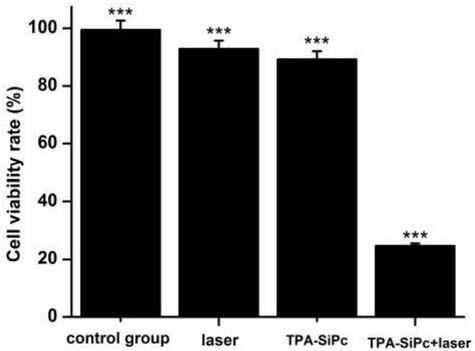
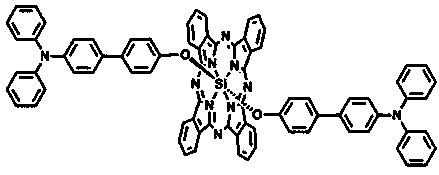
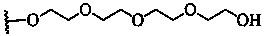
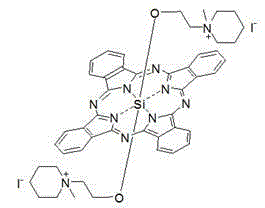
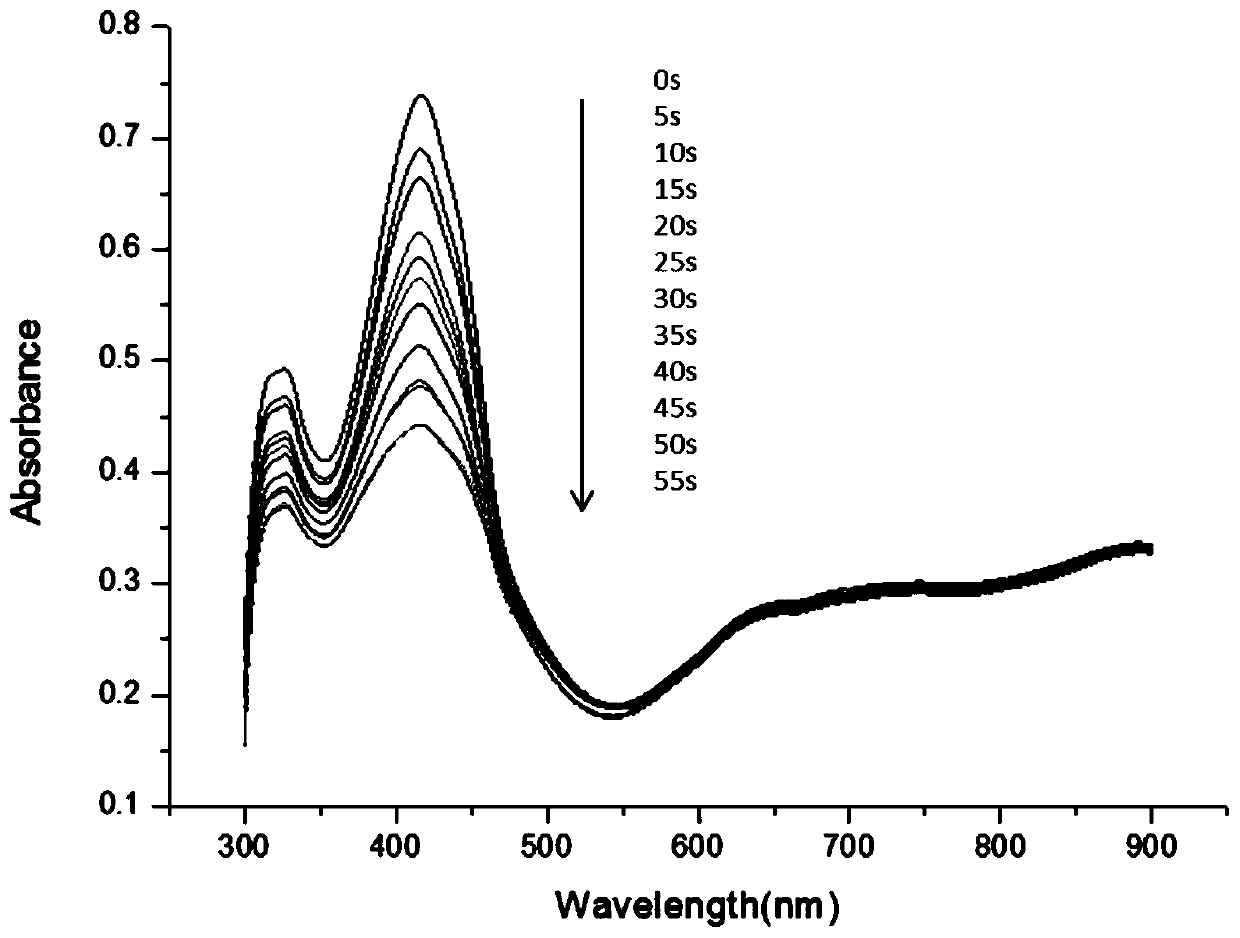
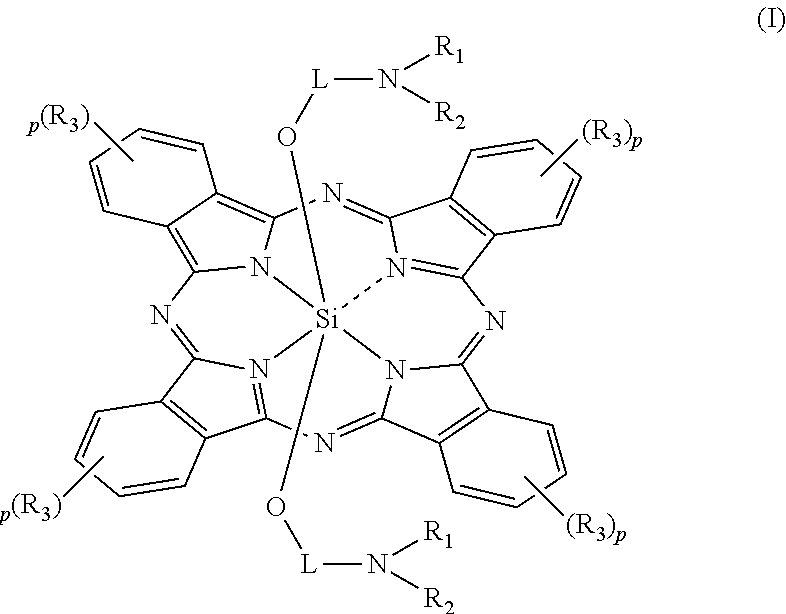
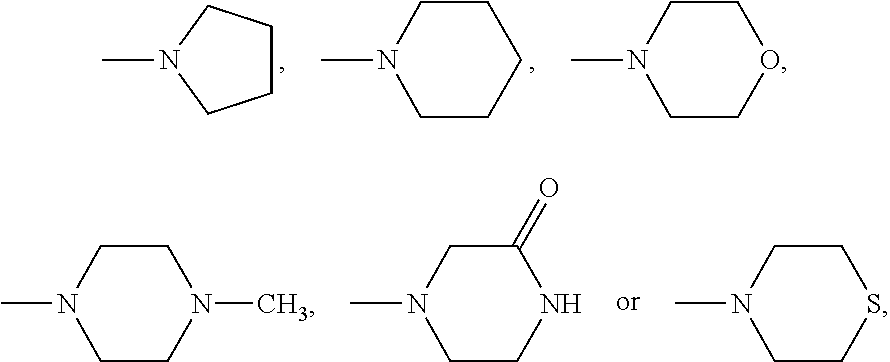
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "Silicon phthalocyanine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
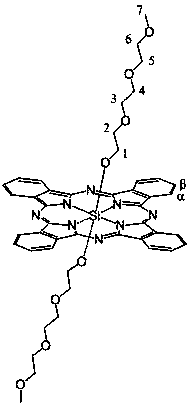
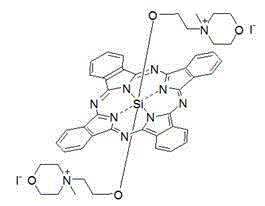
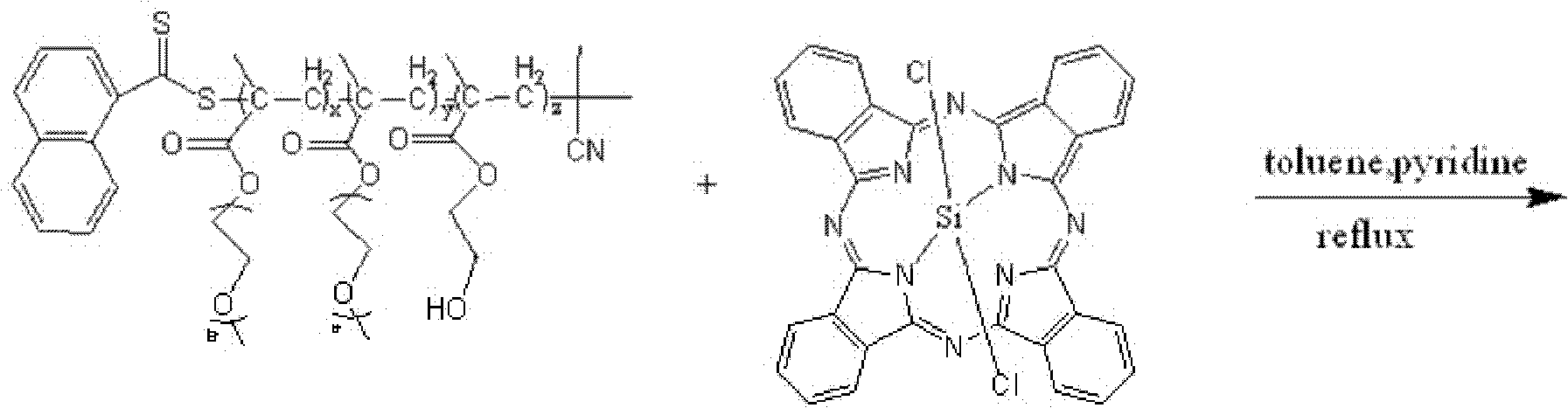
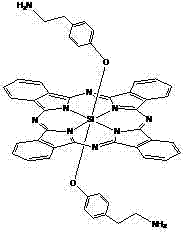
Axial end hydroxyl substituted silicon phthalocyanine and self-assembling body thereof
ActiveCN103864833AHigh photodynamic anticancer activityHigh activityAntibacterial agentsGroup 4/14 element organic compoundsPhotosensPharmaceutical drug
The invention discloses axial end hydroxyl substituted silicon phthalocyanine, a self-assembling body of silicon phthalocyanine, as well as a preparation method and application of silicon phthalocyanine, and belongs to the field of preparation of photodynamic medicines or photosensitizers. The axial end hydroxyl substituted silicon phthalocyanine disclosed by the invention can be used as a photosensitizer for photodynamic treatment, photodynamic diagnosis or photodynamic sterilization, and is high in photodynamic activity.
