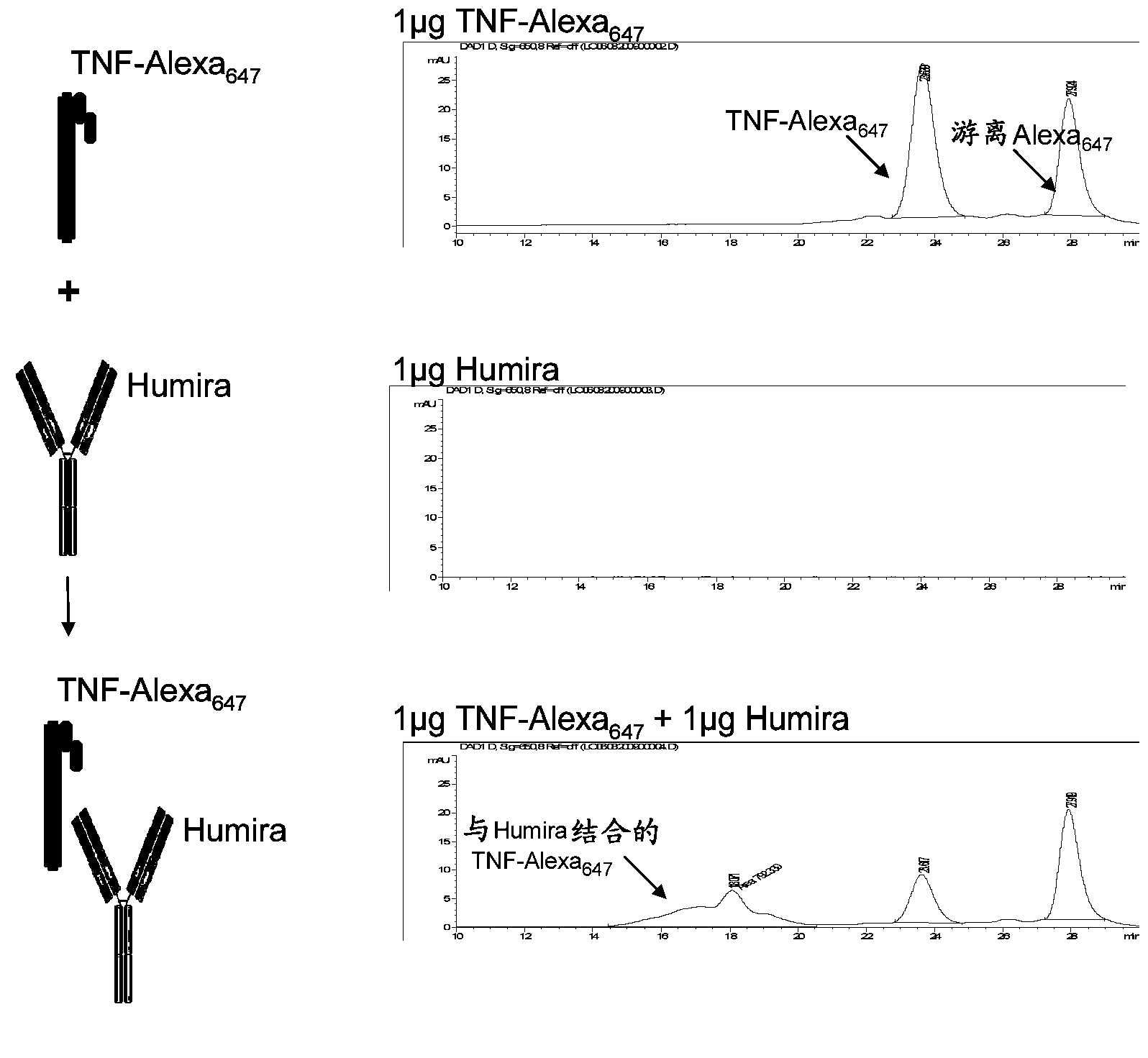
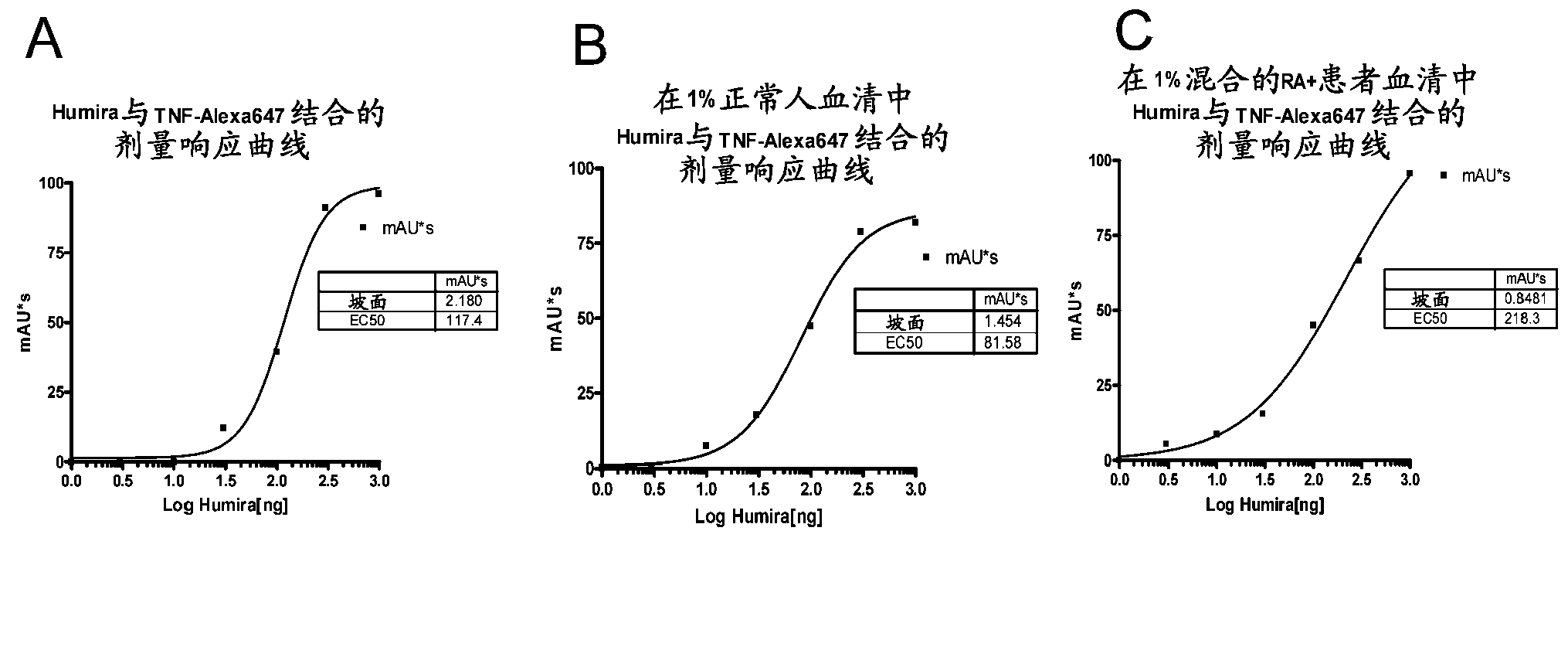
