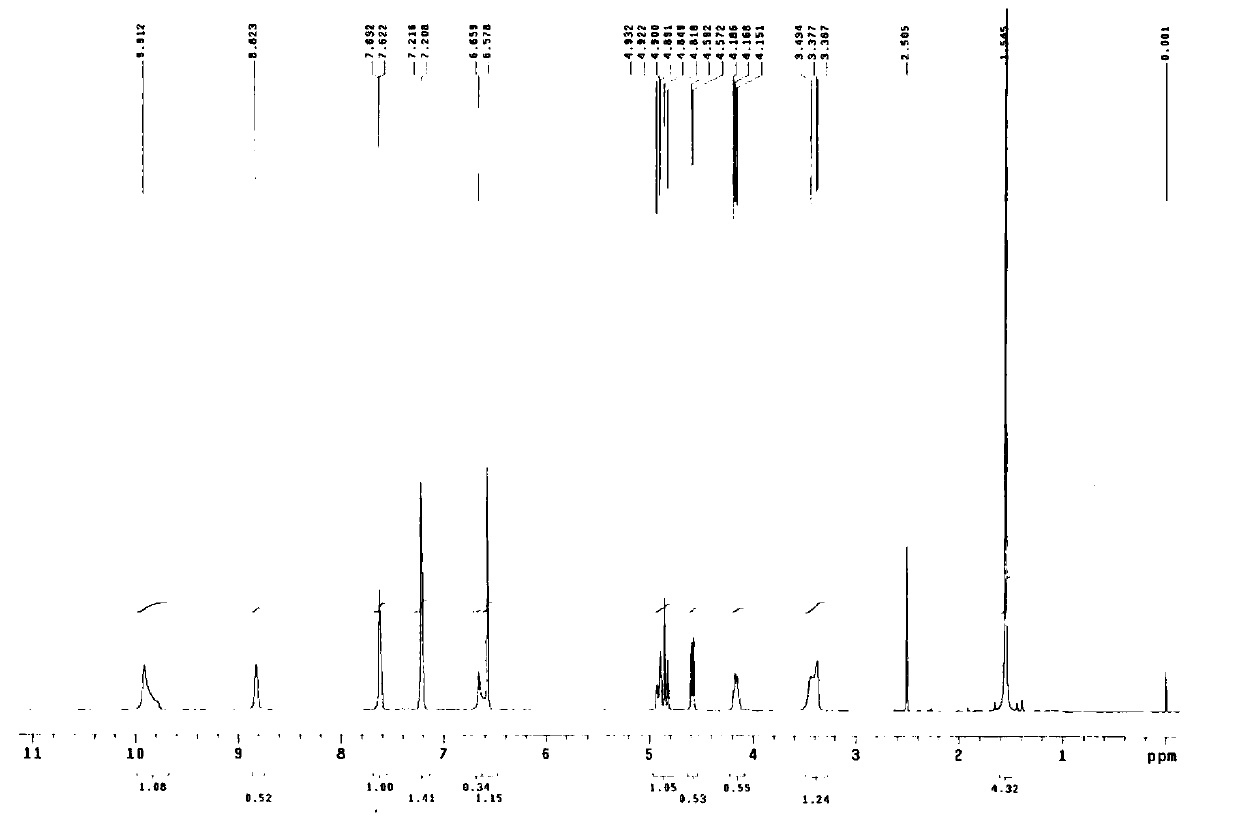
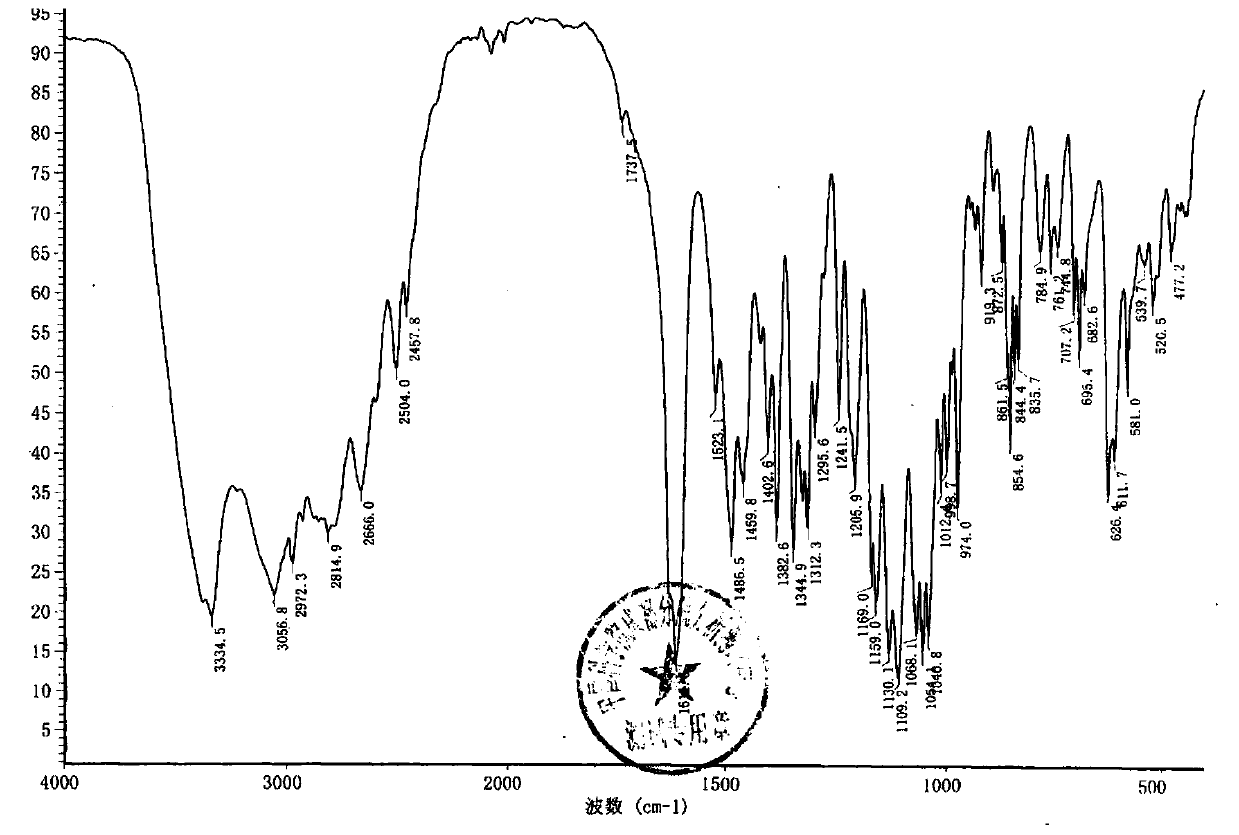
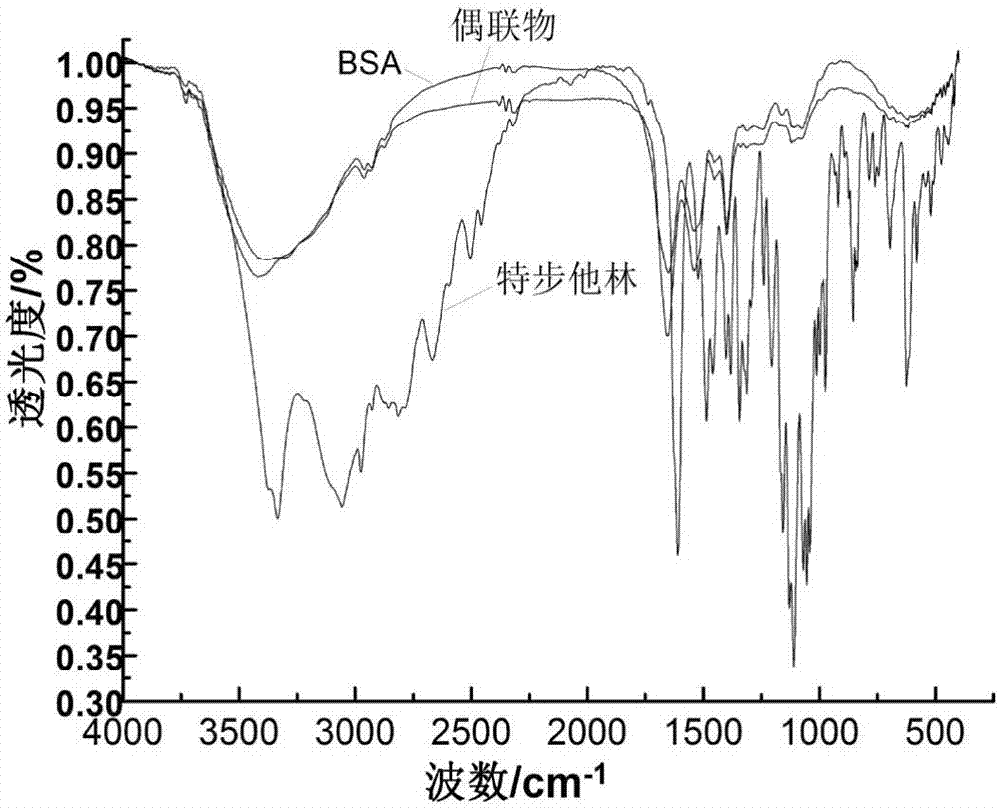
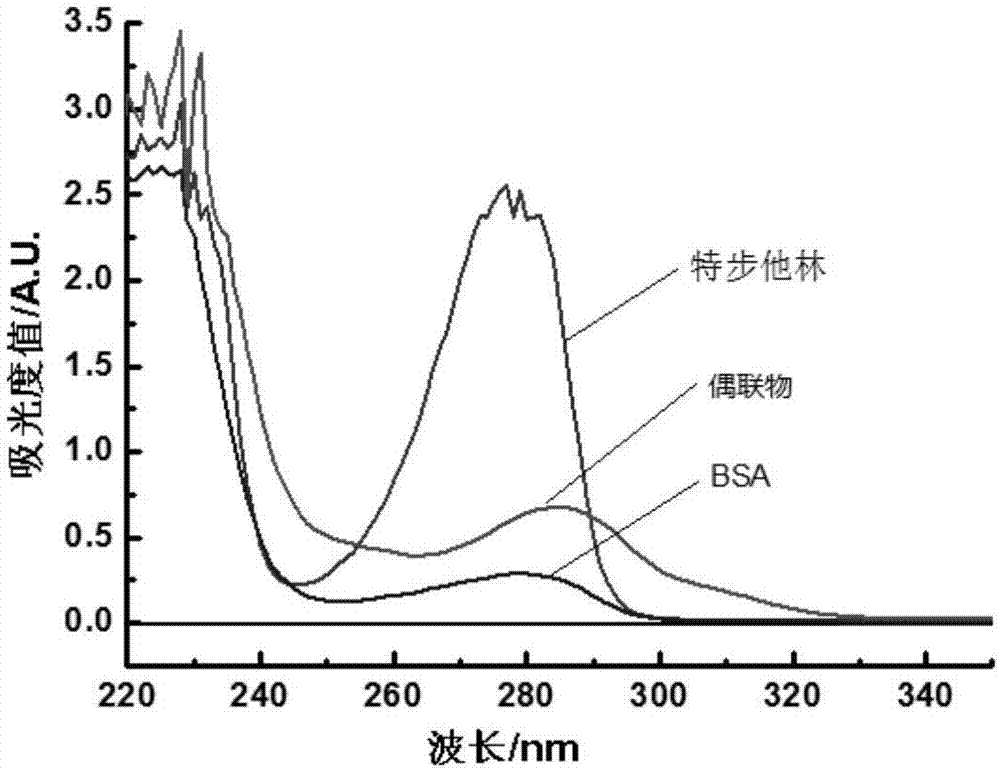
Method for synthesizing terbutaline and bovine serum albumin conjugate
InactiveCN106916221ASolve the cumbersomeSolve the costly synthesis processSerum albuminHybrid peptidesFreeze-dryingPhosphoric acid
The invention provides a method for synthesizing terbutaline and bovine serum albumin conjugate. The method comprises the following steps: 1, dissolving bovine serum albumin (BSA) in a phosphoric acid buffering solution (PBS with the pH value of 6.0), adding terbutaline, stirring the terbutaline and the obtained solution to dissolve the terbutaline, dropwise adding a formaldehyde solution, and carrying out a stirring reaction at room temperature for 24 h to obtain a reaction solution; 2, transferring the reaction solution into a dialysis bag, placing the dialysis bag in a beaker provided with distilled water, and carrying out dialysis under a room temperature stirring condition for 3 days; and 3, centrifuging the above obtained dialysis product by a high speed refrigerated centrifuge, collecting the obtained supernatant, and carrying out vacuum freeze drying to obtain dry powder that is the terbutaline and bovine serum albumin conjugate. The terbutaline and bovine serum albumin conjugate is synthesized through one step, and is of great significance to the immunodetection exploitation and the application of a clenbuterol hydrochloride additive in foods.
Owner:SHANXI UNIV
Preparation method of terbutaline sulfate
ActiveCN111454164AEasy to purifyShorten the total synthetic routeOrganic compound preparationAmino-hyroxy compound preparationAcetophenoneActive ingredient