Owner:FUZHOU UNIV
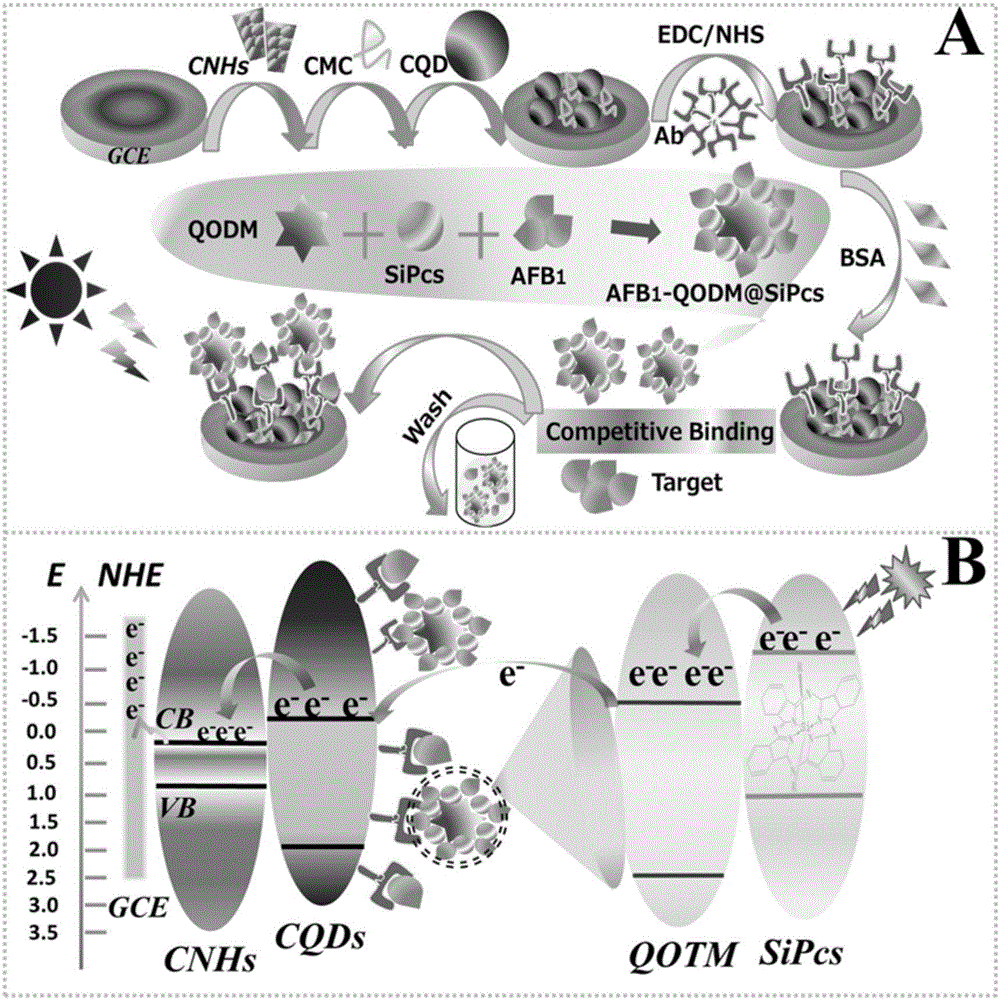
Silicon phthalocyanine functionalized TiO2 mesocrystal based aflatoxin photoelectrochemical detection method
InactiveCN106290514AImprove conductivityEasy transferMaterial electrochemical variablesCarbon nanocompositePhotocurrent
The invention discloses a silicon phthalocyanine functionalized TiO2 mesocrystal based aflatoxin photoelectrochemical detection method. The method is characterized in that a novel carbon nanocomposite formed by carbon nanohorns and carbon quantum dots serves as a sensor support; dendritic phthalocyanine functionalized quasi-octahedral TiO2 mesocrystal is introduced as a bioprobe to realize high-sensitivity detection of aflatoxin. The novel carbon nanocomposite formed by CNHs and CQDs has an excellent photoelectric property; SiPcs functionalized QOTM is superior in photocurrent response and stability as compared with single QOTM. An immunosensor enables mutual competition between standard AFB1 and marked AFB1 in an AFB1 antibody specificity combination process, and target object concentration and photocurrent intensity are in linear relation in a range of 10<-6>-10<2>ng / ml. The method which is universal is provided for ultra-sensitive detection of micromolecular substances.
