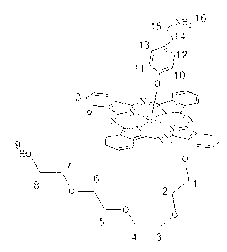
Silicon phthalocyanine axially modified by aminoethyl phenoxyl and polyethylene glycol oligomer
A technology of aminoethylphenoxy and oligoethylene glycol, applied in chemical instruments and methods, botany equipment and methods, active ingredients of silicon compounds, etc., can solve the problem of lack of tumor tissue and cancer cell selectivity, clinical application Limitations, high skin phototoxicity and other issues, to achieve excellent amphiphilicity, improved tissue penetration ability, and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis and physicochemical properties of bis[4-(2-aminoethyl)phenoxy]phthalocyanine silicon (structure shown in the following formula):
[0038]
[0039] Under nitrogen protection, dichlorosilyl phthalocyanine (244.7mg, 0.4mmol), 4-(2-aminoethyl)phenol 1.2~2 mmol (preferably 1.6mmol) and NaH were added to toluene or xylene or dioxane In 20~50ml (preferably toluene, 30ml), reflux for 12~24 hours (preferably 18 hours). Remove the solvent by rotary evaporation in vacuo, dissolve in 100ml of dichloromethane, centrifuge to remove insoluble matter, extract the dichloromethane solution with water (3×100ml), collect the organic layer, then extract with dilute hydrochloric acid (0.1-0.5 mmol), and collect the aqueous layer. The aqueous layer was neutralized with 1M sodium hydroxide, and a blue precipitate was precipitated, centrifuged, washed with water, and dried in vacuum to obtain a blue product with a yield of 45%. The maximum absorption peak of the product in DMSO is...
Embodiment 2
[0042] Synthesis and physicochemical properties of silicon phthalocyanine modified by axial aminoethylphenoxy and oligoethylene glycol with the structure shown in the following formula:
[0043]
[0044] Add 40~100ml (preferably 60ml) of triethylene glycol monomethanol to 1mmol of bis[4-(2-aminoethyl)phenoxy]silyl phthalocyanine in toluene (or xylene or dioxane) Ether, after adding a catalytic amount of NaH, continue the reaction at 110°C, monitor the reaction by TLC, and after 5 hours, rotate the reaction mixture to a small amount, add a small amount of DMF to dissolve, add a large amount of water to precipitate, and remove the solvent and reaction materials by membrane filtration And by-products, dry. Purify the crude product through a silica gel column, use ethyl acetate as the elution solvent, and after the first band is completely eluted, use DMF as the elution solvent to collect the target eluted components, spin to a small amount of solvent, filter, spin Evaporate a...
Embodiment 3
[0047] Synthesis and physicochemical properties of axial aminoethylphenoxy and oligoethylene glycol modified silicon phthalocyanines with the following structure:
[0048]
[0049] Add 40~100ml (preferably 60ml) of triethylene glycol to 1mmol of bis[4-(2-aminoethyl)phenoxy]silyl phthalocyanine in toluene (or xylene or dioxane) solution, add Continue the reaction at 110°C after a catalytic amount of NaH, and monitor the reaction by TLC. After 5 hours, rotate the reaction mixture to a small amount, add a small amount of DMF to dissolve, add a large amount of water to precipitate, and remove the solvent, reaction raw materials and by-products by membrane filtration ,dry. Purify the crude product through a silica gel column, use ethyl acetate as the elution solvent, use ethyl acetate as the elution solvent to pass through the silica gel column, after the first band is completely eluted, use a mixed solvent of DMF and triethylamine (volume ratio is 10 : 1) For the elution solve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com