Preparation method of terbutaline derivatives
A technology for terbutaline derivatives, applied in the field of preparation of terbutaline derivatives, to achieve the effects of high product purity, good environmental protection effect, and reasonable process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
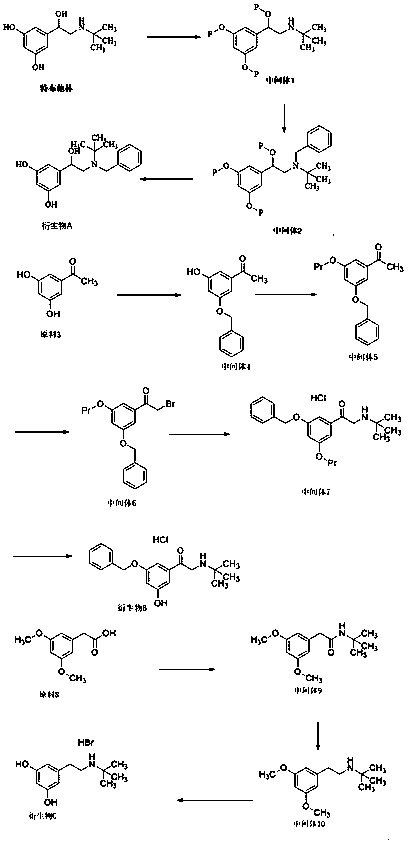
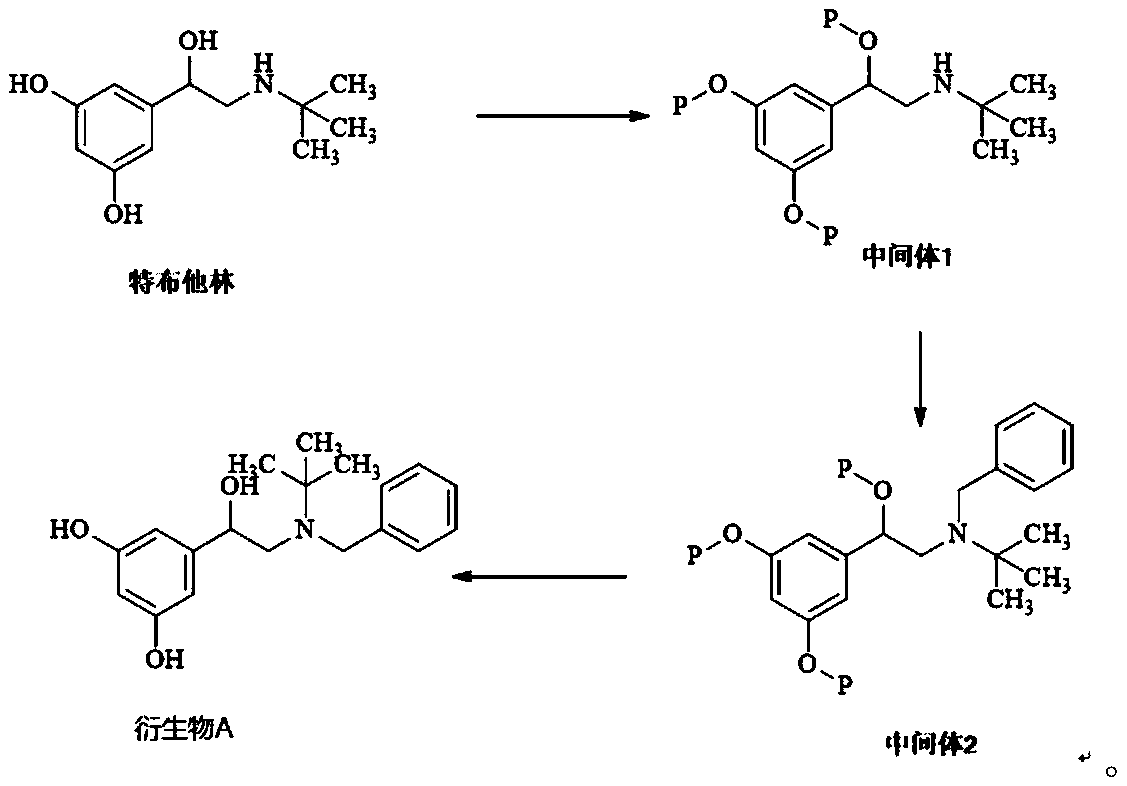
[0037] Such as figure 1 Shown, the preparation method of terbutaline derivative A comprises the following steps:
[0038] (1) Dissolve 20 g of terbutaline in 200 ml of acetonitrile, add 30 g of pyridine and 54 g of tert-butyldimethylchlorosilane to react at room temperature for two hours, concentrate, dilute with dichloromethane, wash with water, and concentrate to obtain 40 g of intermediate 1 , yield 85%;
[0039] (2) Dissolve 20g of intermediate 1 in dimethyl sulfoxide, add 10g of sodium bicarbonate and 9g of benzyl chloride, react at 40°C for 3 hours, add 300ml of water to dilute, extract with ethyl acetate, concentrate, and purify by column chromatography After obtaining about 14g of intermediate 2, the yield is 65%;
[0040] (3) Dissolve 7g of intermediate 2 in 70ml of tetrahydrofuran, add 20ml of dilute hydrochloric acid with a concentration of 4mol / L to reflux for 4 hours, adjust the pH to 7-8 with sodium hydroxide, extract with dichloromethane, and then purify by co...
