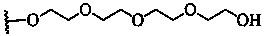
Axial nucleoside asymmetrically-modified silicon phthalocyanine and preparation method and application thereof
An asymmetric, silicon phthalocyanine technology, applied in the field of axial nucleoside asymmetrically modified silicon phthalocyanine and its preparation, can solve the problems of lack of tumor tissue and cancer cell selectivity, clinical application limitations, and high skin phototoxicity , to achieve the effect of excellent amphiphilicity, unique asymmetric substitution characteristics, and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of Bis[5'-(2', 3'-O-isopropyl)-uridineoxy]silicone phthalocyanine
[0043] (1) Synthesis of 2’, 3’-O-isopropyl-uridine
[0044] Dissolve 245 mg (1 mmol) of uridine in 10-30 ml (preferably 20 ml) of acetone, and 8-12 mmol (preferably 10 mmol) of p-toluenesulfonic acid in 10-30 ml (preferably 20 ml) of acetone. Slowly add p-toluenesulfonic acid acetone solution to uridine acetone solution dropwise under ice-water bath, and stir at room temperature for 2-10 h (preferably 6 h). The reaction mixture was added to 4% sodium bicarbonate ice solution, extracted several times with dichloromethane (or trichloromethane), the organic layer was collected, dried over magnesium sulfate, filtered, concentrated, and dried to obtain a yellow powder product. Yield 85%.
[0045] The characterization data of the product are as follows: MS (EI-60) m / z: 283.4 [M-H] - .
[0046] IR (KBr, cm -1 ): 1467, 2935 (CH 3 ); 1703 (C=O); 1671 (C=C); 1467, 2935 (CH 2 ); 3245 (NH,OH); 1121...
Embodiment 2
[0056] Synthesis of Bis[5'-(2', 3'-O-isopropyl)-cytidineoxy]silyl phthalocyanine
[0057] (1) Synthesis of 2’, 3’-O-isopropyl-cytidine
[0058] Dissolve 250 mg (1 mmol) of cytidine in 10-30 ml (preferably 20 ml) of acetone, and 8-12 mmol (preferably 10 mmol) of p-toluenesulfonic acid in 10-30 ml (preferably 20 ml) of acetone. Slowly add the acetone solution of p-toluenesulfonate to the acetone solution of cytidine in an ice-water bath, stir at room temperature for 4-10 hours (preferably 6 hours), centrifuge to collect the white precipitate, wash the precipitate with acetone three to four times, and dissolve it with a small amount of DMF , add ethyl acetate to precipitate, membrane filtration, vacuum drying to obtain a white powder product with a yield of 95%.
[0059] The characterization data of the product are as follows: IR (KBr, cm -1 ): 1383 (CH 3 ); 1123 (-O-); 1727 (C=O); 3067, 1204, 1650 (NH 2 ); 1693 (C=C); 3067, 1204 (-OH).
[0060] 1 H NMR (DMSO-d6, 400MHz, pp...
Embodiment 3
[0068] Synthesis of Bis[5'-(2', 3'-O-isopropyl)-adenosyloxy]silyl phthalocyanine
[0069] (1) Synthesis of 2’, 3’-O-isopropyl-adenosine
[0070] Dissolve adenosine (1 mmol) in 10-30 ml (preferably 20 ml) of acetone, and 8-12 mmol (preferably 10 mmol) of p-toluenesulfonic acid in 10-30 ml (preferably 20 ml) of acetone. Add p-toluenesulfonic acid acetone solution slowly dropwise to 2-aminoadenosine acetone solution under ice-water bath, stir at room temperature for 24~72 h (preferably 6 h), pour into 4% sodium bicarbonate ice water solution, and precipitate white precipitate, Suction filter and dry. Purified by Soxhlet extraction with chloroform, and dried to obtain a white powder product. Yield 85%.
[0071] (2) Synthesis of bis[5'-(2',3'-O-isopropyl)-adenosyloxy]silicone phthalocyanine
[0072] Under nitrogen protection, dichlorosilicon phthalocyanine (40 mg, 0.065mmol), 2',3'-O-isopropyl-adenosine (0.260~0.650mmol, preferably 0.52mmol) and NaH (0.48~0.60mmol , preferably...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com