The invention relates to the field of preparation of chemicals, in particular to a preparation method of terbutaline sulfate. The invention provides a preparation method of terbutaline sulfate. With simple and low-cost acetophenone as an initial raw material, the terbutaline is prepared through five-step reaction; then the terbutaline is subjected to salifying and purification to obtain terbutaline sulfate. According to the method disclosed by the invention, the total synthesis route of terbutaline is effectively shortened, so that the method is simple in intermediate purification, single in reaction solvent, simple in process, mild in reaction condition, easy to operate, high in total yield and more suitable for industrial production; the burden of workshop waste liquid treatment and purification is relieved, the three wastes and reaction energy consumption are reduced, the whole route is combined, research and control of raw material medicine impurities are better facilitated, and working hours are shortened technically and the three wastes and reaction energy consumption are reduced technically.
Owner:SHANDONG MEITAI PHARMA CO LTD
A kind of preparation method of terbutaline sulfate
ActiveCN105254512BMild responseImprove securityAmino preparation from aminesBulk chemical productionLithiumHigh pressure
The invention relates to a preparation method of terbutaline sulphate. The technical problems that according to an existing preparation method of terbutaline sulphate, high-pressure hydrogenation and other high-risk operations, and lithium methide, azomethane and other high-risk reagents exist, and cost is high are solved through the preparation method. 