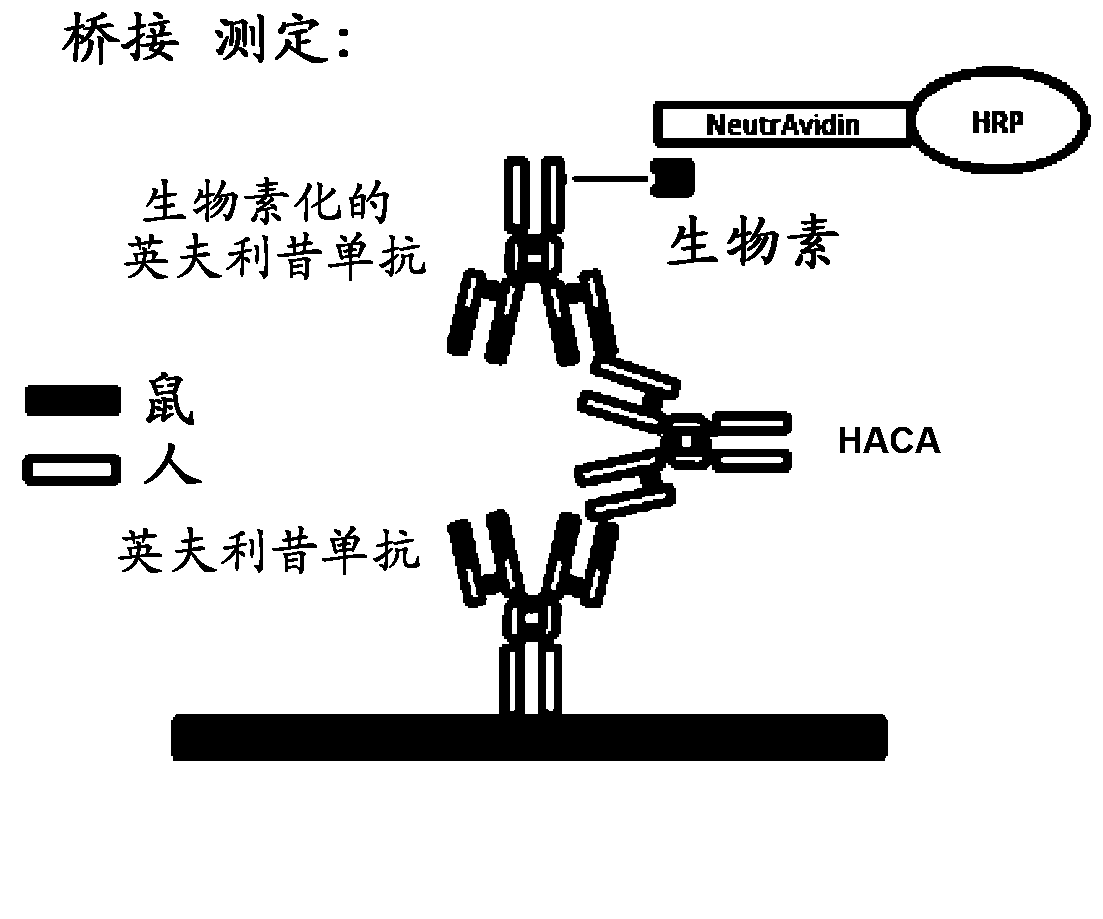
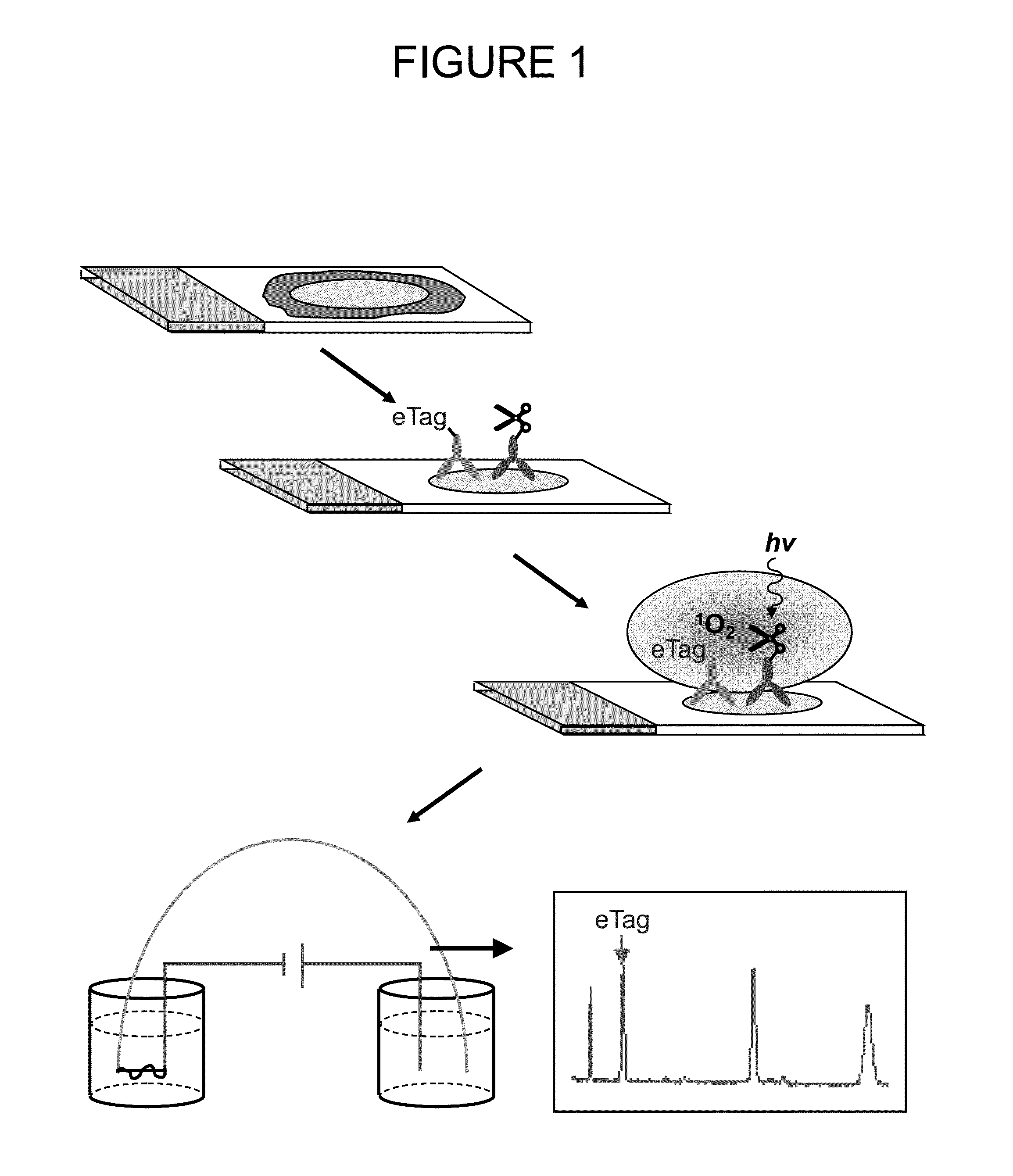
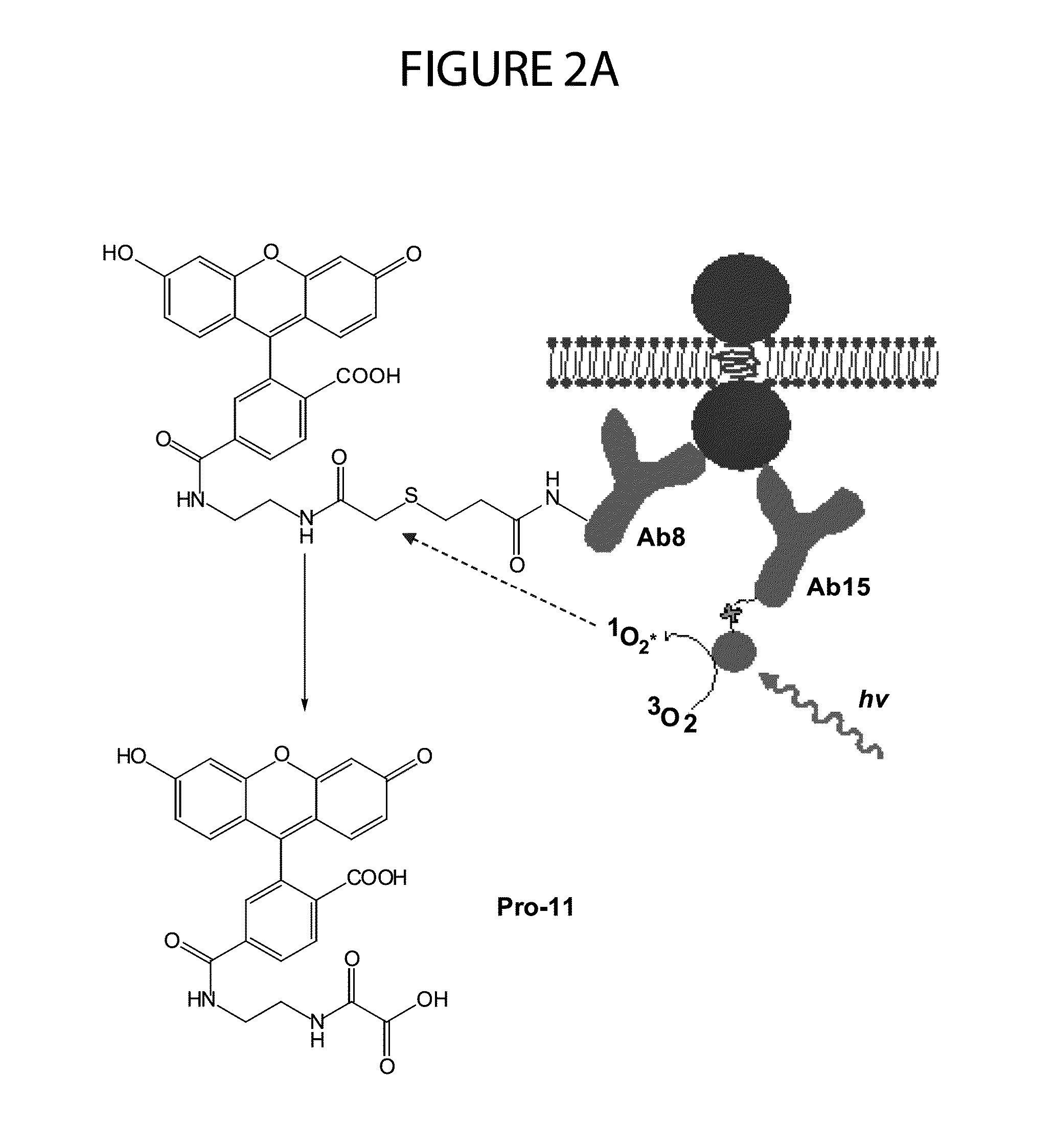
