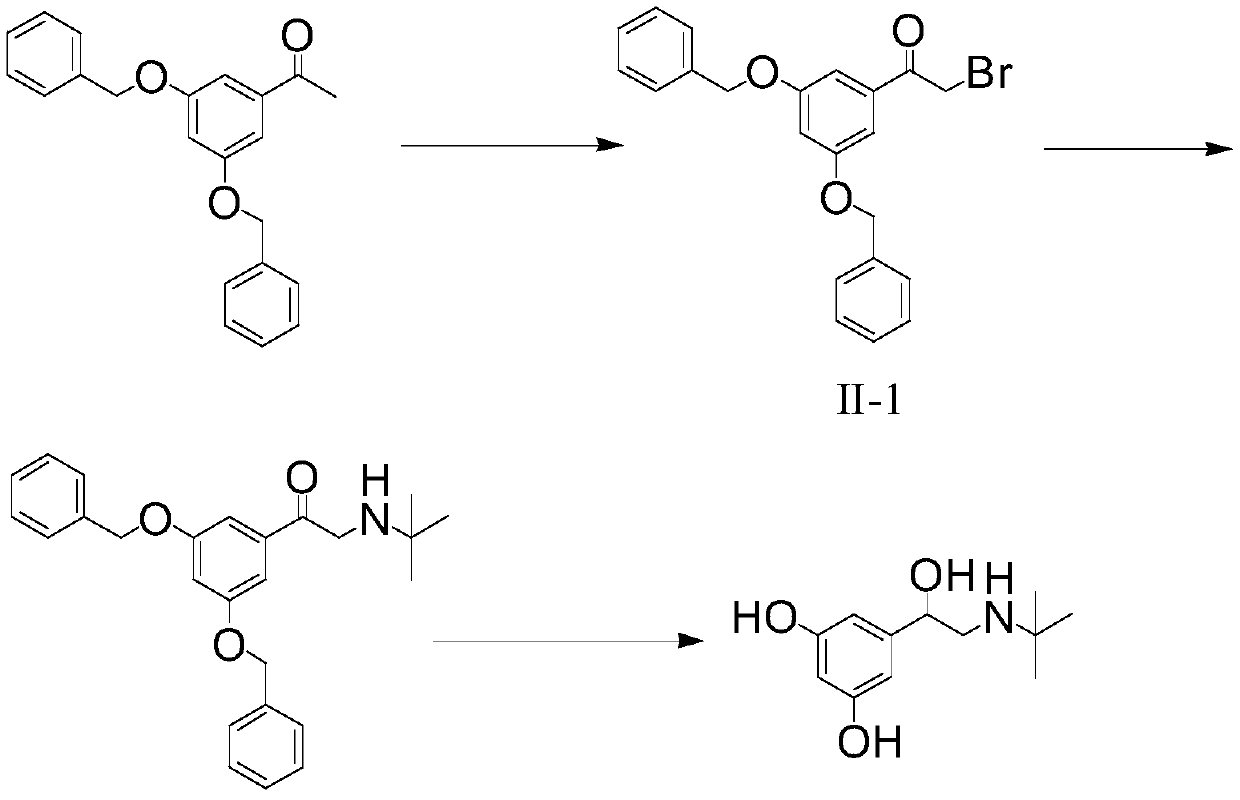
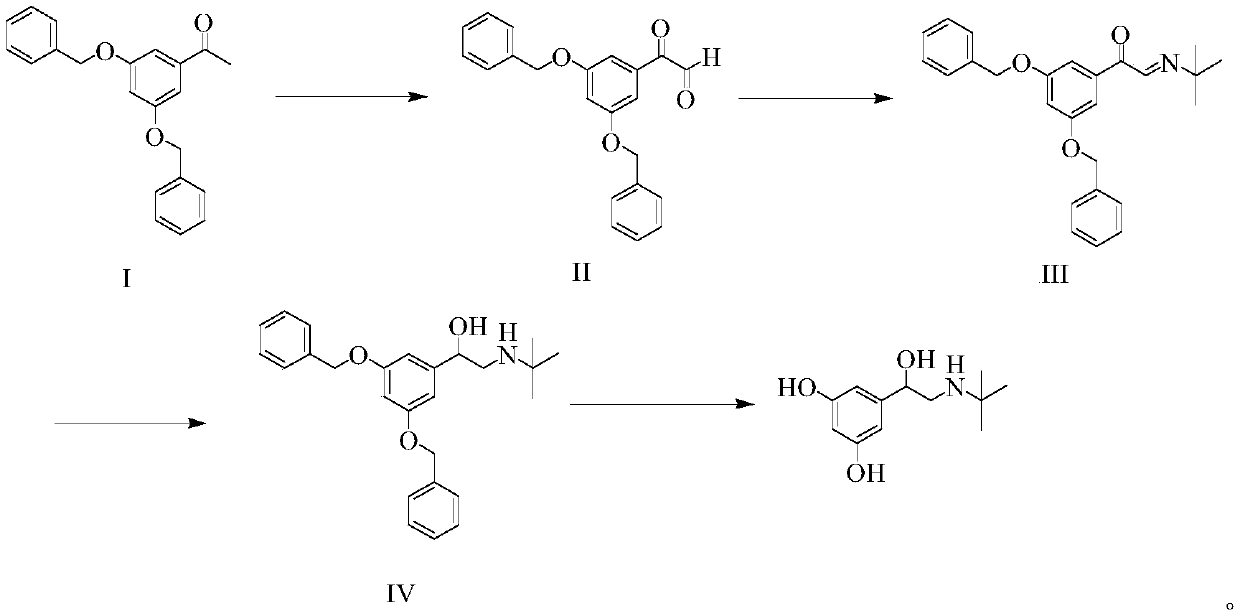
Method for synthesizing terbutaline
A technology of terbutaline and compound, applied in the field of synthesizing terbutaline, can solve the problems of carcinogenicity and residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of compound II:
[0036] Add 40g of compound I to a 200mL reaction flask, then add 100mL of tetrahydrofuran, add 10g of selenium dioxide at room temperature, and react at room temperature for 24 hours. After the reaction is completed, the reaction solution is filtered, and the filtrate is washed with saturated saline and used directly for the next reaction.
[0037] (2) Preparation of compound III:
[0038] Add the treatment solution obtained in step (1) into a 200mL reaction bottle, raise the temperature to 40-45°C, add dropwise a tetrahydrofuran solution of 0.4g / mL tert-butylamine (12g tert-butylamine dissolved in 30mL tetrahydrofuran), and add dropwise for about 30 minutes After the dropwise reaction, continue the heat preservation reaction for 2 hours. After the reaction, control the temperature at 40-45° C. to evaporate the solvent under reduced pressure, add 80 mL of methanol to the distillation residue, and stir for 30 minutes to completely dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com