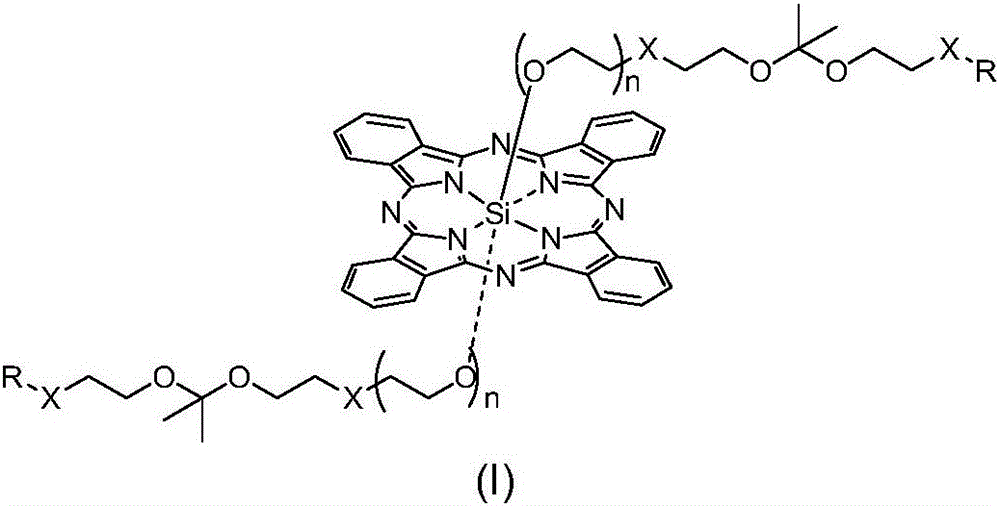
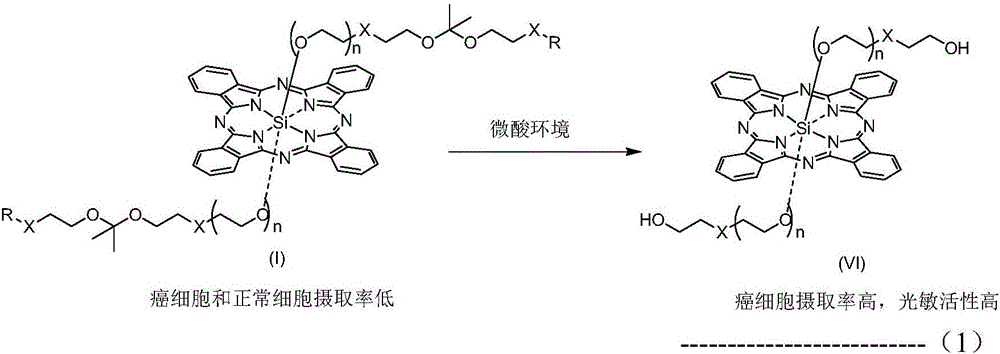
pH sensitive axially substituted silicon phthalocyanine complex, preparing method of pH sensitive axially substituted silicon phthalocyanine complex and application of pH sensitive axially substituted silicon phthalocyanine complex to medicines
A technology of dichlorosilicon phthalocyanine and compound, which is applied in the field of medicine and can solve the problems of incapable of photodynamic damage, weak targeting effect, complex components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
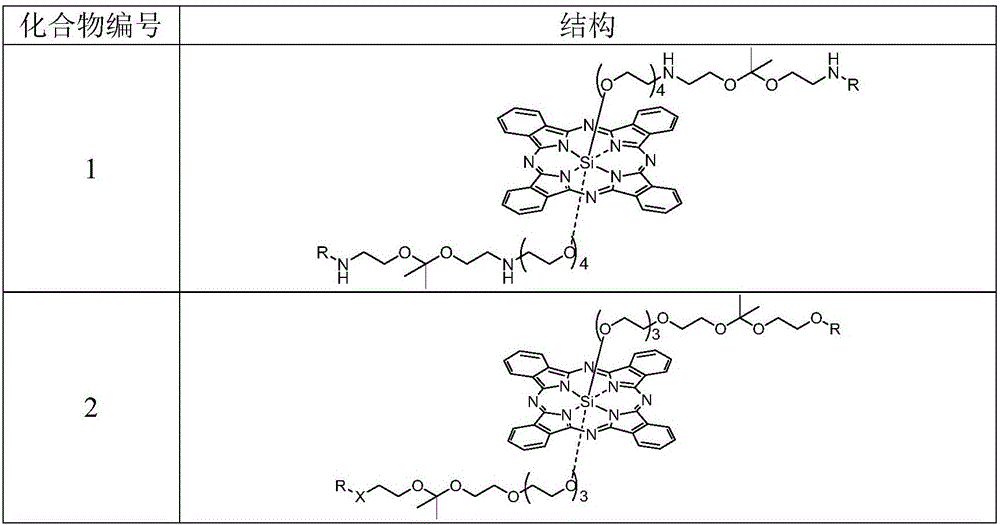
[0067] Example 1 Synthesis of Compound 1
[0068]
[0069] step 1
[0070] In an ice-water bath, add compound 1-1 (1.62g, 10mmol), cholesterol chloroformate (2.2g, 4.9mmol) and triethylamine (2g, 20mmol) into dichloromethane (30mL), continue to stir and react for 3 hours, Stop the reaction, add water (200 mL) to the reaction solution, stir and stand, collect the organic phase, dry the organic phase with anhydrous sodium sulfate, and distill under reduced pressure. The crude product is separated and purified by a silica gel column. The eluent is trichloro Methane / methanol (9:1) to obtain 1-2 as a white solid (1.45 g, 51%). MS(ESI): m / z=598[M+Na] + .
[0071] Step 2
[0072] In an ice-water bath, add compound 1-2 (1.2g, 2.1mmol), compound 1-3 (0.52g, 1.5mmol), pyridine (1.1g, 14mmol) into dichloromethane (35mL), remove the ice-water bath, The temperature was raised to room temperature and the reaction was continued with stirring for 6 hours. The reaction was stopped. Water (100mL) wa...
Embodiment 2
[0075] Example 2 Synthesis of Compound 2
[0076]
[0077] step 1
