A kind of synthesis method of terbutaline and its application in preparing terbutaline sulfate
A technology of terbutaline and a synthesis method, which is applied in the application field of preparing terbutaline sulfate, can solve the problems of high price of bambuterol hydrochloride, expensive starting materials, increased operation difficulty and the like, and achieves reaction conditions. More, low production cost, convenient operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
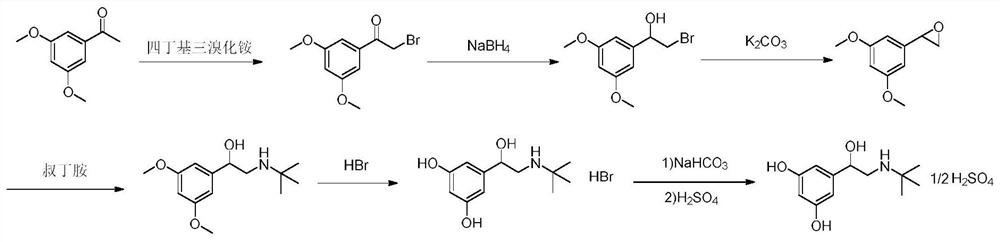
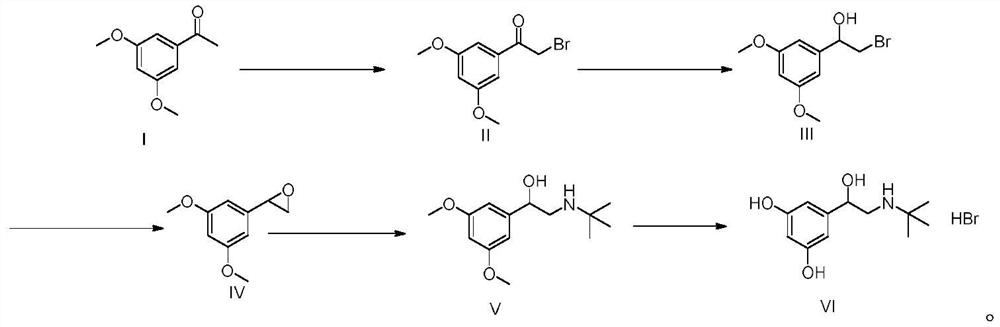
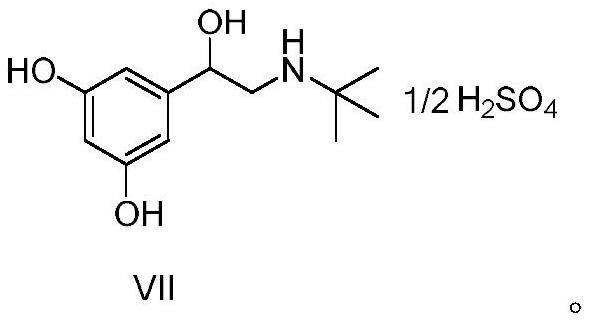
[0055] A kind of preparation method of terbutaline sulfate, refer to figure 1 Shown synthetic route, concrete steps are as follows:
[0056] (1) Add 100g (MW: 180.20, 0.55mol) of 3,5-dimethoxyacetophenone into a three-necked flask, add 300mL tetrahydrofuran and 100mL methanol, add 294g (MW: 482.18, 0.61mol) tetrabutyltri Ammonium bromide, stirred at room temperature for 2h. After the reaction was completed, 1L of isopropanol and 1L of purified water were added to the system, stirred and crystallized in an ice-water bath for 2h, filtered under reduced pressure, and the filter cake was air-dried at 50°C for 8h to obtain 129g of a light yellow solid (molar yield 90% ).
[0057] (2) Add 120g of 2-bromo-1-(3,5-dimethoxyphenyl)ethanone (MW: 259.10, 0.46mol) into a three-necked flask, add 600mL of methanol, and add boron in batches under an ice-water bath Add 8.76 g of sodium hydride (MW: 37.83, 0.23 mol). After the addition, stir at room temperature for 2 h. After the reaction, ...
Embodiment 2
[0061] Embodiment 2: the terbutaline sulfate synthetic route in the present invention has been fully optimized for parameters, and the parameter optimization results are as follows:
[0062] 1. Bromination reaction
[0063] (1) bromination reaction solvent type optimization result is as follows (table 1), and other parameters are with step (1) in embodiment 1:
[0064] Table 1
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com