3,5-resacetophenone serves as a raw material, and terbutaline sulphate is obtained through hydroxyl protection, the bromination reaction, carbonyl reduction, the condensation reaction and sulphating. The preparation method can be suitable for industrially-produced terbutaline sulphate.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
Preparation method of terbutaline sulfate and B crystal form thereof
PendingCN112250586AReduce usageShort reaction pathOrganic compound preparationOrganic chemistry methodsPtru catalystPhenyl group
The invention relates to a preparation method of terbutaline sulfate and a B crystal form thereof, which comprises the following steps: sequentially carrying out bromination, reduction reaction and substitution reaction to obtain 1-[3, 5-bis (benzyloxy) phenyl]-2-(tert-butylamino) ethanol; reacting 1-[3, 5-di (benzyloxy) phenyl]-2-(tert-butylamino) ethanol with a reducing agent and a catalyst, performing salifying with sulfuric acid to obtain a terbutaline sulfate crude product, and crystallizing the terbutaline sulfate crude product under a heating reflux condition to obtain the terbutaline sulfate medicinal B crystal form. Raw materials and auxiliary materials used in the method are cheap and easy to obtain, highly toxic and explosive reagents are avoided in the reaction process, the whole reaction route is short, operation is easy and convenient, reaction conditions are mild and safe, the yield of the obtained finished product terbutaline sulfate and the medicinal B crystal form thereof is high, the yield of terbutaline sulfate is 83% or above, and the yield of the medicinal B crystal form is 70% or above, and industrialized production can be achieved easily.