Owner:FUJIAN NORMAL UNIV
Electrostatic latent image developing toner and method of image forming
ActiveUS20090291382A1High saturationQuality improvementDevelopersElectrographic processes using charge patternWaxLatent image
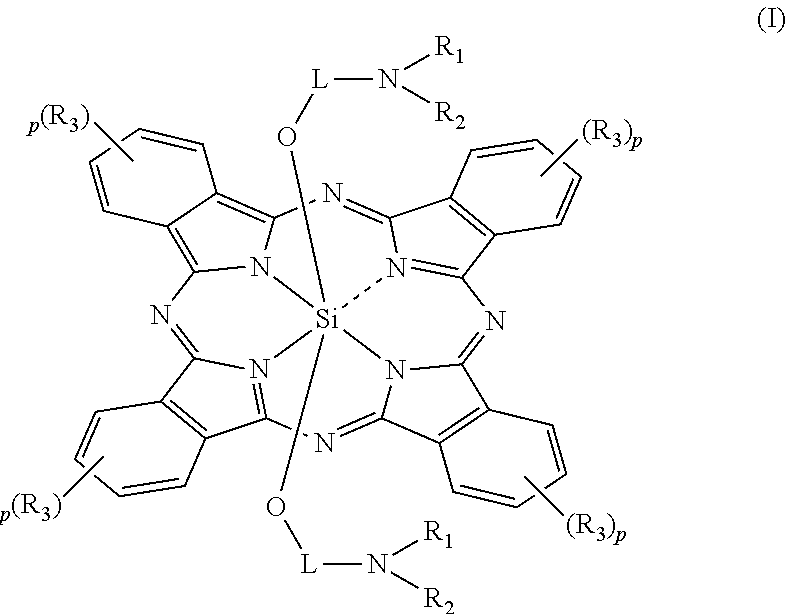
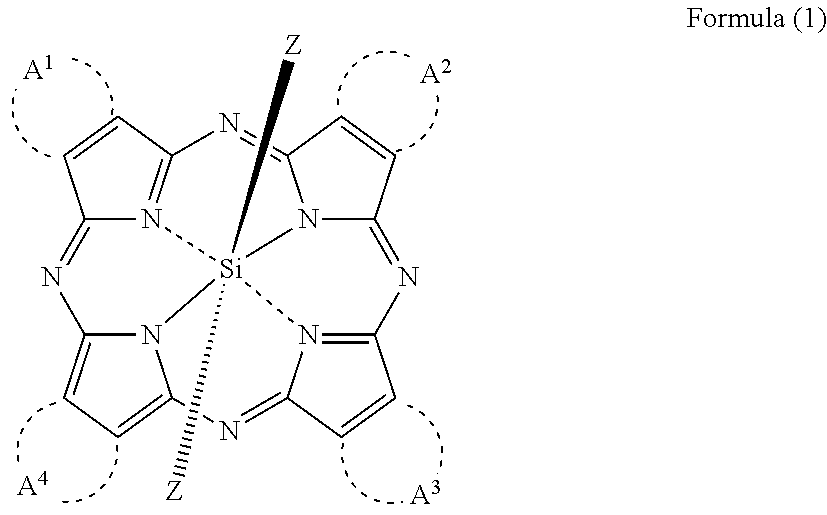
An electrostatic latent image developing toner comprising at least a resin, a wax and a colorant, wherein the wax comprises 40 to 98% by mass of a first release agent comprising an ester wax and 2 to 60% by mass of a second release agent comprising a hydrocarbon having at least one of a branched chain structure and a cyclic structure; and the colorant comprises a silicon phthalocyanine represented by Formula (I):
Owner:KONICA MINOLTA BUSINESS TECH INC
Electrostatic latent image developing toner and method of image forming
An electrostatic latent image developing toner comprising at least a resin, a wax and a colorant, wherein the wax comprises 40 to 98% by mass of a first release agent comprising an ester wax and 2 to 60% by mass of a second release agent comprising a hydrocarbon having at least one of a branched chain structure and a cyclic structure; and the colorant comprises a silicon phthalocyanine represented by Formula (I):
Owner:KONICA MINOLTA BUSINESS TECH INC
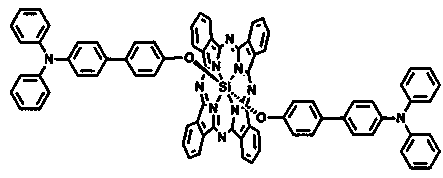
Triphenylamine-based branch ligand substituted silicon phthalocyanine, preparation method and application thereof
ActiveCN108948060AInhibition of self-aggregationImproved Aggregation Fluorescence Quenching PerformanceSilicon organic compoundsEnergy modified materialsTetrakis(triphenylphosphine)palladium(0)Aggregation-induced emission
The invention discloses a triphenylamine-based branch ligand substituted silicon phthalocyanine, a preparation method and an application thereof. 4-bromotriphenylamine and 4-hydroxy phenylboronic acidare catalytically coupled through tetrakis (triphenylphosphine) palladium, so as to acquire a branch precursor 4'-(diphenyl amino)-[1,1'-biphenyl]-4-alcohol; the 4'-(diphenyl amino)-[1,1'-biphenyl]-4-alcohol (TPA-OH) reacts with SiPcCl2 in the presence of methylbenzene and K2CO3, so as to acquire bi-(4-(diphenyl amino)-1-biphenylyloxy) axially substituted silicon phthalocyanine; steric hindranceof a triphenylamine substituted branch structure is capable of restraining the gathering of phthalocyanine to some extent; the triphenylamine with an aggregation-induced emission characteristic is introduced into the branch structure, so that the 'aggregation-induced quenching' effect of phthalocyanine is improved, the optical physical property of phthalocyanine is regulated and the simultaneous execution of fluorescence imaging and photodynamic therapy is realized; the triphenylamine-based branch ligand substituted silicon phthalocyanine can be used as a fluorescence imaging agent and a photodynamic therapy photosensitizer.
Owner:FUJIAN NORMAL UNIV
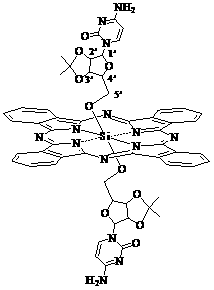
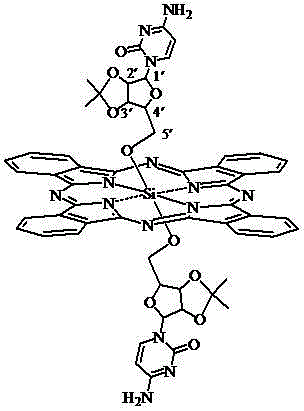
Axial nucleoside asymmetrically-modified silicon phthalocyanine and preparation method and application thereof
ActiveCN103435639AGood amphipathyGood biocompatibilityAntibacterial agentsGroup 4/14 element organic compoundsPhotosensSilicon phthalocyanine
The invention discloses axial nucleoside asymmetrically-modified silicon phthalocyanine and a preparation method and application thereof, belonging to the preparation field of a photodynamic medicine or a photosensitizer. The axial nucleoside asymmetrically-modified silicon phthalocyanine disclosed by the invention can be applied to photodynamic treatment, photodynamic diagnosis or photodynamic disinfection as a photosensitizer, has the structural characteristic of axial asymmetric substitution, and shows good amphipathicity and excellent photodynamic activity.
Owner:FUZHOU UNIV
Axial ALA-modified silicon phthalocyanine and preparation method and application thereof
ActiveCN104844645AGood sexGood synergistic anticancer effectSilicon organic compoundsEnergy modified materialsPhotosensSilicon phthalocyanine
The invention discloses axial ALA-modified silicon phthalocyanine and a preparation method and an application thereof, and belongs to the field of photodynamic drug or photosensitizer preparation. The silicon phthalocyanine provided by the invention can be used in photodynamic treatment, photodynamic diagnosis or photodynamic disinfection as a photosensitizer. The silicon phthalocyanine has a structure containing a second-generation photosensitizer ALA. Cells can release two photosensitizers, namely ALA and silicon phthalocyanine. Photodynamic therapy window is expanded. The silicon phthalocyanine provided by the invention shows a good synergistic anticancer effect and a high photodynamic activity.