Embodiment 2
[0042] Such as figure 1 Shown, the preparation method of terbutaline derivative A comprises the following steps:
[0043] (1) Dissolve 20 g of terbutaline in 200 ml of dimethyl sulfoxide, add 30 g of pyridine and 54 g of tert-butyldimethylchlorosilane to react at room temperature for two hours, concentrate, dilute with dichloromethane, wash with water, and concentrate to obtain 38g intermediate 1, yield 81%;
[0044] (2) Dissolve 20g of intermediate 1 in dimethyl sulfoxide, add 10g of sodium bicarbonate and 9g of benzyl chloride, react at 60°C for 2 hours, add 300ml of water to dilute, extract with ethyl acetate, concentrate, and purify by column chromatography After obtaining about 15g of intermediate 2, the yield is 61%;
[0045](3) Dissolve 14g of intermediate 2 in 150ml of tetrahydrofuran, add 40ml of dilute hydrochloric acid with a concentration of 4mol / L to reflux for 4 hours, adjust the pH to 7-8 with sodium hydroxide, extract with dichloromethane, and then purify by ...
Embodiment 3
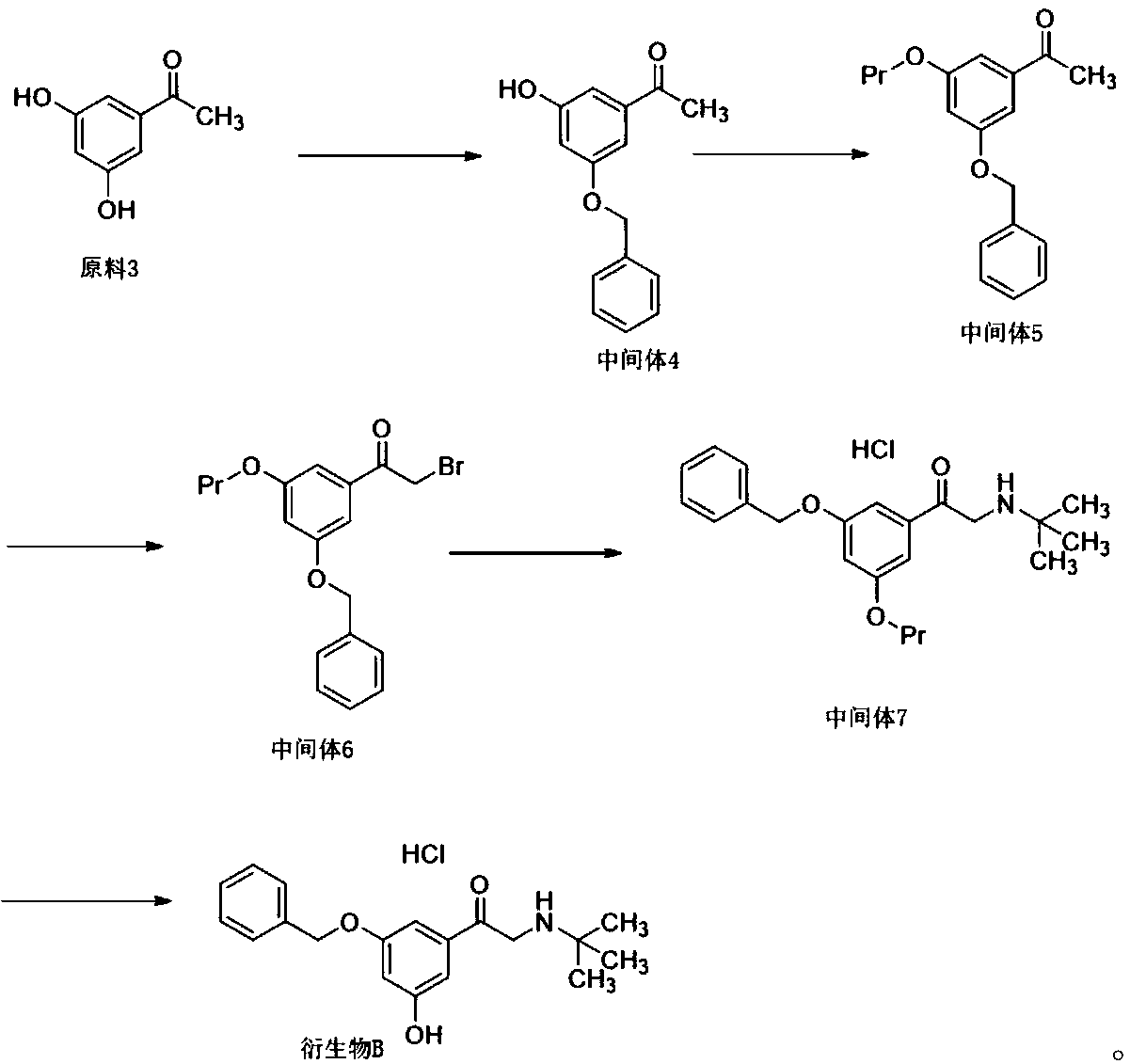
[0047] Such as figure 1 Shown, the preparation method of terbutaline derivative B comprises the following steps:
[0048] (1) Dissolve 60g of raw material 3 in 500ml of acetonitrile, add 100g of potassium carbonate and 67g of benzyl bromide to react at 40°C for 3 hours, add 600ml of water to dilute, extract with ethyl acetate, concentrate, and obtain about 80g of intermediate after purification by column chromatography 4. The yield is 84%;
[0049] (2) Dissolve 55g of intermediate 4 in 550ml of acetonitrile, add 51g of pyridine and 51g of tert-butyldimethylchlorosilane to react at room temperature for two hours, concentrate, dilute with dichloromethane, wash with water and concentrate to obtain 70g of intermediate 5, Yield 86%;
[0050] (3) Dissolve 70g of intermediate 5 in 700ml of acetonitrile, add 100g of N-bromosuccinimide, react at 60°C for 4 hours, concentrate and purify by column chromatography to obtain 70g of intermediate 6, with a yield of 82%;
[0051] (4) 25g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com