Owner:NINGBO TEAM PHARMA
Preparation method of terbutaline derivatives
PendingCN111440078AReasonable process designImprove protectionGroup 4/14 element organic compoundsOrganic compound preparationBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method of terbutaline derivatives, belongs to the field of compound preparation, and provides a synthesis method of a novel drug molecule acotiamide derivative, which is reasonable in process design, high in yield and convenient and controllable in operation process. The invention provides a preparation method of three terbutaline derivatives. The preparationmethod provided by the invention has the advantages of reasonable process design, strong operability, mild reaction conditions and high yield, and can realize industrial production. The series of terbutaline derivatives prepared by the method provide important basis for scientific evaluation of quality, safety and efficiency of the series of terbutaline derivatives, and have important applicationvalue.
Owner:TLC NANJING PHARMA RANDD CO LTD
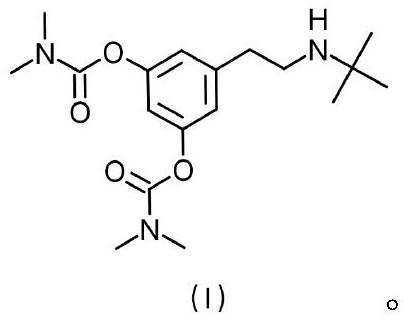
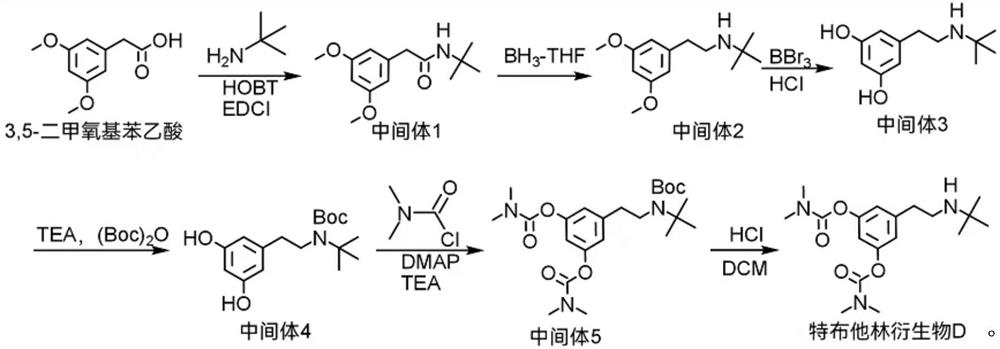
Terbutaline derivative D as well as preparation method and application thereof
PendingCN114539100AThe synthesis method is simpleCarbamic acid derivatives preparationOrganic compound preparationTert-ButylamineCombinatorial chemistry
The invention discloses a terbutaline derivative D and a synthesis method thereof, the structure of the terbutaline derivative D is as shown in a structural formula (I), and the specific synthesis route is as follows: 3, 5-dimethoxyphenylacetic acid and tert-butylamine are taken as raw materials, and the terbutaline derivative D is obtained through condensation, reduction, deprotection and substitution reaction. The terbutaline derivative D disclosed by the invention is simple in synthesis method, an intermediate generated in the synthesis process can be used as a terbutaline impurity, and a final product can be used as a bambuterol impurity for quality research.
Owner:HARVEST PHARMA HUNAN CO LTD
Preparation method of terbutaline
ActiveCN111960955AReduce polarityHigh selectivityOrganic compound preparationAmino-hyroxy compound preparationDistillationEthyl acetate
The invention belongs to the field of pharmacy, and particularly relates to a preparation method of terbutaline. The method comprises: S1, adding a compound I and dichloromethane into a reaction kettle, stirring and dissolving, and then adding anhydride, S2, after the reaction is finished, adding dilute alkaline water into the system, fully stirring and washing, collecting a dichloromethane phase,and carrying out reduced pressure distillation to obtain a yellow oily matter, namely a compound II, S3, adding the compound II, ethyl acetate, tert-butylamine, sodium hydroxide and an oxidation protective agent into the reaction kettle, and S4, after the reaction in the step S3 is finished, adding purified water into the system to wash the reaction solution in the step S3, collecting an ethyl acetate phase, and carrying out reduced pressure distillation on the ethyl acetate phase to obtain terbutaline. According to the technical scheme provided by the invention, the post-reaction treatment difficulty is reduced, so that an intermediate compound is easily separated from a reaction system, meanwhile, side reactions such as epoxy bond hydrolysis are avoided, and the stability and yield of the final product terbutaline are improved.