Owner:FUZHOU UNIV
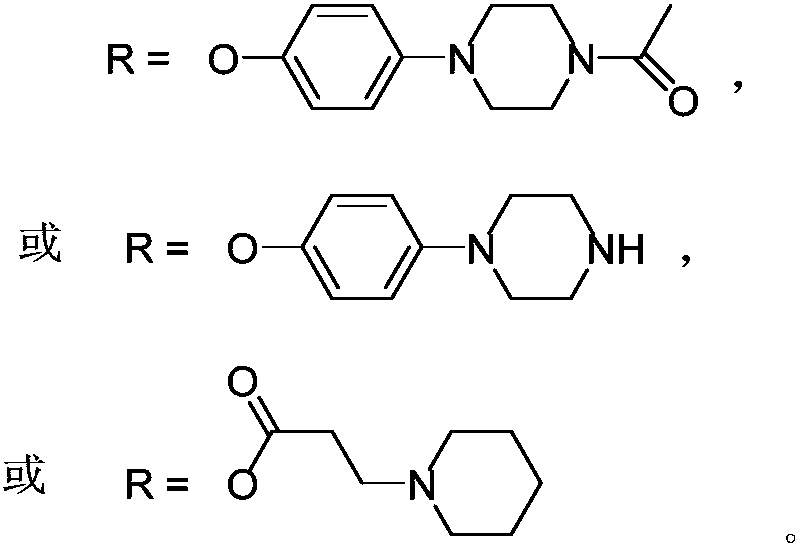
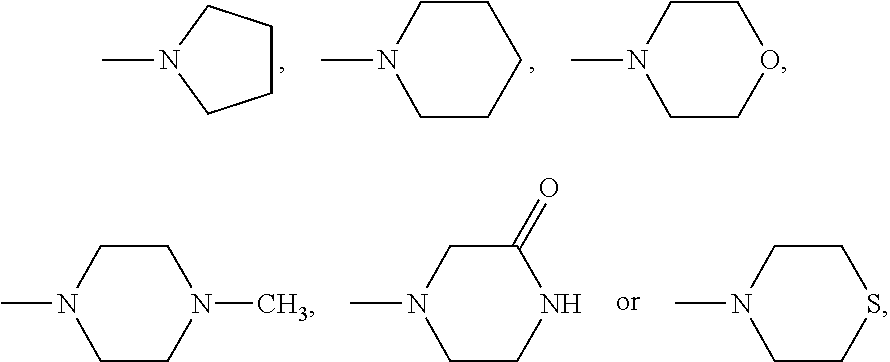
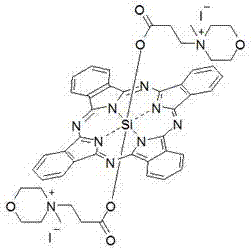
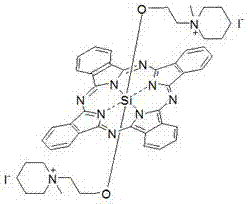
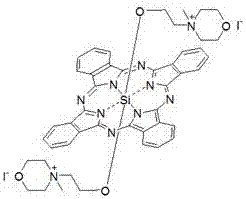
Silicon phthalocyanine axially bonded with piperidine or morpholine derivative with ester bond
ActiveCN104650129AGood amphipathyStrong penetrating powerAntibacterial agentsGroup 4/14 element organic compoundsPhotosensMorpholine
The invention discloses a silicon phthalocyanine axially bonded with a piperidine or morpholine derivative with an ester bond as well as a preparation method and application of the silicon phthalocyanine and belongs to the field of preparing photodynamic drugs or photosensitizers. The silicon phthalocyanine can be used as a photosensitizer used for carrying out photodynamic antibacterium, photodynamic cancer resistance, photodynamically treating other diseases, photodynamic disinfection and photodynamic diagnosis and has the advantages of simple preparation, strong photosensitization and the like, and the maximum absorption spectrum is located in a near infrared region.
Owner:FUZHOU UNIV
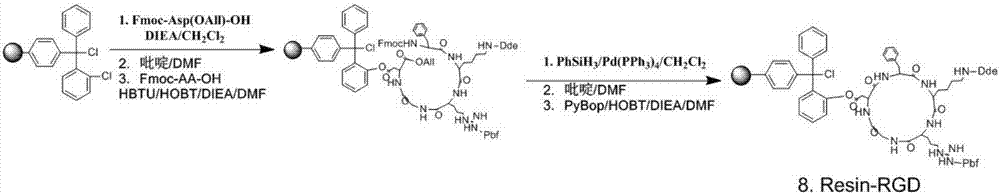
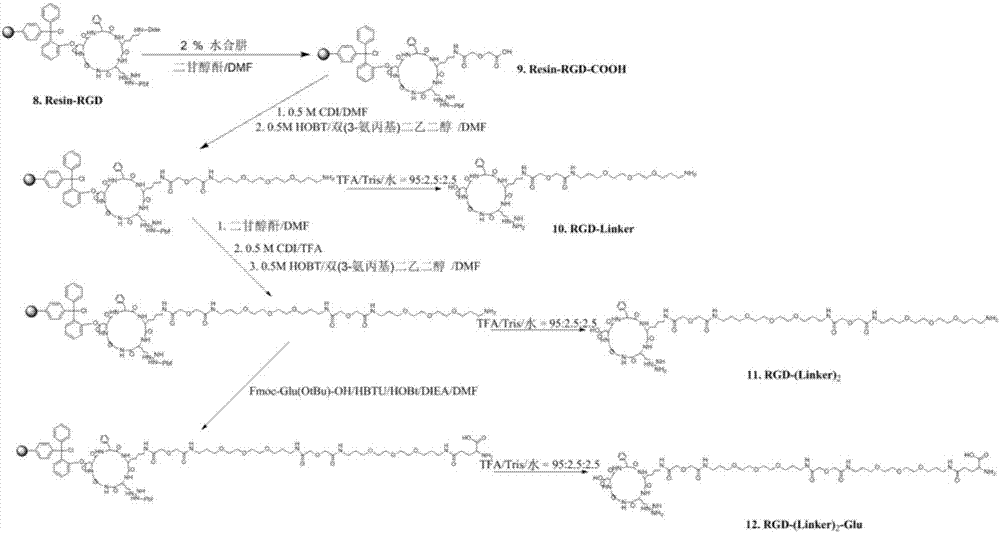
Synthesis and application of RGD polypeptide coupled SiPc (silicon phthalocyanine) photosensitizer
ActiveCN107226839AGood water solubilityResolve aggregationEnergy modified materialsPeptide preparation methodsTumor targetTumor targeting
The invention relates to a SiPc (silicon phthalocyanine) photosensitizer using RGD polypeptide as a targeted group, a preparation method of the SiPc photosensitizer and application of the SiPc photosensitizer to an aspect of tumor photodynamics therapy. The SiPc is used as a photosensitive part to be connected with an RGD polypeptide ligand; PEG (polyethylene glycol) and fragments containing carboxy groups are introduced into the structure; a series of novel photosensitizers with the tumor targeting performance can be prepared. One coupling compound RGD-(Linker)2-Glue-SiPc has good optical physic and photodynamic performance; the EC50 value on the receptor positive tumor cells is 10 to 20nm; under the condition of once medication, the spongioblastoma on the mouse body can be cured; after the continuous observation for 35 days, recurrence does not occur; good application prospects are shown in the field of tumor photodynamics therapy.
