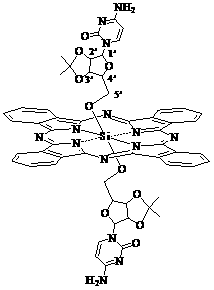
Silicon phthalocyanine modified by cytidine derivative and preparation method and application thereof
A technology of silicon phthalocyanine and derivatives, which is applied in the field of silicon phthalocyanine modified by cytidine derivatives and its preparation, can solve the problems of lack of tumor tissue and cancer cell selectivity, clinical application limitations, and large skin phototoxicity, etc., to achieve Fast preparation, easy separation and purification, high biocompatibility and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of cytidine derivative modified silicon phthalocyanine of the present invention is: (1) under ice-water bath ~ room temperature, cytidine (or 5-azacytidine, or 5-fluorocytidine, or 5-methylcytidine) and p-toluenesulfonic acid were placed in acetone and stirred for 2~20 hours, the molar ratio of the two was 1:8~12, and purified by solvent method, extraction method and chromatography to obtain 2', 3'-O-iso Propyl-cytidine (or 2', 3'-O-isopropyl-5-azacytidine, or 2', 3'-O-isopropyl-5-fluorocytidine, or 2', 3' -O-isopropyl-5-methylcytidine).
[0033] (2) with dichlorosilyl phthalocyanine and 2', 3'-O-isopropyl-cytidine (or 2', 3'-O-isopropyl-5-azacytidine, or 2', 3' -O-isopropyl-5-fluorocytidine, or 2', 3'-O-isopropyl-5-methylcytidine) as the reactant, the molar ratio of the two is 1:4~10; Using toluene, xylene or dioxane as a solvent, the amount of solvent used is 40-400ml for 1mmol of dichlorosilicon phthalocyanine, and reacted at 100-130°C for 18...
Embodiment 1
[0041] Synthesis of Bis[5'-(2', 3'-O-isopropyl)-cytidineoxy]silyl phthalocyanine
[0042] (1) Synthesis of 2’, 3’-O-isopropyl-cytidine
[0043] Dissolve 250 mg (1 mmol) of cytidine in 10-30 ml (preferably 20 ml) of acetone, and 8-12 mmol (preferably 10 mmol) of p-toluenesulfonic acid in 10-30 ml (preferably 20 ml) of acetone. Slowly add the acetone solution of p-toluenesulfonate to the acetone solution of cytidine in an ice-water bath, stir at room temperature for 4-10 hours (preferably 6 hours), centrifuge to collect the white precipitate, wash the precipitate with acetone three to four times, and dissolve it with a small amount of DMF , add ethyl acetate to precipitate, membrane filtration, vacuum drying to obtain a white powder product with a yield of 95%.
[0044] The characterization data of the product are as follows: IR (KBr, cm -1 ): 1383 (CH 3 ); 1123 (-O-); 1727 (C=O); 3067, 1204, 1650 (NH 2 ); 1693 (C=C); 3067, 1204 (-OH).
[0045] 1 H NMR (DMSO-d6, 400MHz, pp...
Embodiment 2
[0053]Synthesis of Bis[5'-(2', 3'-O-isopropyl)-5-azacytidineoxy]silyl phthalocyanine
[0054] (1) Synthesis of 2’, 3’-O-isopropyl-5-azacytidine
[0055] Dissolve 244 mg (1 mmol) of 5-azacytidine in 10-30 ml (preferably 20 ml) of acetone, and dissolve 8-12 mmol (preferably 10 mmol) of p-toluenesulfonic acid in 10-30 ml (preferably 20 ml) of acetone. Slowly add the p-toluenesulfonic acid acetone solution dropwise into the 5-azacytidine acetone solution under an ice-water bath, and stir at room temperature for 12-36 h (preferably 24 h). Sodium bicarbonate solution was slowly added to the reaction mixture with stirring until the bubbles disappeared and a white precipitate formed. Filter, wash twice with acetone, and dry to obtain a white powder product with a yield of 90%.
[0056] The characterization data of the product are as follows: IR (KBr, cm -1 ): 1110 (C-O-C); 1693 (-CO-N); 3414.7, 1212, 1610 (NH 2 ); 1693 (C=C); 3291, 1158 (-OH); 1470 (-CH 2 -).
[0057] 1 H NMR (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com