Owner:南京恒道医药科技股份有限公司
Method for synthesizing terbutaline
PendingCN110734382AOrganic compound preparationCarbonyl compound preparation by oxidationTert-ButylamineCombinatorial chemistry
The invention discloses a method for synthesizing terbutaline. The method comprises the following steps: reacting a compound I with selenium dioxide to obtain a compound II; reacting the compound II with tert-butylamine to obtain a compound III; reacting the compound III with a reducing agent to obtain a compound IV; and reacting the compound IV with a catalyst to remove benzyloxy to obtain terbutaline. The synthesis method has the advantage that the generation of impurity alpha-bromo-3, 5-dibenzyloxyacetophenone is avoided.
Owner:SUZHOU HOMESUN PHARMA
A kind of preparation method of terbutaline sulfate
ActiveCN107513023BPrevent oxidationHigh purityOrganic compound preparationAmino-hyroxy compound preparationInorganic saltsPtru catalyst
The invention relates to a preparation method of terbutaline sulfate, which comprises the following steps: dissolving a compound of formula I or formula II into a solvent A, adding a metal catalyst for catalytic hydrogenation, so as to obtain 5-[2-[(1,1-dimethylethyl)amido]-1-ethoxyl]-1,3-resorcinol; after the reaction is finished, filtering, collecting filter liquor, adding a certain amount of concentrated sulfuric acid in the filter liquor, after stirring is stopped, recycling the solvent A, adding a solvent B into a residue, stirring to separate out a white crystal substance, performing suction filtration, thus obtaining the terbutaline sulfate. According to the method provided by the invention, the treating difficulty after the reaction is greatly reduced, the introduction of water and inorganic salt is avoided, and the product content is high; furthermore, the stability of the terbutaline sulfate is obviously improved by distilling the product under acidic conditions, the catalyst and the reaction solvents are recyclable, so that the environmental protection pressure is greatly reduced.
Owner:SHIJIAZHUANG NO 4 PHARMA
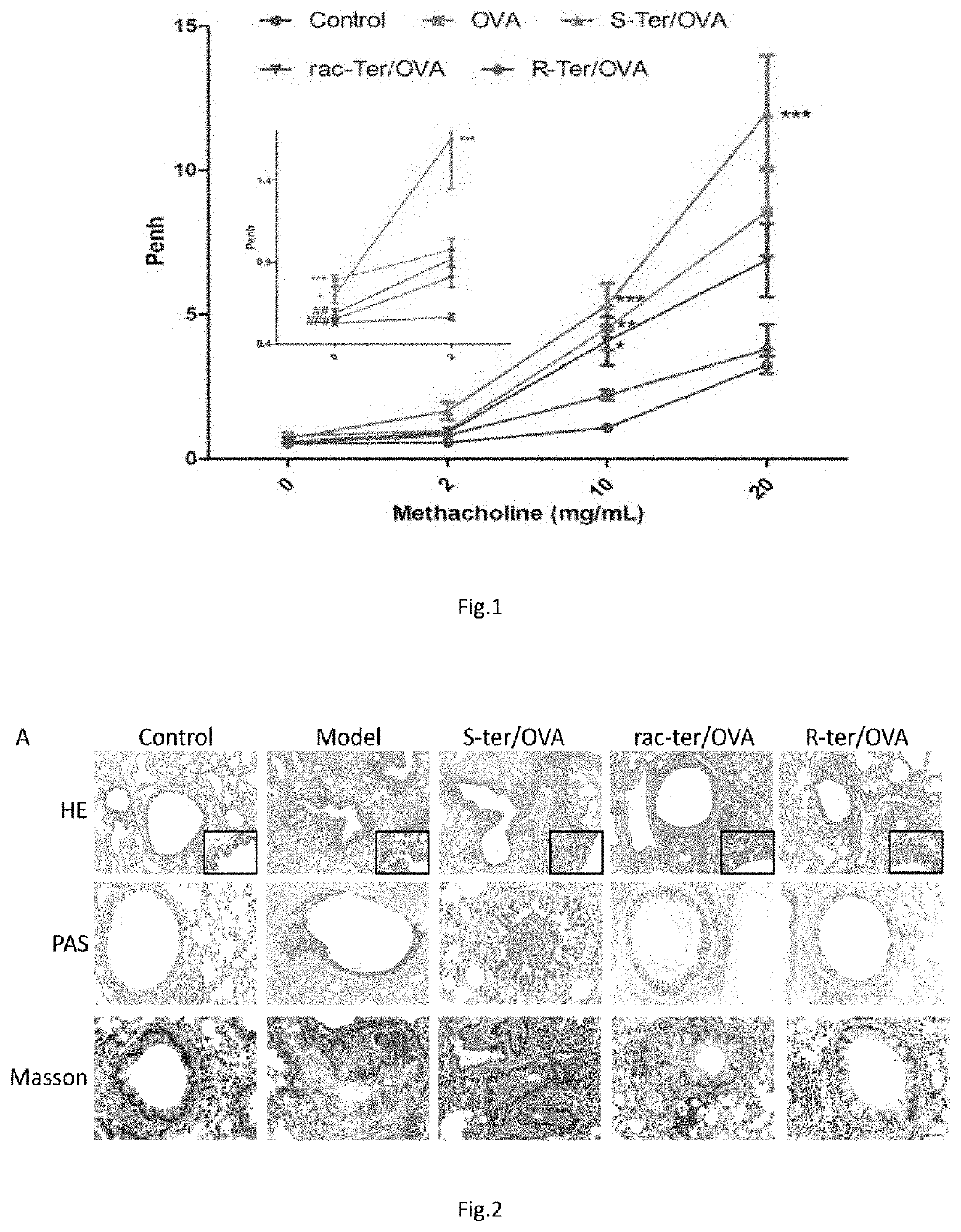
Use of R-enantiomer Beta2-agonists for prevent and treatment of pulmonary inflammation and inflammatory remodeling for reduced adverse effects
InactiveUS20220362174A1Reduce adverse effectsPharmaceutical delivery mechanismRespiratory disorderImmune modulatorFibrosis
This invention disclosed new use of R-terbutaline and other R-enantiomer β2-agonists as immune-modulators for treatment of bronchia-lung inflammatory symptoms or inflammatory fibrosis remolding. This invention also disclosed new use of R-terbutaline and R-β2-agonists for reduced adverse effects related to racemic or S-enantiomer β2-agonists including airway hyper responsiveness and airway fibrosis.