Owner:KANGHONG YAOYUAN TECH CO LTD TIANJIN
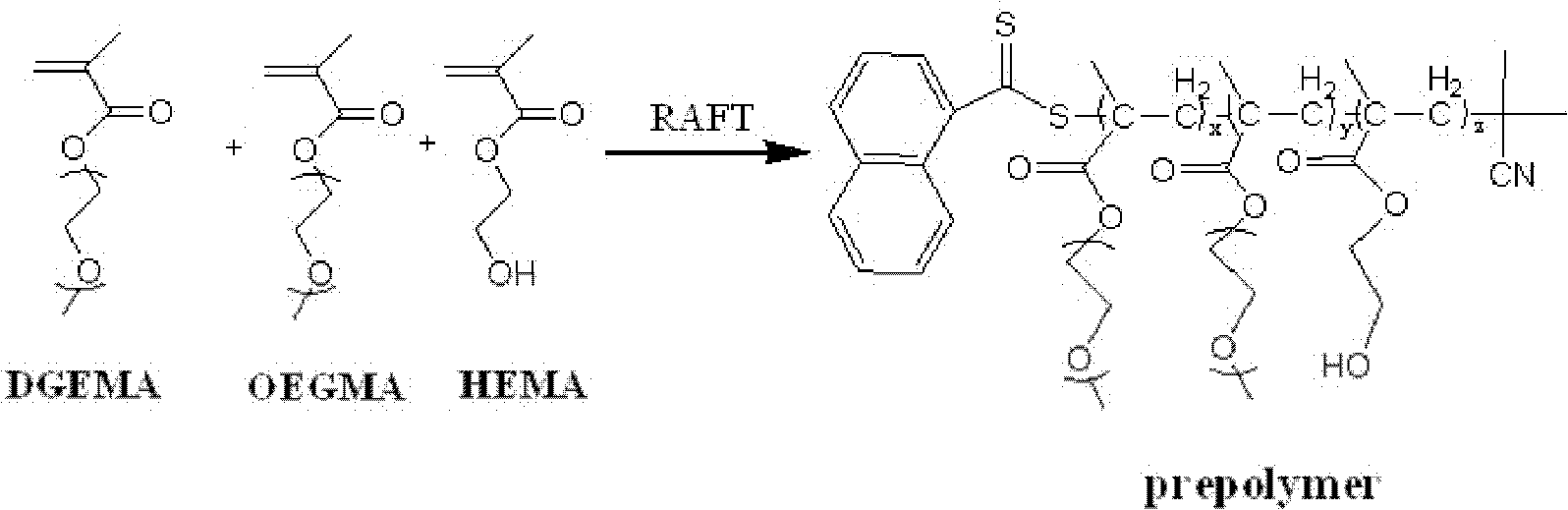
Multi-targeted photodynamic therapy polymer carrier and preparation method thereof
InactiveCN102432755AThere will be no problem with partial releaseImprove stabilityEnergy modified materialsPharmaceutical non-active ingredientsQuantum yieldCancer cell
The invention discloses a preparation method of a multi-targeted photodynamic therapy polymer carrier. The preparation method comprises the following steps of: firstly, preparing a thermosensitive prepolymer: carrying out reversible addition-fragmentation chain transfer polymerization on diethylene glycol dimethacrylate, polyethylene glycol methacrylate and methacrylic-2-ethyl hydroxyl as reactants to obtain the thermosensitive prepolymer; and secondly, by taking the thermosensitive prepolymer and silicon phthalocyanine dichloride as reactants, connecting the silicon phthalocyanine dichloride onto a thermosensitive prepolymer chain segment by a chemical bond method to obtain the multi-targeted photodynamic therapy polymer carrier. According to the preparation method disclosed by the invention, the multi-targeted photodynamic therapy polymer carrier is synthesized and is a water-soluble thermosensitive nano-carrier; the multi-targeted photodynamic therapy polymer carrier can be gathered in cells by regulating and controlling the temperature and a high-transparent retention effect of solid tumors in a targeted manner and further the photodynamic therapy is carried out; and singlet oxygen has quantum yield as high as 0.55 and can be used for further photodynamic therapy.
Owner:SUZHOU UNIV
Silicon phthalocyanine modified by cytidine derivative and preparation method and application thereof
ActiveCN102827228AEasy to manufactureFast productionAntibacterial agentsOrganic active ingredientsPhotosensPharmaceutical drug
The invention discloses silicon phthalocyanine modified by a cytidine derivative and a preparation method and an application thereof, which belong to the field of preparation of photodynamic medicaments or photosensitizers. The silicon phthalocyanine modified by the cytidine derivative provided by the invention can be taken as a photosensitizer for use in photodynamic therapy, photodynamic diagnosis or photodynamic disinfection, has the advantages of high selectivity, high photodynamic activity and the like, has definite constitution, is easy and convenient to prepare, and is easy to be industrialized.
Owner:FUZHOU UNIV
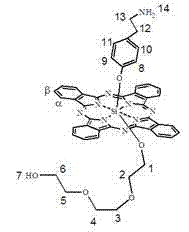
Silicon phthalocyanine axially modified by aminoethyl phenoxyl and polyethylene glycol oligomer
ActiveCN103254223AGood amphipathyAsymmetric instead of uniqueAntibacterial agentsBiocidePhotosensOligomer
The invention discloses silicon phthalocyanine axially modified by aminoethyl phenoxyl and polyethylene glycol oligomer as well as a preparation method and application of silicon phthalocyanine, belonging to the field of preparation of photodynamic medicaments or photosensitizers. The silicon phthalocyanine provided by the invention can be used as a photosensitizer for photodynamic treatment, photodynamic diagnosis or photodynamic sterilization, has the structural characteristic of axially asymmetrical substitution and shows favorable amphipathy and extremely high photodynamic activity.
Owner:FUZHOU UNIV
Silicon phthalocyanine modified by uridine derivatives and preparation method and application of silicon phthalocyanine
ActiveCN102827226AEasy to manufactureFast productionAntibacterial agentsSenses disorderPhotosensPharmaceutical drug
The invention discloses silicon phthalocyanine modified by uridine derivatives and a preparation method and application of the silicon phthalocyanine, and belongs to the field of preparation of photodynamic drugs or photosensitizer. The silicon phthalocyanine modified by the uridine derivatives can be used as a photosensitizer for photodynamic therapy, photodynamic diagnosis or photodynamic disinfection, has the advantages of high selectivity, high photodynamic activity and the like, and is explicit in composition and easy to prepare and industrialize.