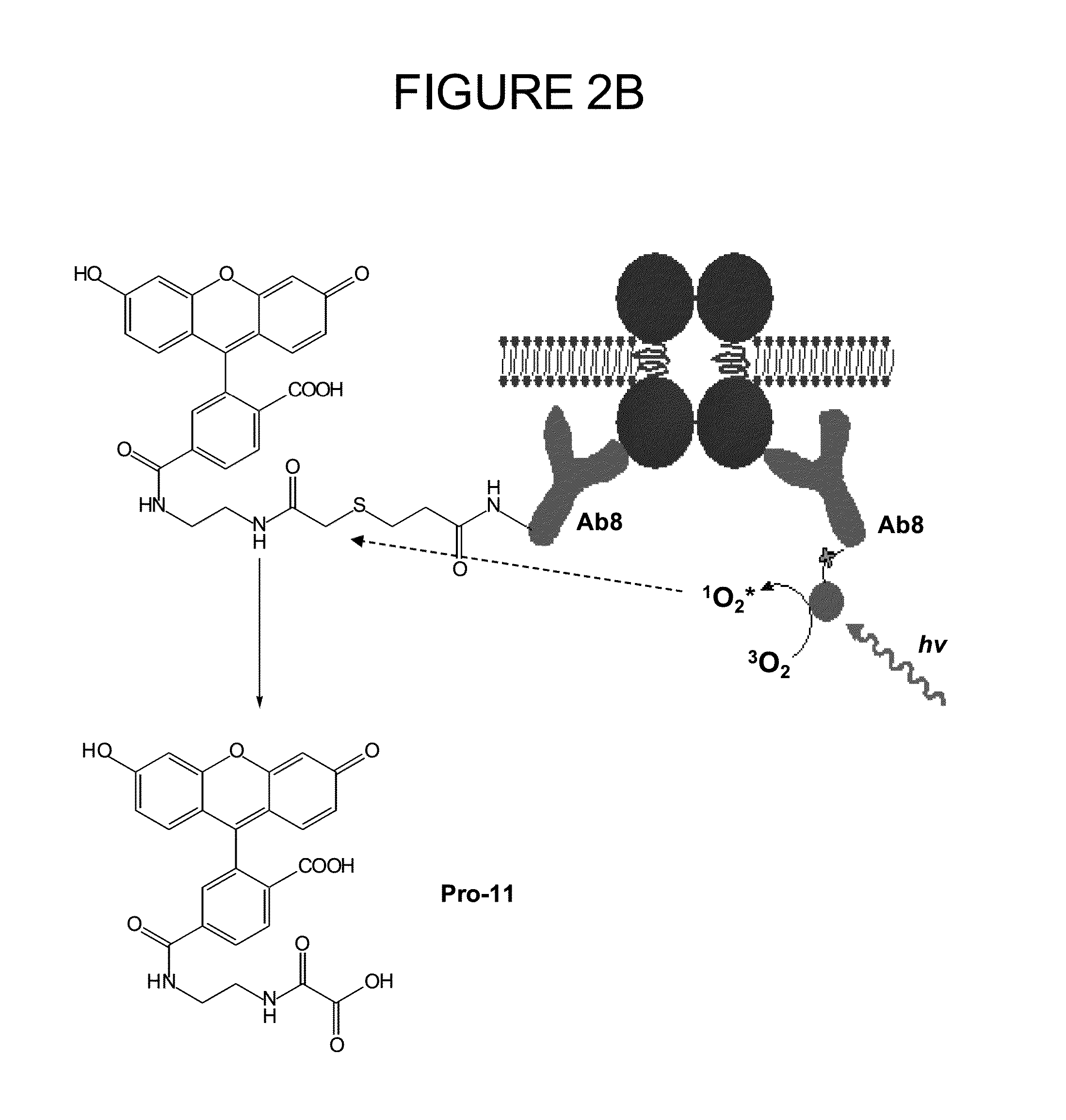
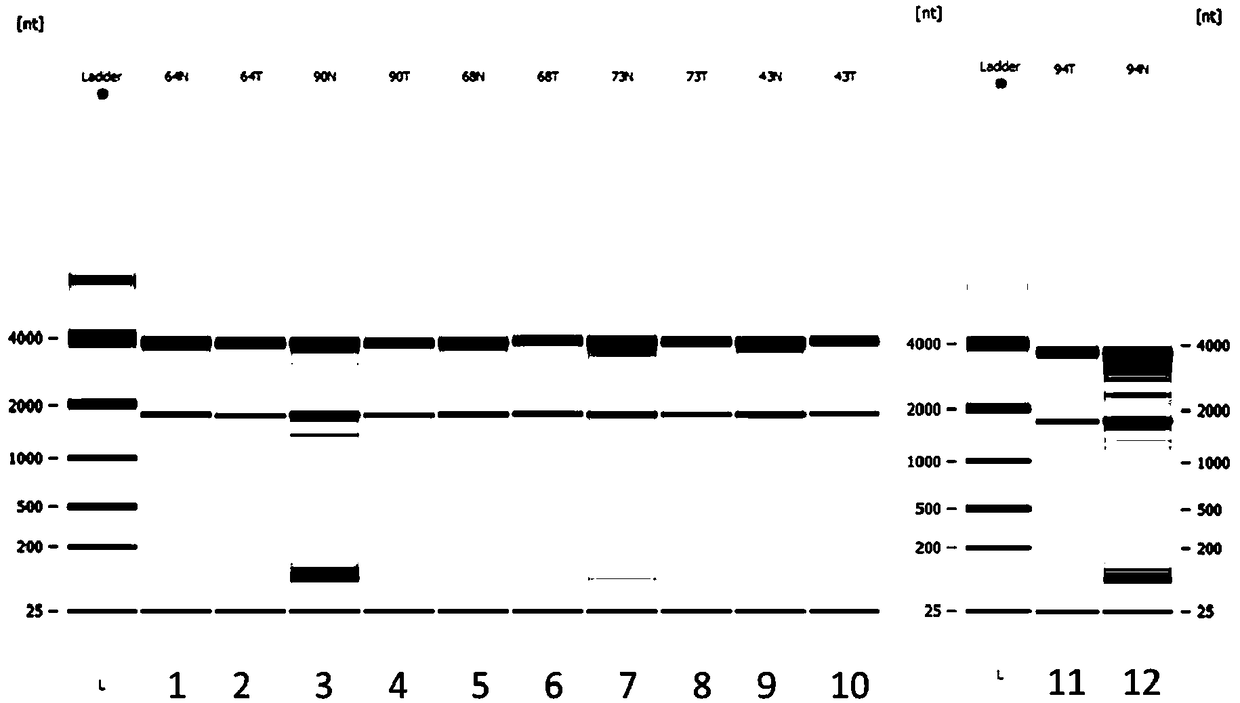
