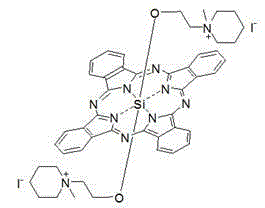
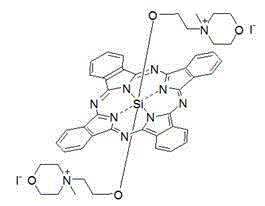
Silicon phthalocyanine axially bonded with piperidine or morpholine derivative with ester bond
A technology of morpholine derivatives and silicon phthalocyanine, which is applied in the field of photodynamic drug or photosensitizer preparation, can solve the problems of high skin phototoxicity, limited clinical application, complex synthesis route, etc., and achieves clear structure and improved tissue penetration ability. , easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis and physical and chemical properties of bis[3-(N-piperidinyl)propyl ester group]silicone phthalocyanine (structure shown in the following formula):
[0038]
[0039] Under nitrogen protection, add dichlorosilyl phthalocyanine (244.7mg, 0.4mmol), 3-(N-piperidine)propionic acid 1.2-2.4mmol (preferably 2.0mmol) to toluene or xylene or dioxane 20 -50ml (preferably toluene, 30ml), reflux for 20-36 hours (preferably 24 hours). Remove the solvent by rotary evaporation in vacuo, dissolve with 100ml of dichloromethane, centrifuge to remove insoluble matter, extract the dichloromethane solution with water (3×100ml), collect the organic layer, then extract with dilute hydrochloric acid (0.1-0.5 mmol), and collect the aqueous layer. The aqueous layer was neutralized with 1M sodium hydroxide, and a blue precipitate was precipitated, centrifuged, washed with water, and dried in vacuum to obtain a blue product with a yield of 45%. The maximum absorption peak of the produ...
Embodiment 2
[0042] Synthesis and physical and chemical properties of bis[3-(N-methyl-N-piperidinyl) propyl ester group] silicon phthalocyanine diiodide (structure shown in the following formula):
[0043]
[0044] Under the protection of nitrogen, bis[3-(N-piperidinyl) propyl ester group] silicon phthalocyanine (0.023mmol), excess methyl iodide was added in chloroform (20ml), after reflux for 1~4 hours (preferably 2 hours) Stir at room temperature for 16-48 hours (preferably 24 hours). After filtering, the filter cake was washed three times with 50 ml of chloroform, and vacuum-dried to obtain the product with a yield of 73%. The maximum absorption peak of the product in DMF is located at 684 nm, and the maximum absorption wavelength in aqueous solution is located at 691-700 nm.
[0045] The structural characterization data of the product are as follows: HR-MS (ESI) m / z: 441.1888 [M-2I] 2+ ; 1 H NMR (DMSO-d6, 400MHz, ppm): δ9.70~9.82 (m, 8H, Pc-H α ), δ8.55~8.66 (m, 8H, Pc-H β ), δ...
Embodiment 3
[0047] Synthesis of bis[3-(N-morpholine) propyl ester group] silicon phthalocyanine (structure shown in the following formula):
[0048]
[0049] Under the protection of nitrogen, dichlorosilyl phthalocyanine (244.7mg, 0.4mmol), 3-(N-morpholine) propionic acid 1.2-2.4mmol (preferably 2.0mmol) was added to toluene or xylene or dioxane 20 -50ml (preferably toluene, 30ml), reflux for 20-36 hours (preferably 24 hours). Remove the solvent by rotary evaporation in vacuo, dissolve with 100ml of dichloromethane, centrifuge to remove insoluble matter, extract the dichloromethane solution with water (3×100ml), collect the organic layer, then extract with dilute hydrochloric acid (0.1-0.5 mmol), and collect the aqueous layer. The aqueous layer was neutralized with 1M sodium hydroxide, and a blue precipitate was precipitated, centrifuged, washed with water, and dried in vacuum to obtain a blue product with a yield of 43%. The maximum absorption peak of the product in DMF is located at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com