Owner:FUZHOU UNIV
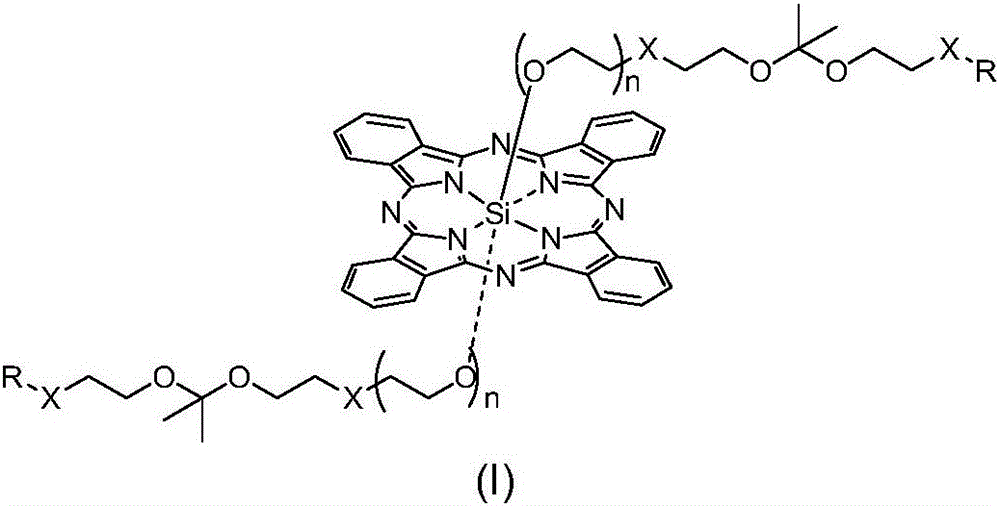
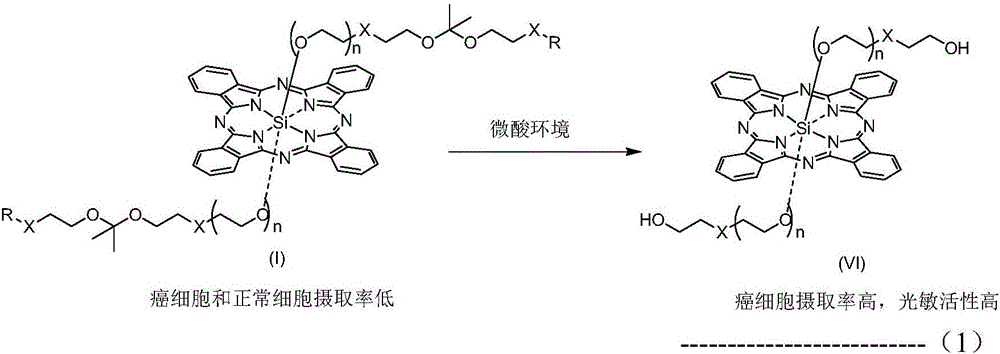
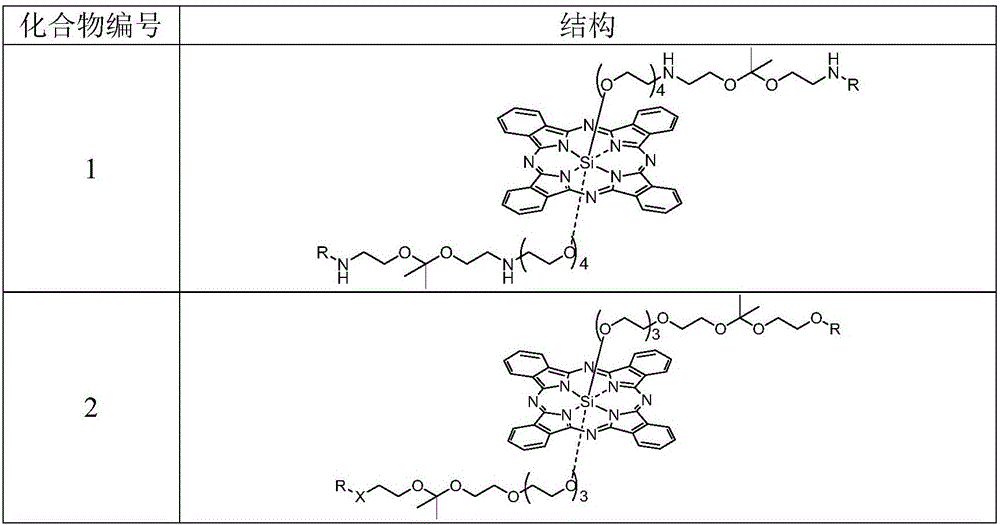
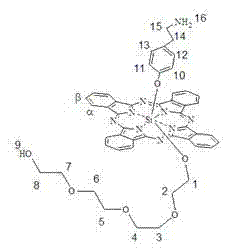
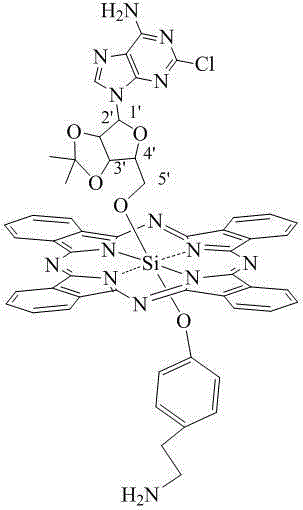
pH sensitive axially substituted silicon phthalocyanine complex, preparing method of pH sensitive axially substituted silicon phthalocyanine complex and application of pH sensitive axially substituted silicon phthalocyanine complex to medicines
The invention relates to a pH sensitive ketal connection cholesterol-silicon phthalocyanine complex, a preparing method of the pH sensitive ketal connection cholesterol-silicon phthalocyanine complex and application of the pH sensitive ketal connection cholesterol-silicon phthalocyanine complex to medicines. In particular, the invention relates to a silicon phthalocyanine complex shown in the general formula (I), a preparing method of the silicon phthalocyanine complex, a medicine component containing the complex and application of the silicon phthalocyanine complex as a photosensitizer, in particular to application to cancer treatment. All substituting groups in the general formula (I) are the same as definitions in the specification. Due to existence of cholesterol groups, the complex in the series are difficultly taken by cancer cells and normal cells, but in the cancer tissue extracellular micro acid environment, ketal keys are subject to hydrolysis reactions, hydrolysis derivatives of silicon phthalocyanine can be easily taken by the cancer cells, and the extremely-high photosensitive activity is shown; and the complex and the component can be prepared into cancer cell extracellular micro acid environment target photosensitive medicines.
Owner:SHENZHEN SONO PHOTODYNAMIC BIO MED TECH LTD
Method for preparing silica particles containing a phthalocyanine derivative, said particles and uses thereof
InactiveUS20120156495A1Silicon organic compoundsIndividual molecule manipulationSilica particleEmulsion
A method for preparing a silica particle incorporating at least one phthalocyanine derivative is provided. In the method, the particle may be prepared from at least one silicon phthalocyanine derivative via an inverse micro-emulsion. In addition, the silica particles and their uses are provided.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
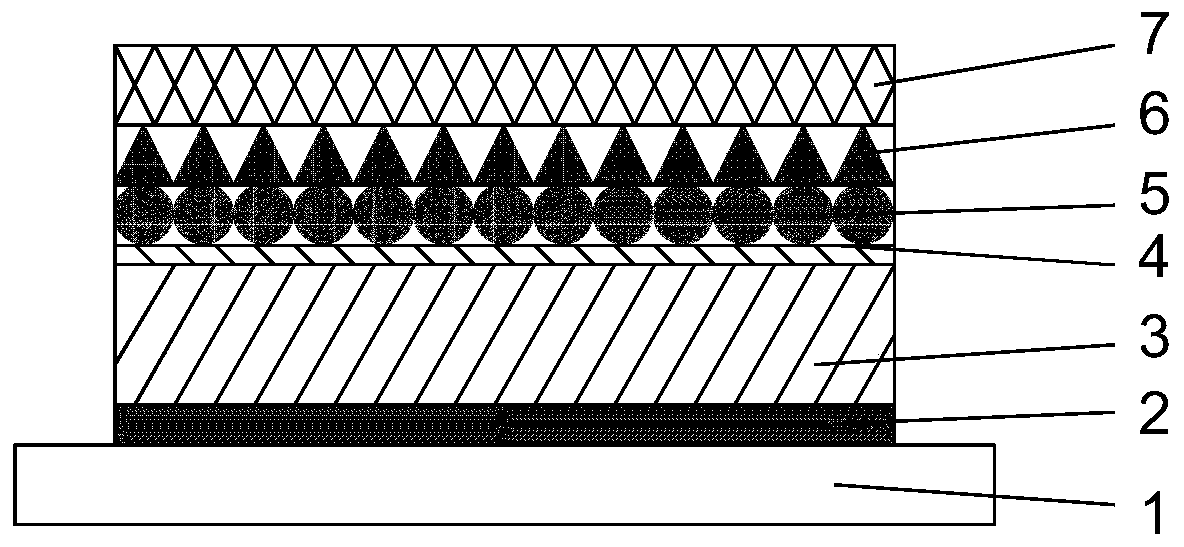
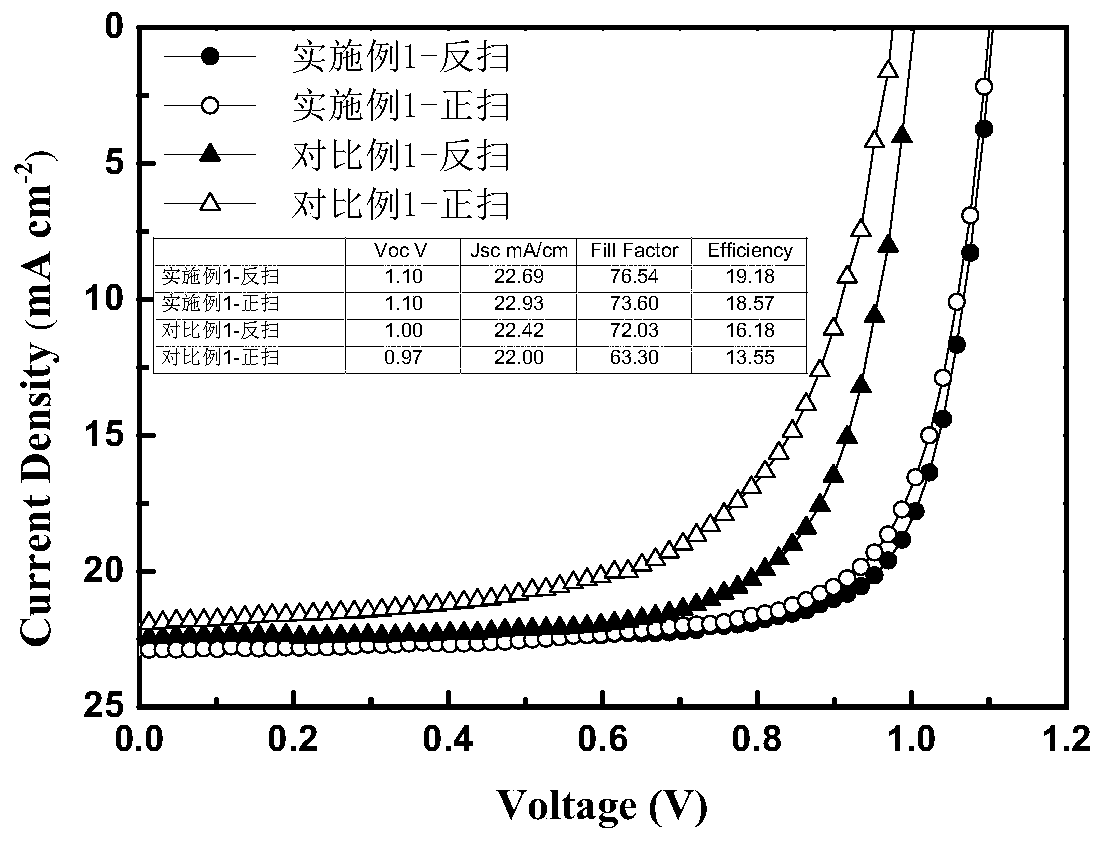
Non-fullerene perovskite solar cell and preparation method thereof
ActiveCN110085753AGood chemical stabilityImprove thermal stabilitySolid-state devicesSemiconductor/solid-state device manufacturingPerovskite solar cellHysteresis phenomenon
The invention discloses a non-fullerene perovskite solar cell which comprises a conductive substrate, a hole transmission layer, a light absorption layer, an interface passivation layer, a non-fullerene electron transmission layer, a hole barrier layer and a back electrode which are arranged from the bottom to the top. The interface passivation layer is a silicon phthalocyanine layer. The siliconphthalocyanine is used as the interface passivation layer material to construct the high-efficiency non-fullerene perovskite solar cell so as to improve the hysteresis phenomenon of photocurrent, remarkably improve the photoelectric conversion efficiency and stability and provides a new idea for constructing the low-cost, high-efficiency and stable non-fullerene perovskite solar cell.
Owner:CENT SOUTH UNIV
Axial galactose/lactose modified silicon phthalocyanine as well as preparation method and application thereof
InactiveCN111393465AGood medicineGood biocompatibilitySilicon organic compoundsEnergy modified materialsPhotosensMedicine