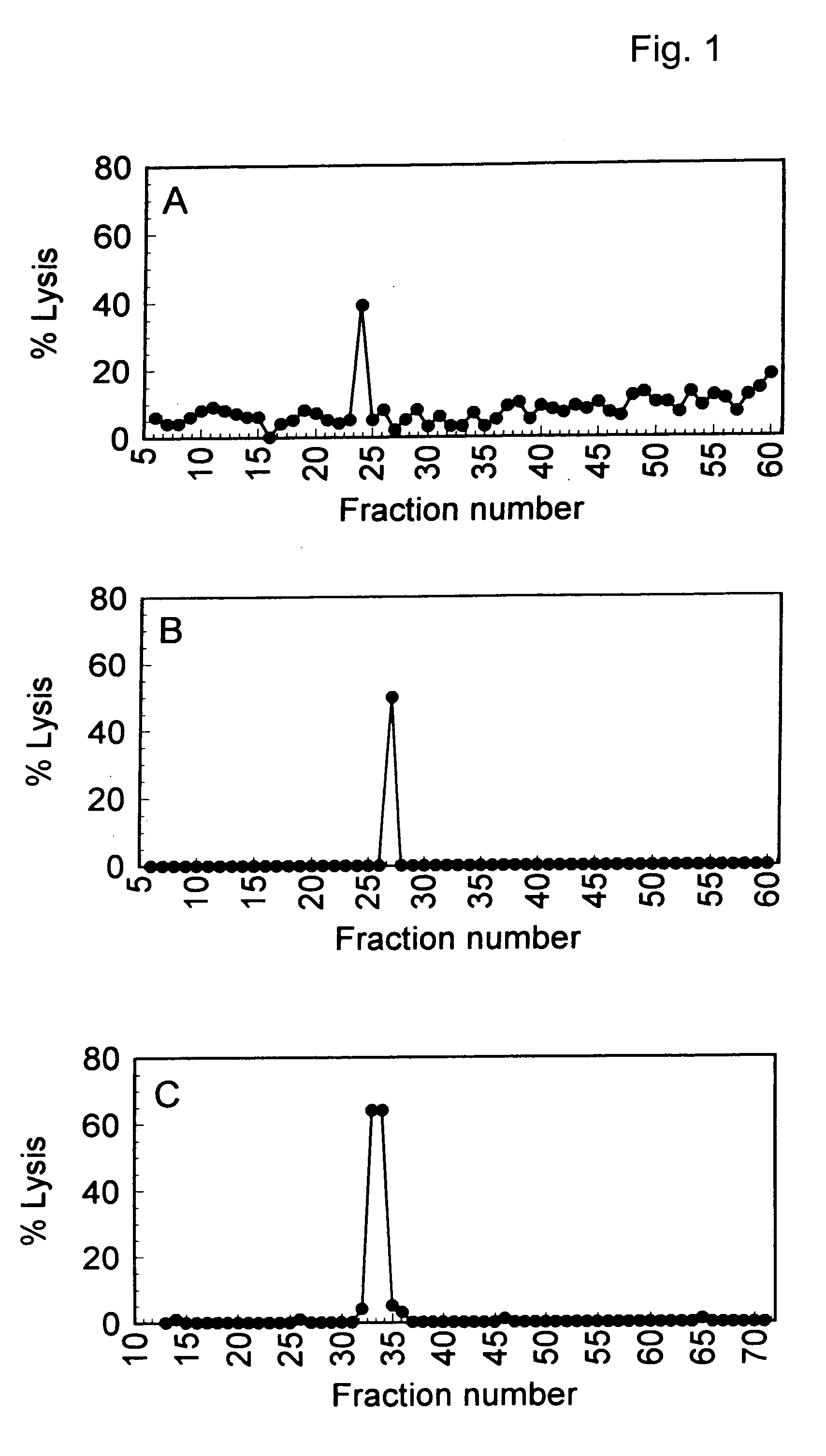
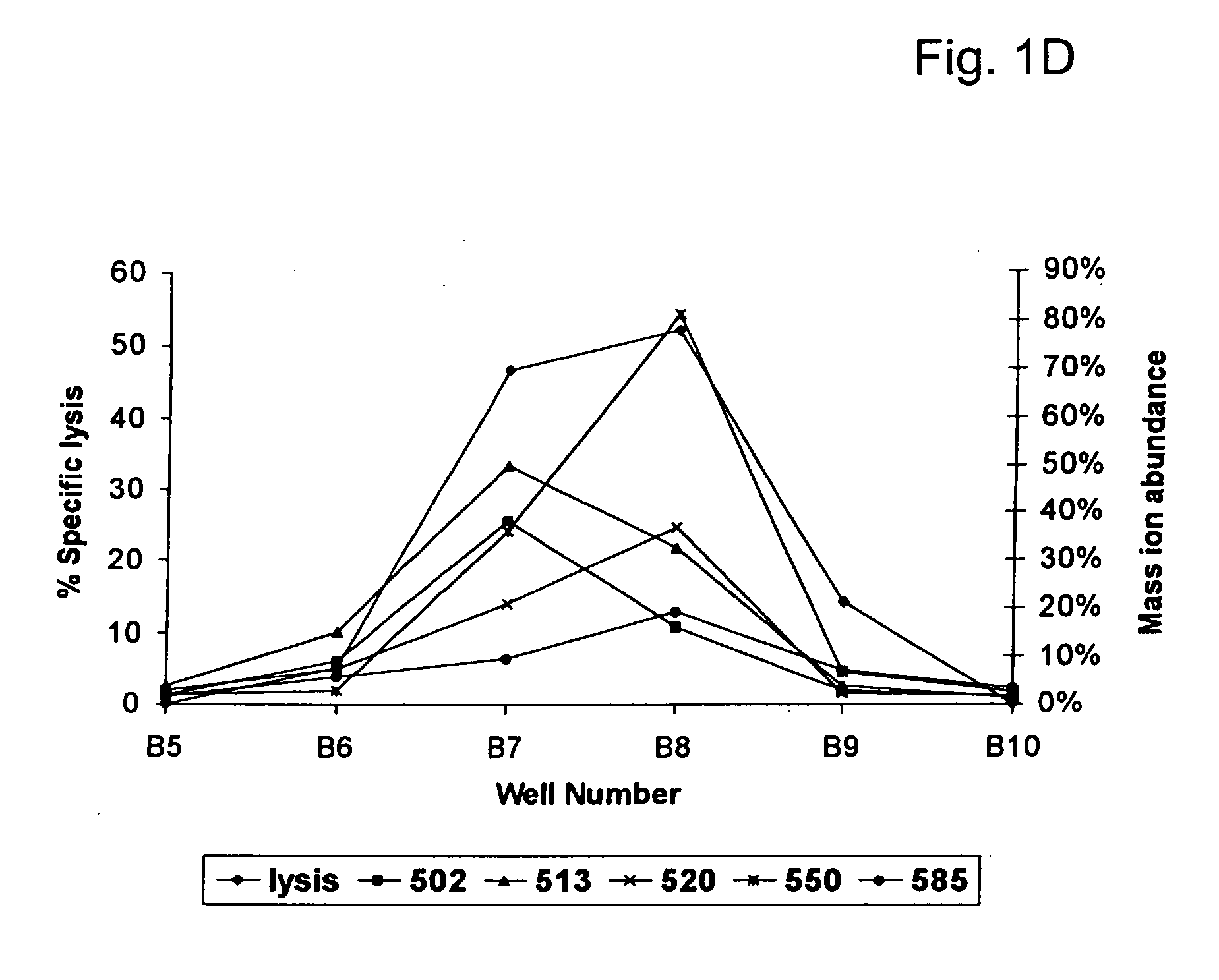
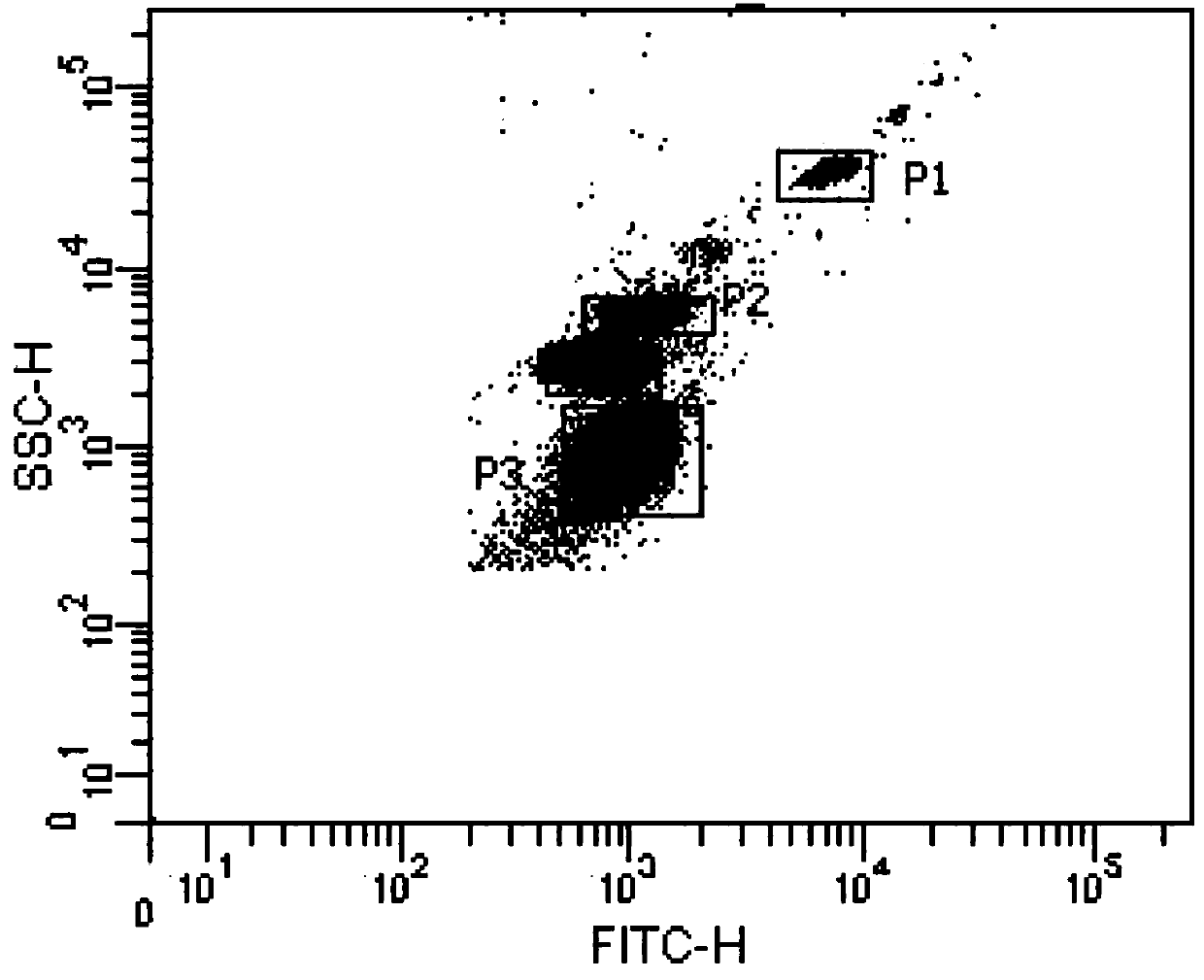