[0078] In an ice-water bath, add compound 2-1 (0.57g, 3.5mmol), cholesterol chloroformate (1.55g, 3.5mmol) and pyridine (0.8g, 10.1mmol) into dichloromethane (35mL), the temperature is raised to room temperature, Continue to stir the reaction for 2 hours, stop the reaction, add water (100mL) to the reaction solution, stir and stand, collect the organic phase, dry the organic phase with anhydrous sodium sulfate, distill under reduced pressure, and separate and purify the crude product with silica gel chromatography. The eluent was chloroform / methanol (15:1) to obtain 2-2 (0.92 g, 46%) as a white solid. MS(ESI): m / z=599[M+Na] + .
[0079] Step 2
[0080] In an ice-water bath, add compound 2-2 (0.9g, 1.6mmol), compound 2-3 (0.9g, 3.0mmol) to tetrahydrofuran (30mL), and slowly add sodium hydride (0.3g, 12.5mmol) to the reaction solution ), remove the ice-water bath, raise the temperature to room temperature and continu...
Embodiment 3
[0083] Example 3 Synthesis of Compound 3
[0084]
[0085] step 1
[0086] In an ice-water bath, add compound 1-2 (1.2g, 2.1mmol), compound 3-1 (0.5g, 2.4mmol), pyridine (0.8g, 10.1mmol) into dichloromethane (25mL), and raise the temperature to room temperature Continue to stir the reaction for 8 hours, stop the reaction, add water (100mL) to the reaction solution, stir and stand, collect the organic phase, dry the organic phase with anhydrous sodium sulfate, distill under reduced pressure, and separate and purify the crude product with a silica gel chromatography column. The eluent was chloroform / methanol (9:1) to obtain a pale yellow oil 3-2 (0.85 g, 62%). MS(ESI): m / z=663[M] + . Step 2
[0087] Dichlorosilicon phthalocyanine (0.21g, 0.34mmol), compound 1-4 (1.5g, 2.2mmol) and pyridine (1g, 12.7mmol) were added to toluene (30mL), the temperature was raised to 120℃ and reacted for 24 hours. The solvent was distilled off by pressure, and the solid residue was added with 100 ml of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com