The invention discloses axial galactose / lactose modified silicon phthalocyanine as well as a preparation method and an application thereof, which belong to the field of preparation of photodynamic drugs or photosensitizers. The axial galactose / lactose modified silicon phthalocyanine provided by the invention has the advantages of non-aggregation in a physiological solution, high activity, high biological safety and the like, and can be used as a photosensitizer for photodynamic therapy, photodynamic diagnosis or photodynamic disinfection.
Owner:FUZHOU UNIV
Triphenylamino dendrimer ligand substituted silicon phthalocyanine and its preparation method and application
ActiveCN108948060BInhibit aggregation behaviorImproved quenching (ACQ)" effectSilicon organic compoundsEnergy modified materialsDendrimerImaging agent
The invention discloses a triphenylamine-based branch ligand substituted silicon phthalocyanine, a preparation method and an application thereof. 4-bromotriphenylamine and 4-hydroxy phenylboronic acidare catalytically coupled through tetrakis (triphenylphosphine) palladium, so as to acquire a branch precursor 4'-(diphenyl amino)-[1,1'-biphenyl]-4-alcohol; the 4'-(diphenyl amino)-[1,1'-biphenyl]-4-alcohol (TPA-OH) reacts with SiPcCl2 in the presence of methylbenzene and K2CO3, so as to acquire bi-(4-(diphenyl amino)-1-biphenylyloxy) axially substituted silicon phthalocyanine; steric hindranceof a triphenylamine substituted branch structure is capable of restraining the gathering of phthalocyanine to some extent; the triphenylamine with an aggregation-induced emission characteristic is introduced into the branch structure, so that the 'aggregation-induced quenching' effect of phthalocyanine is improved, the optical physical property of phthalocyanine is regulated and the simultaneous execution of fluorescence imaging and photodynamic therapy is realized; the triphenylamine-based branch ligand substituted silicon phthalocyanine can be used as a fluorescence imaging agent and a photodynamic therapy photosensitizer.
Owner:FUJIAN NORMAL UNIV
Application of piperazidine substituted silicon phthalocyanine and application thereof to photothermal therapy
ActiveCN107789623AHigh biosecurityHigh photothermal conversion efficiencyGroup 4/14 element organic compoundsEnergy modified materialsNear infrared laserMedicine
The invention discloses application of piperazidine substituted silicon phthalocyanine and application thereof to photothermal therapy, and belongs to the field of a photothermal agent or photothermaltherapy medicine. A solution containing the silicon phthalocyanine provided by the invention has the obvious photoinduced temperature rising effect under the illumination of near infrared laser; thephotothermal therapy can be realized; meanwhile, the photodynamic active oxygen generation effect is also achieved; the cooperated effect of photodynamic therapy and photodynamic therapy can be achieved.
Owner:FUZHOU UNIV
Method for preparing silica particles containing a phthalocyanine derivative, said particles, and uses thereof
InactiveCN102639541ASilicon organic compoundsPorphines/azaporphinesSilica particlePhthalocyanine derivatives
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Silicon phthalocyanine axially modified by aminoethyl phenoxyl and polyethylene glycol oligomer
ActiveCN103254223BGood amphipathyAsymmetric instead of uniqueAntibacterial agentsBiocidePhotosensOligomer
The invention discloses silicon phthalocyanine axially modified by aminoethyl phenoxyl and polyethylene glycol oligomer as well as a preparation method and application of silicon phthalocyanine, belonging to the field of preparation of photodynamic medicaments or photosensitizers. The silicon phthalocyanine provided by the invention can be used as a photosensitizer for photodynamic treatment, photodynamic diagnosis or photodynamic sterilization, has the structural characteristic of axially asymmetrical substitution and shows favorable amphipathy and extremely high photodynamic activity.