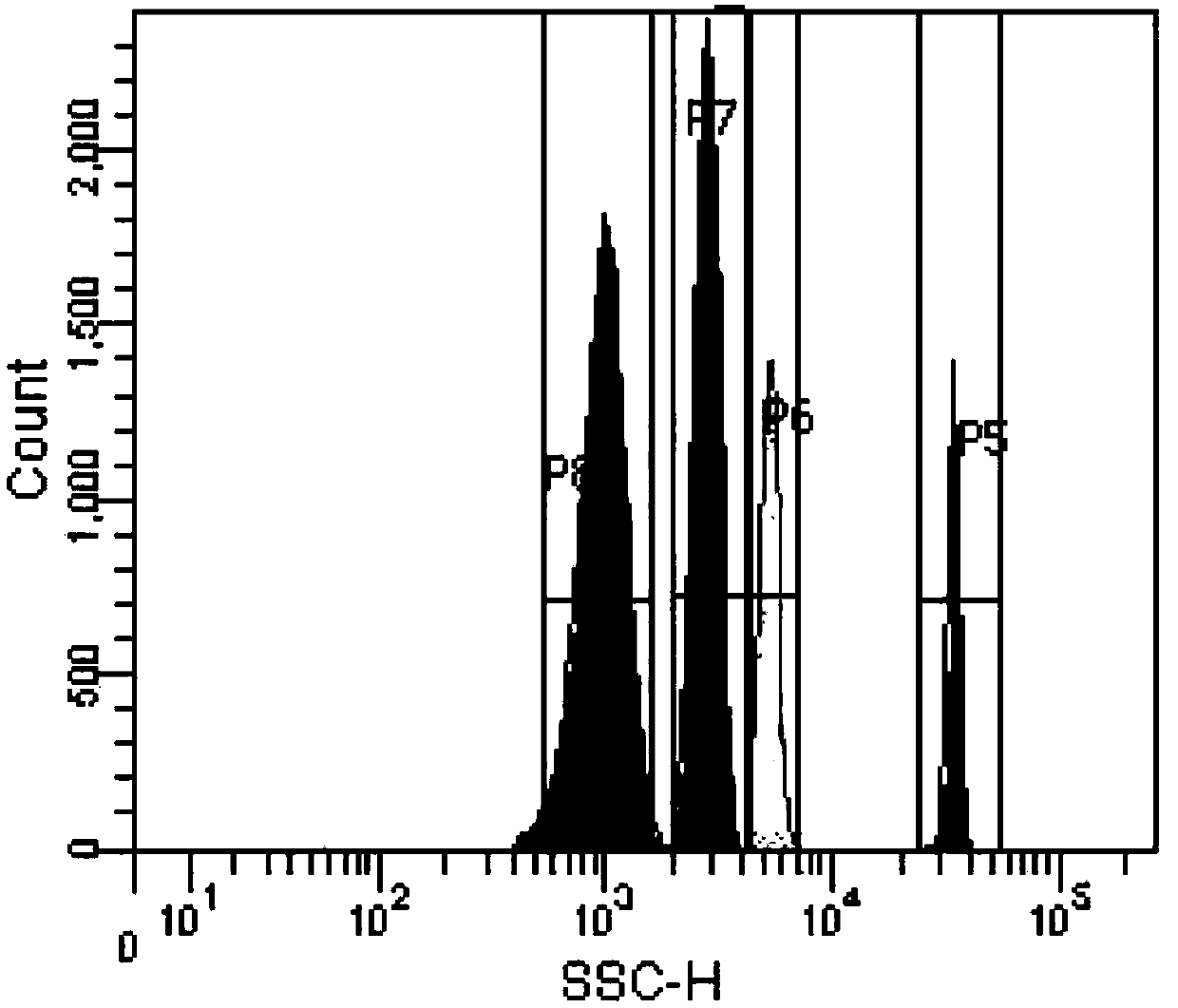
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