Preparation method of terbutaline sulfate
A technology of terbutaline sulfate and compound, applied in the field of medicine, can solve the problems of long steps, low yield, large environmental pollution, etc., and achieve the effects of low production cost, easy availability of materials, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
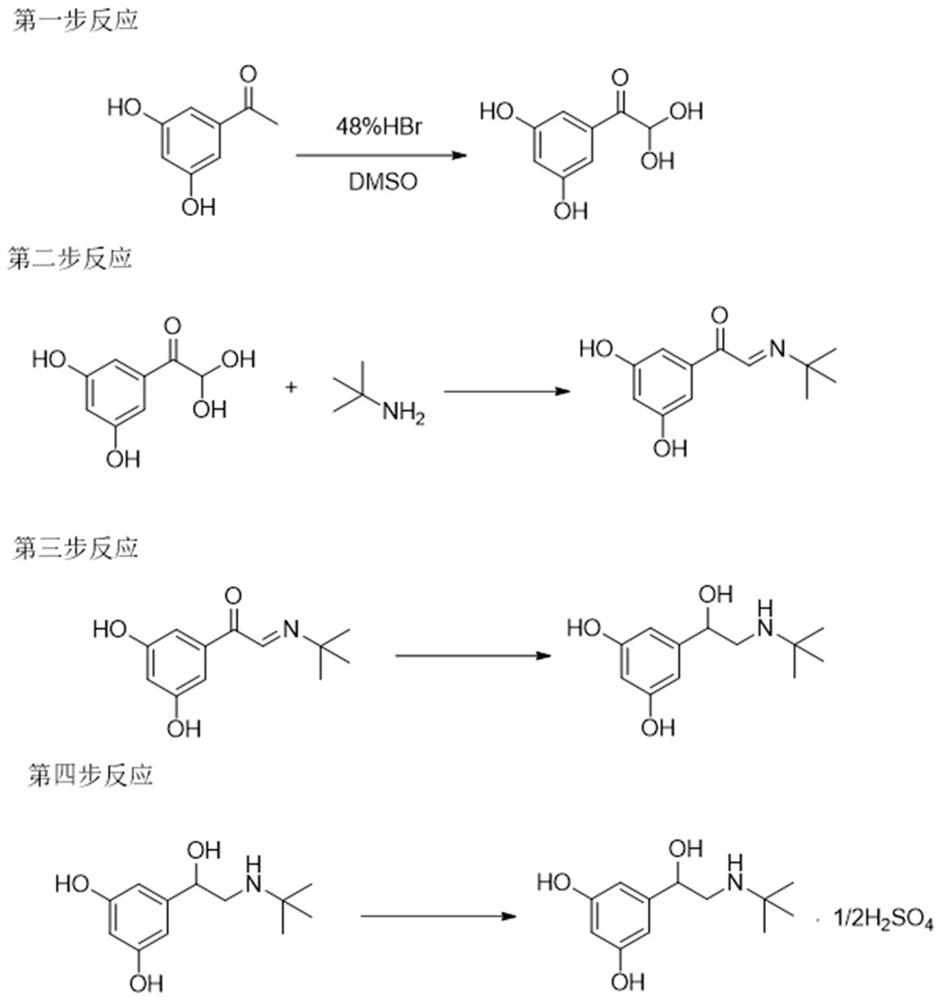
[0033] This example provides a preparation method of terbutaline sulfate, using 3,5-dihydroxyacetophenone as a raw material, through oxidation reaction, condensation reaction, carbonyl reduction reaction, and sulfuric acid salt formation, to obtain terbutaline sulfate Lin includes the following methods:
[0034] Step 1, oxidation reaction:
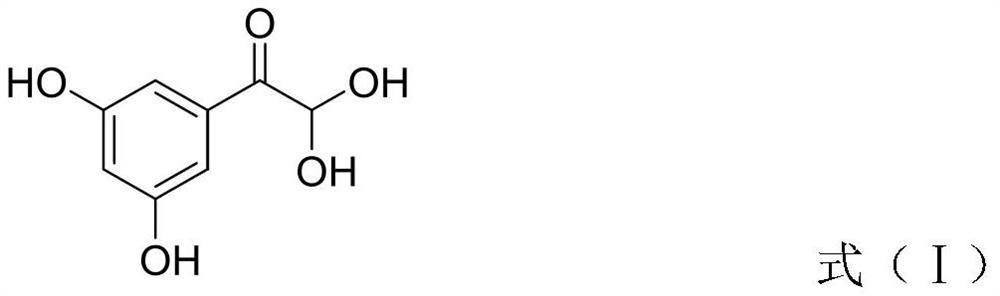
[0035] 50.0g (0.33mol) of 3,5-dihydroxyacetophenone was dissolved in 400ml DMSO, and 112ml of hydrobromic acid (HBr) (0.99mol) with a mass concentration of 48% was added at 30°C to 40°C to keep The temperature was reacted for 13 to 16 hours, and after it was lowered to room temperature, it was added to ice water, extracted with an organic solvent including dichloromethane, and the organic phase was concentrated under reduced pressure to dryness to obtain a solid, which was 3,5 as shown in formula (I). -44.54 grams of dihydroxyphenylglyoxal, and the molar yield reaches 73.3%.
[0036]
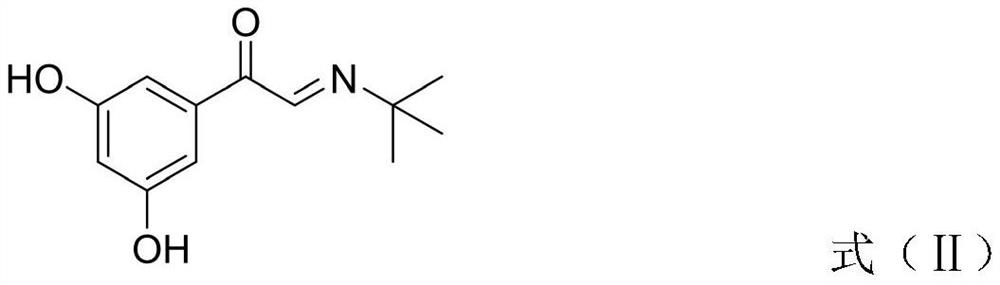
[0037] Step 2, condensation reaction:
[0038] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com