Owner:TAN KS
Method for preparing terbutaline sulphate crystal form B meeting medicinal requirements
InactiveCN101475497BChemically stableStable physical propertiesOrganic compound preparationRespiratory disorderDissolutionSolvent
The invention provides a method for preparing terbutaline sulphate B crystal form. The method comprises: selecting a solvent, dissolving 1-(3,5-dibenzyloxy phenyl)-2-n-tert-butyl aminoethanol into the solvent, removing benzyl groups through hydrogenolysis by a general method, obtaining terbutaline free alkali, and adding sulfuric acid into the terbutaline free alkali to form terbutaline sulphate of crystal form B; or dissolving terbutaline sulphate of non-crystal form B into the solvent, stirring the terbutaline sulphate of the non-crystal form B in the solvent at a certain temperature until the terbutaline sulphate of the non-crystal form B is converted into the terbutaline sulphate B crystal form through dissolution and lixiviation, and reclaiming the leached terbutaline sulphate B crystal form. The method has the advantage of obtaining the terbutaline sulphate B crystal form which has stable chemical and physical properties and meets the requirements of medical preparation.
Owner:李勤耕
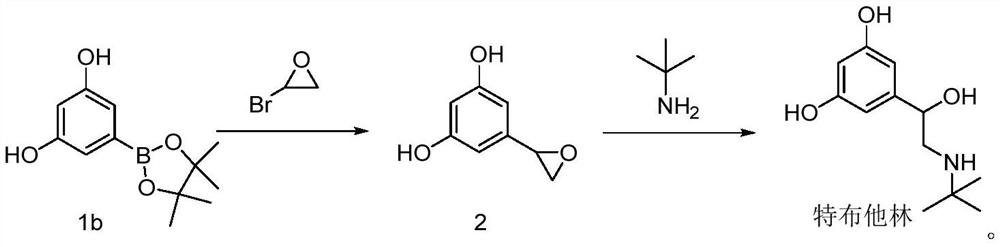
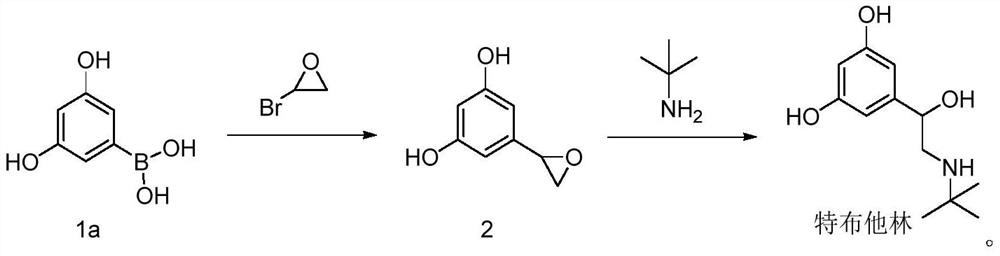
Preparation method of terbutaline
PendingCN113461555AReduce usageFew reaction stepsOrganic compound preparationBulk chemical productionChemical synthesisTert-Butylamine
The invention provides a preparation method of terbutaline, and relates to the technical field of chemical synthesis. The preparation method of terbutaline comprises the following steps: (a) carrying out Suzuki reaction on a compound 1 and halogenated ethylene oxide to obtain a compound 2; and (b) reacting the compound 2 with tert-butylamine to obtain terbutaline. The Suzuki reaction is creatively applied to the preparation of terbutaline, so that the reaction steps are greatly shortened, the processes of bromination reaction, protection group loading on hydroxyl and deprotection are avoided, catalytic hydrogenation is not needed, the reaction conditions are mild, the reaction process is easy to control, and the safety coefficient is high. Raw materials are simple and easy to obtain, and the industrialization cost is saved. The product yield is high, the purity is high, and a process route capable of industrially producing a product with higher quality is provided.