Positive patient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
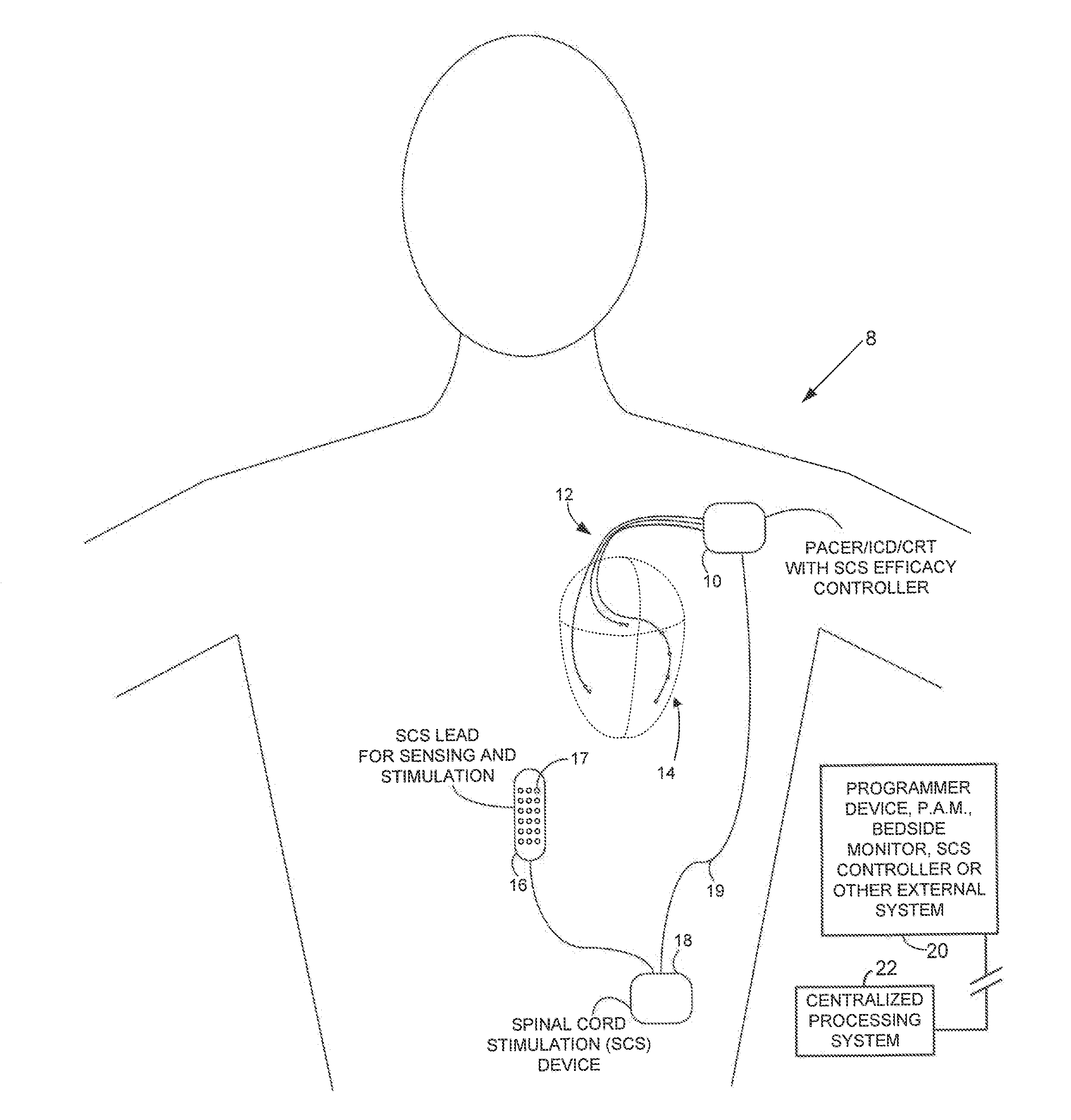
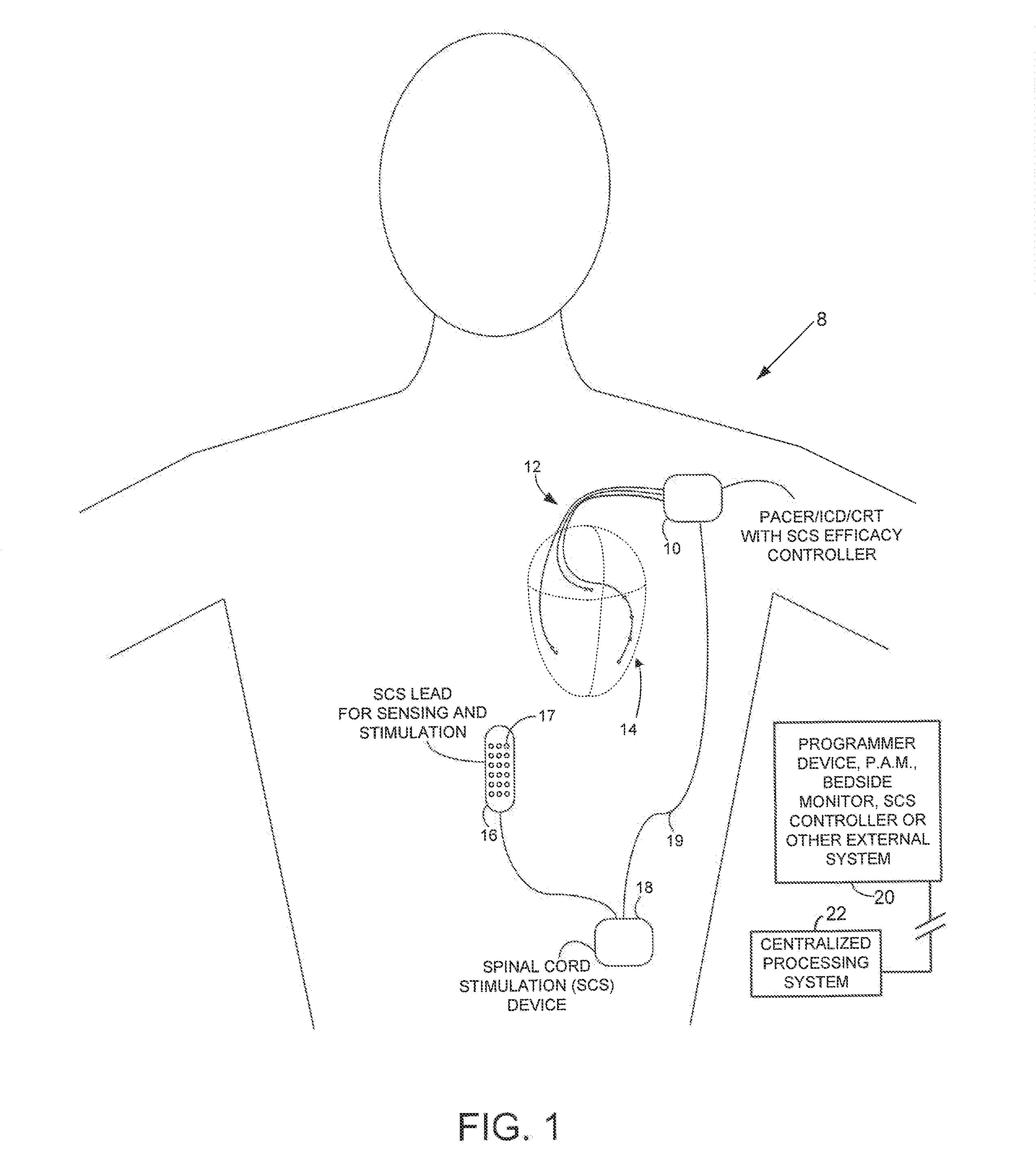
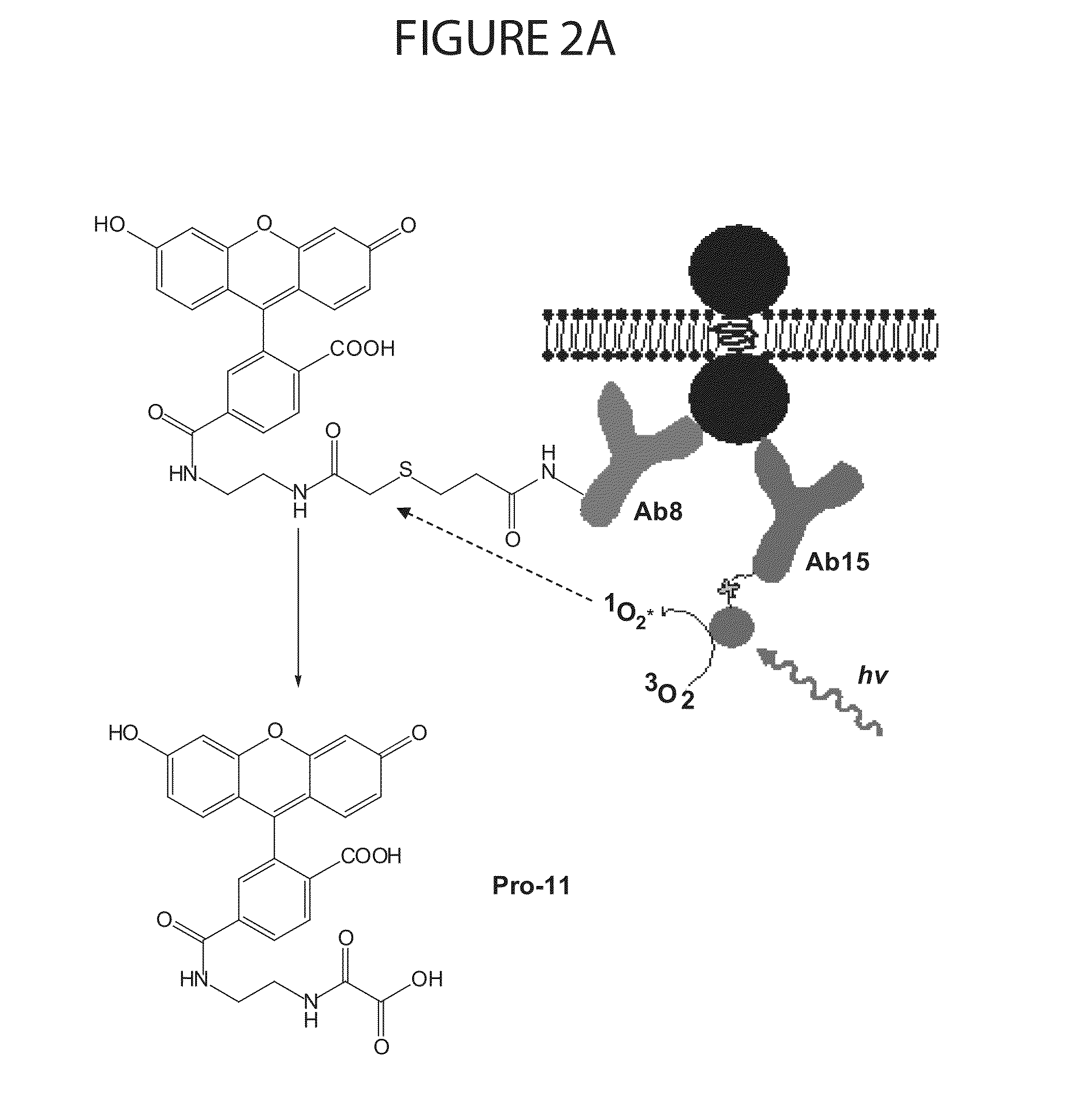