Owner:FUZHOU UNIV
Positively charged water-soluble arm type dendritic ligand silicon phthalocyanine complexes as well as preparation methods and application thereof
ActiveCN107474064AGood water solubilityInhibition of self-aggregationAntibacterial agentsSilicon organic compoundsIsoquinolineQuinoline
The invention discloses positively charged water-soluble arm type dendritic ligand silicon phthalocyanine complexes as well as preparation methods and an application thereof. The complexes comprise di-(3,5-di(1',3'-methylpyridine propoxy)butanyloxy) axially substituted silicon phthalocyanine and di-(3,5-di(1',4'-isoquinoline butanyloxy)butanyloxy) axially substituted silicon phthalocyanine, wherein di-(3,5-di(1',3'-methylpyridine propoxy)butanyloxy) axially substituted silicon phthalocyanine is prepared from 3,5-di(1',3'-methylpyridine propoxy) phenyl methanol and silicon(IV) phthalocyanine dichloride or from 3,5-di-bromopropoxy) phenyl methanol and excessive methylpyridine and silicon(IV) phthalocyanine dichloride; di-(3,5-di(1',4'-isoquinoline butanyloxy)butanyloxy) axially substituted silicon phthalocyanine is prepared from 3,5-di(1',4'-quinoline butoxy) phenyl methanol and silicon(IV) phthalocyanine dichloride, or from 3,5-di(bromobutoxy) phenyl methanol and excessive isoquinoline and silicon(IV) phthalocyanine dichloride. The application of the complexes as a photosensitizer in photodynamic therapy is disclosed, especially the application to photoinactivation of MDRAB (multidrug-resistant acinetobacter baumannii).
Owner:FUJIAN NORMAL UNIV
Method and system for aromatic macrocyclic compounds (phthalocyanines) as cathode additives for inhibition of transition metal dissolution and stable solid electrolyte interphase formation
ActiveUS11456457B2Negative electrodesPositive electrodesAluminum phthalocyanine chlorideCobalt phthalocyanine
Owner:ENEVATE CORP
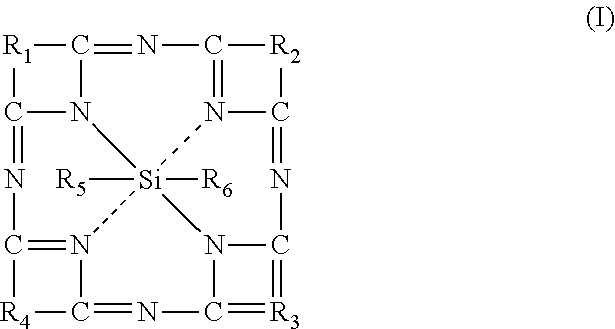
Silicon phthalocyanine complex, preparation method and medicinal application thereof
ActiveUS20170002028A1Improve accessibilityImprove propertiesSilicon organic compoundsPhotodynamic therapySilicon phthalocyanineCoordination complex
The present invention relates to a silicon phthalocyanine complex, the preparation method and the medicinal application thereof. The present invention particularly relates to a silicon phthalocyanine complex of formula (I), the preparation method thereof and a pharmaceutical composition comprising the same, as well as the use thereof as a photosensitizer, in particular the use in the treatment of cancers, wherein each substituent in formula (I) is the same as defined in the description.
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD
Aminosilicone phthalocyanine-graphene oxide composite nanomaterial, its preparation method and application
ActiveCN107746411BGood treatment effectExcellent photothermal performanceGroup 4/14 element organic compoundsEnergy modified materialsCancer cellOxide composite
The invention relates to an aminophthalocyaninosilicon-graphene oxide composite nano-material, and a preparation method and an application thereof. The aminophthalocyaninosilicon-graphene oxide composite nano-material is a compound formed through bonding aminophthalocyaninosilicon to graphene oxide through a covalent bond; and the composite nano-material has good photodynamic therapy and photothermal therapy synergism in in-vitro cell experiments and mouse in-vivo experiments, and improves the fatality and the therapy efficiency of cancer cells. The invention also provides the preparation method of the aminophthalocyaninosilicon-graphene oxide composite nano-material. The method has the advantages of simple process and wide application range.
Owner:SHANDONG UNIV
Silicon phthalocyanine complex, preparation method and medicinal application thereof
The present invention relates to a silicon phthalocyanine complex, the preparation method and the medicinal application thereof. The present invention particularly relates to a silicon phthalocyanine complex of formula (I), the preparation method thereof and a pharmaceutical composition comprising the same, as well as the use thereof as a photosensitizer, in particular the use in the treatment of cancers, wherein each substituent in formula (I) is the same as defined in the description.
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD
A silicon phthalocyanine with an axial ester bond linking piperidine or morpholine derivatives
ActiveCN104650129BGood amphipathyStrong penetrating powerAntibacterial agentsGroup 4/14 element organic compoundsDiseaseMorpholine
The invention discloses a silicon phthalocyanine axially bonded with a piperidine or morpholine derivative with an ester bond as well as a preparation method and application of the silicon phthalocyanine and belongs to the field of preparing photodynamic drugs or photosensitizers. The silicon phthalocyanine can be used as a photosensitizer used for carrying out photodynamic antibacterium, photodynamic cancer resistance, photodynamically treating other diseases, photodynamic disinfection and photodynamic diagnosis and has the advantages of simple preparation, strong photosensitization and the like, and the maximum absorption spectrum is located in a near infrared region.
Owner:FUZHOU UNIV
A kind of axial nucleoside asymmetrically modified silicon phthalocyanine and its preparation method and application
ActiveCN103435639BGood amphipathyGood biocompatibilityAntibacterial agentsGroup 4/14 element organic compoundsPhotosensPharmaceutical drug
The invention discloses a silicon phthalocyanine asymmetrically modified by axial nucleosides, a preparation method and application thereof, and belongs to the field of preparation of photodynamic drugs or photosensitizers. The axial nucleoside asymmetrically modified silicon phthalocyanine provided by the present invention can be used as a photosensitizer for photodynamic therapy, photodynamic diagnosis or photodynamic disinfection. It has the structural characteristics of axial asymmetric substitution and shows good amphiphile and high photodynamic activity.
Owner:FUZHOU UNIV
A kind of axially substituted phthalocyanine silicon complex and its doxorubicin conjugate
ActiveCN105669735BGood amphipathyIncrease uptakeOrganic active ingredientsSilicon organic compoundsChemotherapy effectsDrugs preparations
Owner:FUZHOU UNIV
Production process for colorant, colorant composition, toner, ink for ink jet recording and color filter
InactiveUS20130260306A1Improve performanceHigh color reproductionInksOrganic dyesSilicon phthalocyanineLightfastness
Provided is a production process for a colorant which contains a silicon phthalocyanine compound making it possible to reach a targeted particle diameter even by a conventional dispersing method and which is excellent in performances such as a color reproducibility, a light fastness, an electrostatic property, a transparency and the like, and a colorant composition, a toner, an ink for ink jet recording and a color filter which are excellent in the above performances.The above production process is a production process for a colorant containing a silicon phthalocyanine compound and a copper phthalocyanine compound and is characterized by having a preparing step of reacting raw materials of a silicon compound and phthalocyanine under the presence of a copper salt or the copper phthalocyanine compound described above to prepare the silicon phthalocyanine compound described above.
Owner:KONICA MINOLTA BUSINESS TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com