Owner:TIANJIN PHARMA GROUP CORP
Preparation method of terbutaline sulfate
InactiveCN113045437AShort reaction stepsGood atomic economicsOrganic compound preparationCarbonyl compound preparation by oxidationAcetophenoneEnvironmental chemistry
The invention provides a preparation method of terbutaline sulfate. The preparation method comprises the following steps: with 3,5-dihydroxyacetophenone as a raw material, directly carrying out an oxidation reaction on 3,5-dihydroxyacetophenone without protecting a phenolic hydroxyl group so as to obtain aromatic glyoxal, and then carrying out a condensation reaction, a reduction reaction and sulfuric acid salification on the aromatic glyoxal and tert-butylamine to obtain terbutaline sulfate. According to the method, a high-risk operation unit for hydrogenation debenzylation in an existing terbutaline sulfate preparation process is eliminated; and meanwhile, bromination reaction with relatively large pollution to the environment is avoided, and the whole process is more suitable for industrial production.
Owner:北京睿悦生物医药科技有限公司 +1
Terbutaline sulfate oral liquid and production method thereof
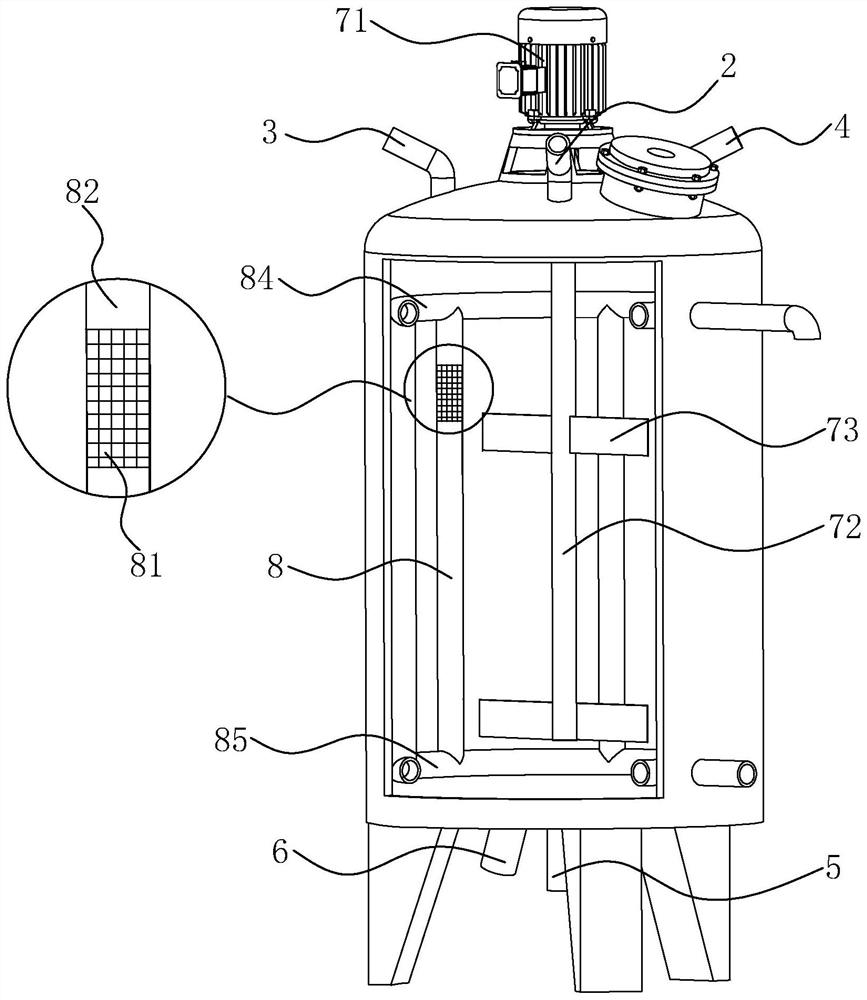
PendingCN113712912AReduce exposureReduce oxidationOrganic active ingredientsDispersion deliveryTerbutaline sulphateChemistry
The invention discloses a production method of a terbutaline sulfate oral liquid. The production method comprises the following steps of: S1, adding terbutaline sulfate and an auxiliary agent into a liquid preparation tank, introducing nitrogen into the tank, completely discharging air in the tank, then performing vacuum suction, discharging the nitrogen, sucking preparation water into the liquid preparation tank by utilizing negative pressure, and stirring for dissolving to obtain a liquid medicine; S2, adding 1 to 3 weight percent of sodium hydroxide solution into the liquid medicine, adjusting the pH value to 4.0 + / -0.5, then adding purified water, diluting the liquid medicine to a prescription dosage, and uniformly mixing to obtain a filling liquid; and S3, introducing the nitrogen into the liquid preparation tank, transferring the filling liquid into a filter for filtering to obtain a filtrate, and filling the filtrate into a medicine bottle to obtain the terbutaline sulfate oral liquid. The production method disclosed by the invention can effectively reduce the oxidation of the oral liquid and reduce the impurity content of the oral liquid.
