Compound 1-(2-benzyl-3,5-bis(benzyloxy)phenyl)ethanone, preparation method and application thereof
A compound, benzyl halide technology, applied in the field of medicinal chemistry, can solve problems such as affecting the purity of drugs, affecting the efficacy of drugs, and toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
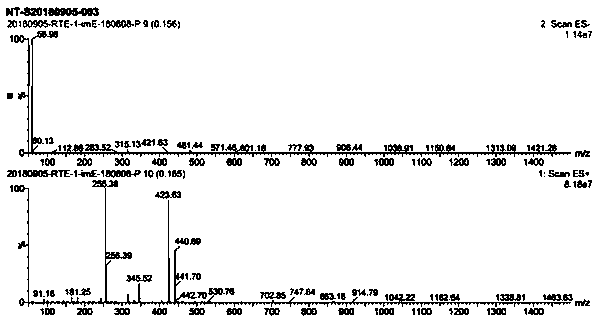
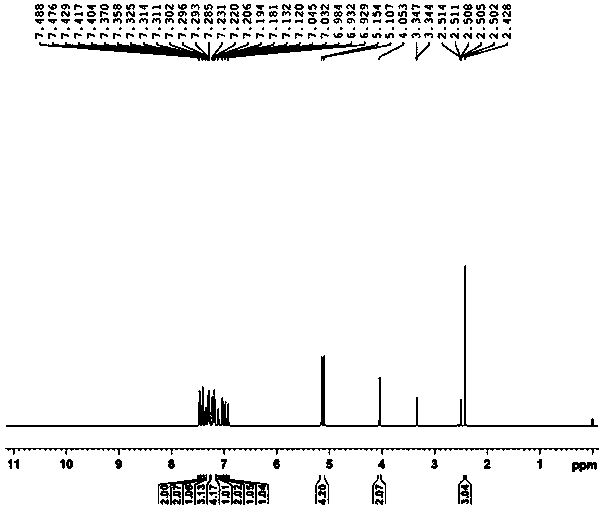
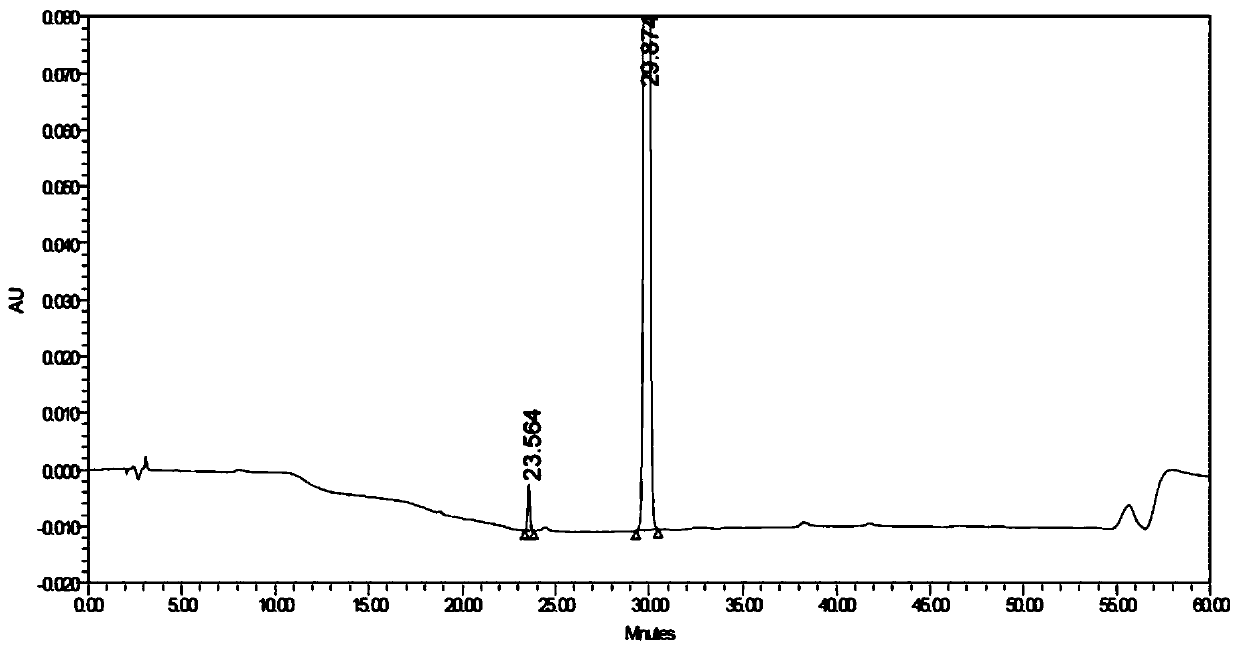
Image
Examples
Embodiment 1
[0040] Example 1 Preparation and purification of 1-(2-benzyl-3,5-bis(benzyloxy)phenyl)ethanone (compound of formula I) (1) Preparation of compound of formula I
[0041] Add 20g of the compound of formula II into a 500ml single-necked bottle, add 200ml of ethanol, 72g of potassium carbonate powder, and 55g of benzyl chloride in sequence, and stir the reaction at 80°C. After the completion of the reaction as detected by TLC, the temperature was lowered and suction filtered, and the filtrate was concentrated to dryness under reduced pressure at 45° C. to obtain a concentrate with a purity of 86.6%.
[0042] (2) purification of formula I compound
[0043] Dissolve 15g of the concentrate prepared in step (1) in 50ml of dichloromethane, add 25g of 200-300 mesh silica gel to mix the sample, use 200-300 mesh of 100g of silica gel to pack the column; elute with 500ml of petroleum ether, and then use 500ml of volume ratio 50:1 petroleum ether: ethyl acetate was used for elution, and fi...
Embodiment 2
[0047] Example 2 Preparation and purification of 1-(2-benzyl-3,5-bis(benzyloxy)phenyl)ethanone (compound of formula I)
[0048] (1) Preparation of formula I compound
[0049] Add 20g of the compound of formula II into a 500ml single-necked bottle, add 200ml of methanol, 60g of sodium hydroxide powder, and 55g of benzyl chloride in sequence, and stir the reaction at 60°C. After the completion of the reaction as detected by TLC, the temperature was lowered and suction filtered, and the filtrate was concentrated to dryness at 45°C under reduced pressure to obtain a concentrate with a purity of 72.3%.
[0050] (2) purification of formula I compound
[0051] Dissolve 15g of the concentrate prepared in step (1) in 50ml of ethyl acetate, add 25g of 200-300 mesh silica gel to mix the sample, use 200-300 mesh of 60g of silica gel to pack the column; elute with 1L petroleum ether, and then use 1L volume ratio 50:1 petroleum ether: ethyl acetate was used for elution, and finally 1 L of...
Embodiment 3
[0052] Example 3 Application of 1-(2-benzyl-3,5-bis(benzyloxy)phenyl)ethanone (compound of formula I) as a reference substance in the determination of impurity content of compound of formula III
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com