Application of piperazidine substituted silicon phthalocyanine and application thereof to photothermal therapy
A technology of photothermal therapy and silicon phthalocyanine, which is applied in the field of photothermal agents and photothermal therapy drugs, can solve the problems that have not yet been seen in the application research of phthalocyanine photothermal therapy, and achieve good industrialization prospects, stable properties, and tissue penetration powerful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
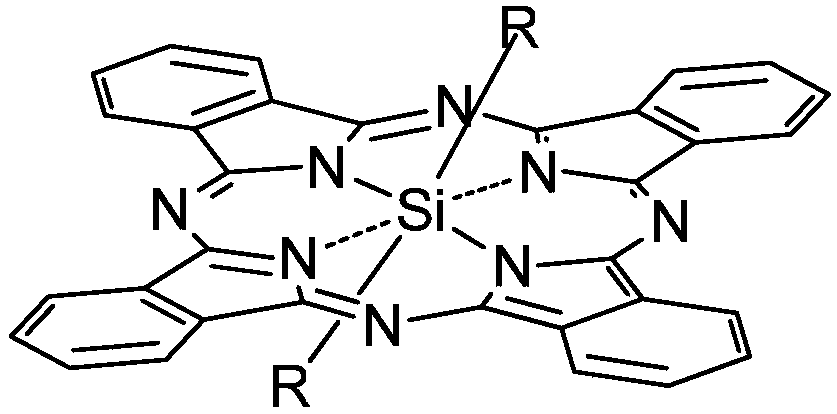
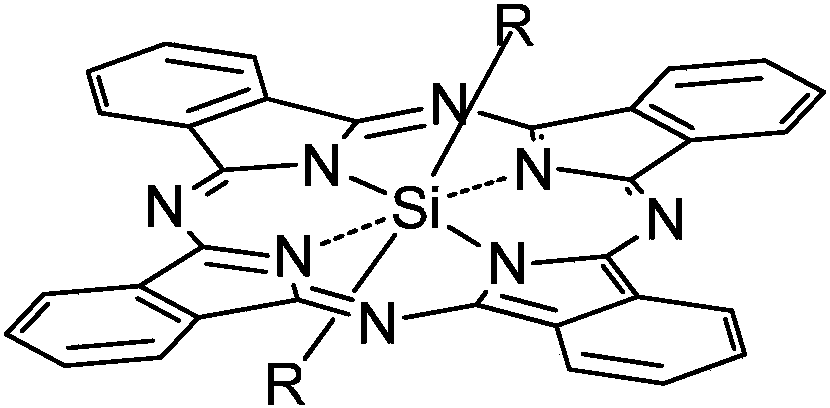
[0035] Synthesis of silicon phthalocyanine with the following structure
[0036] in:
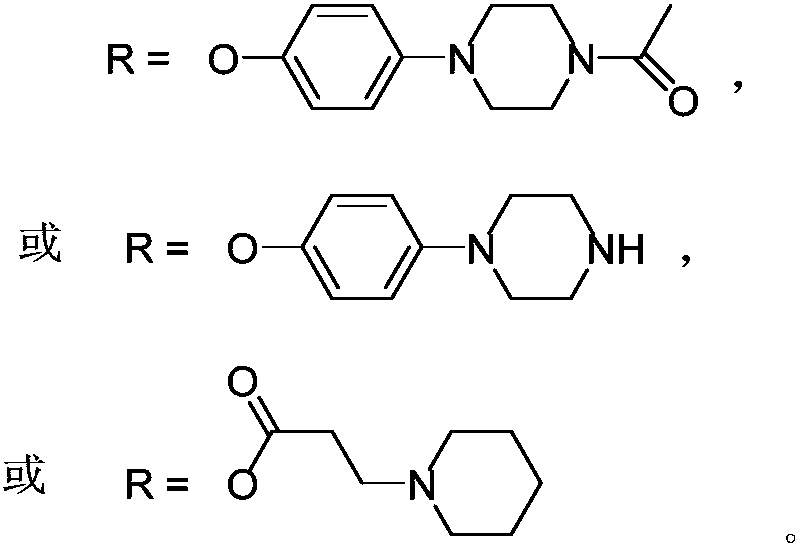
[0037]The compound can be named: bis[4-(4-acetylpiperazine)]silicon phthalocyanine, and the target product was prepared with reference to the inventor's paper published in Bioorg.Med.Chem.Lett., 2006, 16, 2450-2453: Disperse dichlorosilicon phthalocyanine and 1-acetyl-4-(4-hydroxyphenyl)piperazine at a molar ratio of 1:8 to 11 in anhydrous toluene, and reflux for 48 hours in the presence of NaH. After the reaction was complete, the toluene was evaporated to dryness, rinsed with a large amount of water, and dried in vacuum. The crude product was separated and activated by silica gel chromatography, and the eluent was acetone. The yield was 62%. The characterization data of the product are as follows:
[0038] 1 HNMR (CDCl 3 ,400MHz,ppm): δ9.59-9.62(m,8H,Pc-H α ),8.34-8.36(m,8H,Pc-H β ), 5.18 (d, J=8.1Hz, 4H, Ph-H β ),3.35-3.36(m,4H,CH 2 ),3.17-3.18(m,4H,CH 2 ), 2.38 (d, J=8.1Hz...
Embodiment 2
[0042] Synthesis of silicon phthalocyanine with the following structure
[0043] in:
[0044] The compound can be named as: bis[4-(1-methyl-4-acetylpiperazine)] silicon phthalocyanine diiodide. Prepare the target product with reference to the papers published by the inventor in Bioorg.Med.Chem.Lett., 2006, 16, 2450-2453: dissolving bis[4-(4-acetylpiperazine)] silicon phthalocyanine in chloroform , made into a saturated solution, adding excess methyl iodide, refluxed for 1 hour, cooled to room temperature and stirred for 12 hours, a large amount of insoluble matter was precipitated, filtered to obtain the corresponding target product with a yield of 87%.
[0045] The characterization data of the product are as follows:
[0046] 1 HNMR (CDCl 3 , 400MHz, ppm) data and its attribution: δ9.64-9.67 (m, 8H, Pc-H α ),8.50-8.53(m,8H,Pc-H β ), 6.25 (d, J=9.3Hz, 4H, Ph-H β ),3.27-3.29(m,8H,CH 2 ), 2.59 (d, J=9.3Hz, 4H, Ph-H α ),2.56(s,6H,N-CH 3 ),2.48-2.50(m,8H,CH 2 ),2.17...
Embodiment 3
[0051] Synthesis of silicon phthalocyanine with the following structure
[0052] in:
[0053] This compound can be named as: two [4-(1-piperazinyl) phenoxy] silicon phthalocyanine, preparation method is as follows:
[0054] Using dichlorosilicon phthalocyanine and 4-(1-piperazinyl)phenol or 3-(N-piperidine)propionic acid as reactants, the molar ratio of the two is 1:1~20 (optimum 1:3 ), with toluene, xylene or dioxane as a solvent, with NaH as a catalyst, under the protection of nitrogen, react for 1 to 36 hours (optimum 24 hours) at 100 to 130 ° C, and the solvent consumption is every 0.1mmol two Chlorosilicon phthalocyanine needs 6-10mL, and the amount of NaH is 0.3-0.5mmol per 0.1mmol of dichlorosilicon phthalocyanine. After the reaction was complete, the toluene was evaporated to dryness, washed with a large amount of water, and dried under vacuum to obtain a blue crude product. The crude product is separated by silica gel chromatography, the eluent is ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com