Owner:杭州上禾健康科技有限公司
Synthesis method of terbutaline and application of terbutaline in preparation of terbutaline sulfate
ActiveCN112209841AAvoid the process of hydrodebenzylationReduce pollutionOrganic compound preparationCarbonyl compound preparationAcetophenoneHydrolysis
The invention discloses a synthesis method of terbutaline, which is characterized by comprising the following steps: (1) reacting 3, 5-dimethoxyacetophenone with tetrabutylammonium tribromide to obtain 2-bromo-1-(3, 5-dimethoxyphenyl)ethanone; (2) reacting the 2-bromo-1-(3, 5-dimethoxyphenyl)ethanol with a reducing agent in methanol to obtain 2-bromo-1-(3, 5-dimethoxyphenyl)ethanol; (3) carrying out cyclization reaction on the 2-bromo-1-(3, 5-dimethoxyphenyl)ethanol under an alkaline condition to generate 2-(3, 5-dimethyloxy) ethylene oxide; (4) carrying out a reaction on the 2-(3, 5-dimethyloxy) ethylene oxide and tert-butylamine to generate 1-(3, 5-dimethoxyphenyl)-2-tert-butylamino-ethanol; and (5) hydrolyzing under an acidic condition to obtain a salt of 1-(3, 5-dihydroxyphenyl)-2-tert-butylamino-ethanol, namely terbutaline. The total molar yield is about 60% and is far higher than the yield in the prior art, and the synthesis method of terbutaline and the application of terbutaline in the preparation of terbutaline sulfate are suitable for industrial production.
Owner:YANGZHOU ZHONGBAO PHARMA
A kind of synthesis method of terbutaline and its application in preparing terbutaline sulfate
ActiveCN112209841BAvoid the process of hydrodebenzylationReduce pollutionOrganic compound preparationCarbonyl compound preparationAcetophenonePhenyl group
The invention discloses a synthetic method of terbutaline, which is characterized in that it comprises the following steps: (1) reacting 3,5-dimethoxyacetophenone with tetrabutylammonium tribromide to obtain 2- Bromo-1-(3,5-dimethoxyphenyl)ethanone; (2) react it with a reducing agent in methanol to give 2-bromo-1-(3,5-dimethoxyphenyl ) ethanol; (3) it is carried out ring-forming reaction under alkaline condition, generates 2-(3,5-dimethyloxyl) oxirane; (4) it is reacted with tert-butylamine, generates 1-( 3,5-dimethoxyphenyl)-2-tert-butylamino-ethanol; (5) demethylating it under acidic conditions to give 1-(3,5-dihydroxyphenyl)-2-tert-butyl The salt of amino-ethanol is terbutaline. In the present invention, the total molar yield is about 60%, which is much higher than that of the prior art, and is suitable for industrialized production.
Owner:YANGZHOU ZHONGBAO PHARMA
A kind of preparation method of terbutaline
ActiveCN111960955BReduce polarityHigh selectivityOrganic compound preparationAmino-hyroxy compound preparationDistillationEthyl acetate
The invention belongs to the field of pharmacy, and in particular relates to a preparation method of terbutaline. The technical points are as follows: a method for preparing terbutaline, S1. Add compound I and methylene chloride into the reaction kettle, stir and dissolve and then add acid anhydride; S2. After the reaction is completed, add dilute alkaline water into the system to fully stir and wash , collect the dichloromethane phase, and distill under reduced pressure to obtain a yellow oily substance, which is compound II; S3. add compound II, ethyl acetate, tert-butylamine, sodium hydroxide and oxidation protection agent to the reaction kettle; S4. step S3 completes the reaction Then add purified water to the system to wash the reaction solution in step S3, collect the ethyl acetate phase, and distill the ethyl acetate phase under reduced pressure to obtain terbutaline. The technical solution provided by the invention reduces the difficulty of post-reaction treatment, makes the intermediate compound easy to separate from the reaction system, avoids the occurrence of side reactions such as epoxy bond hydrolysis, and increases the stability and stability of the final product terbutaline. yield.
Owner:南京恒道医药科技股份有限公司
Process treatment method for improving stability of terbutaline sulphate solution for nebulization
ActiveCN112274484ALower requirementGuaranteed stabilityOrganic active ingredientsDispersion deliveryProduction lineBiochemical engineering
The invention belongs to the technical field of medicine, and particularly relates to a process treatment method for improving the stability of a terbutaline sulphate solution for nebulization. According to the invention, a metal chelating agent is adopted to passivate production equipment of a production line of the terbutaline sulphate solution for nebulization, and the passivated production equipment is employed to prepare liquid medicine. According to the process treatment method, metal ions on the surface of the production equipment are effectively removed through passivation of the equipment of the production line, and the stability of a product is guaranteed; and meanwhile, through an optimized packaging material, it is synchronously guaranteed that a final packaging material does not affect the product, nitrogen charging protection is not needed in the production process, a requirement on a production line is low, and production cost is saved.
Owner:南京华盖制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com