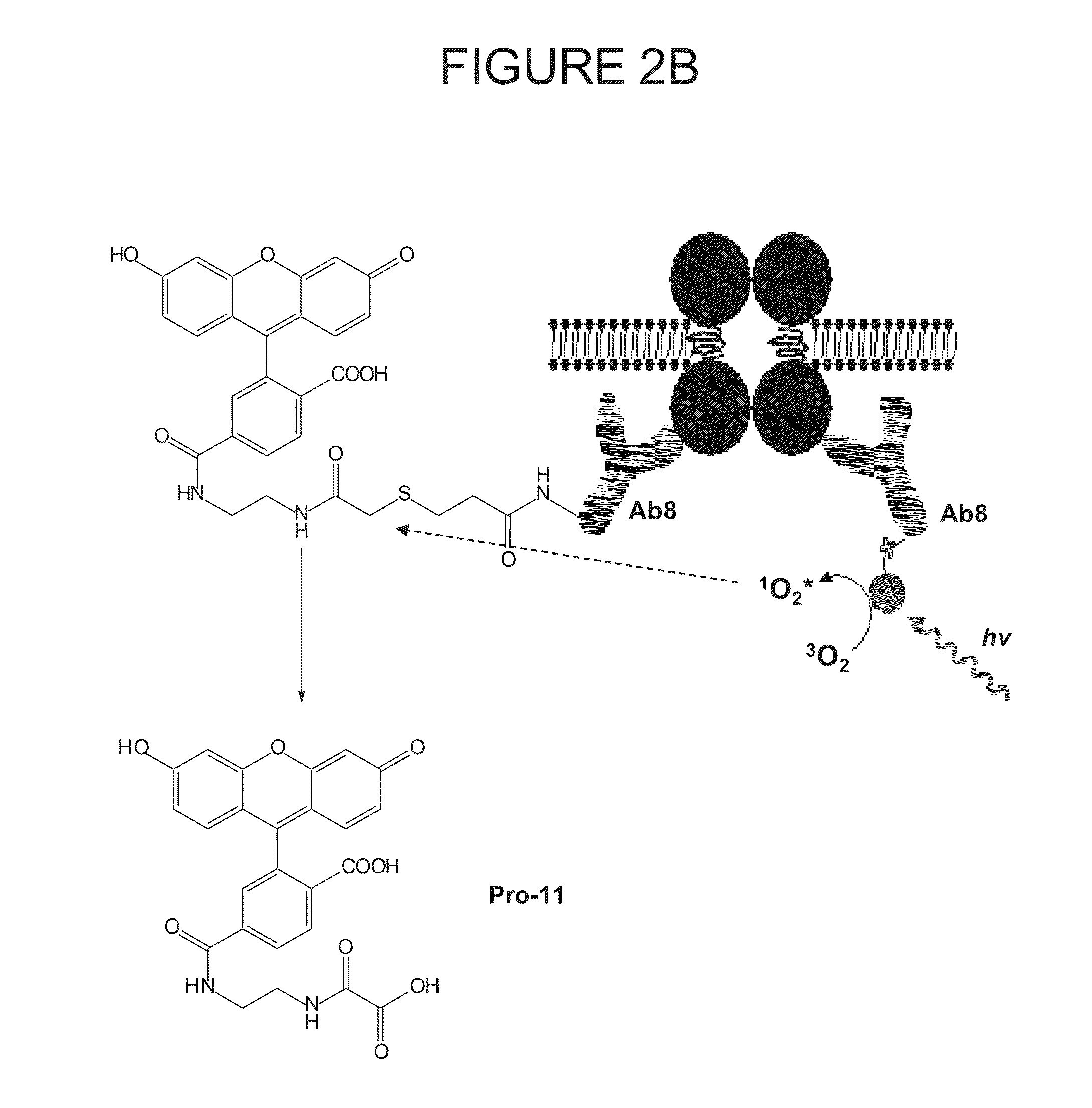
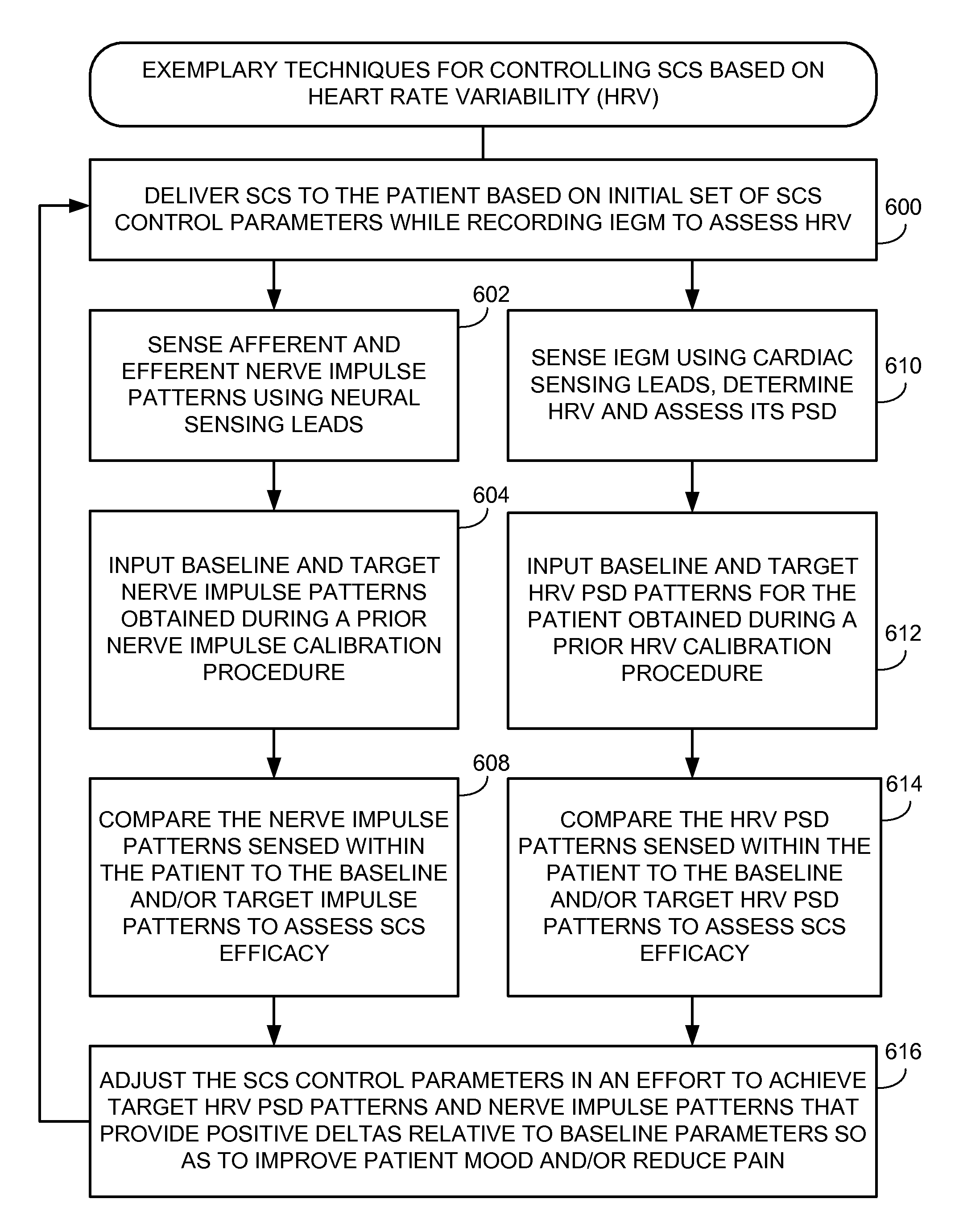
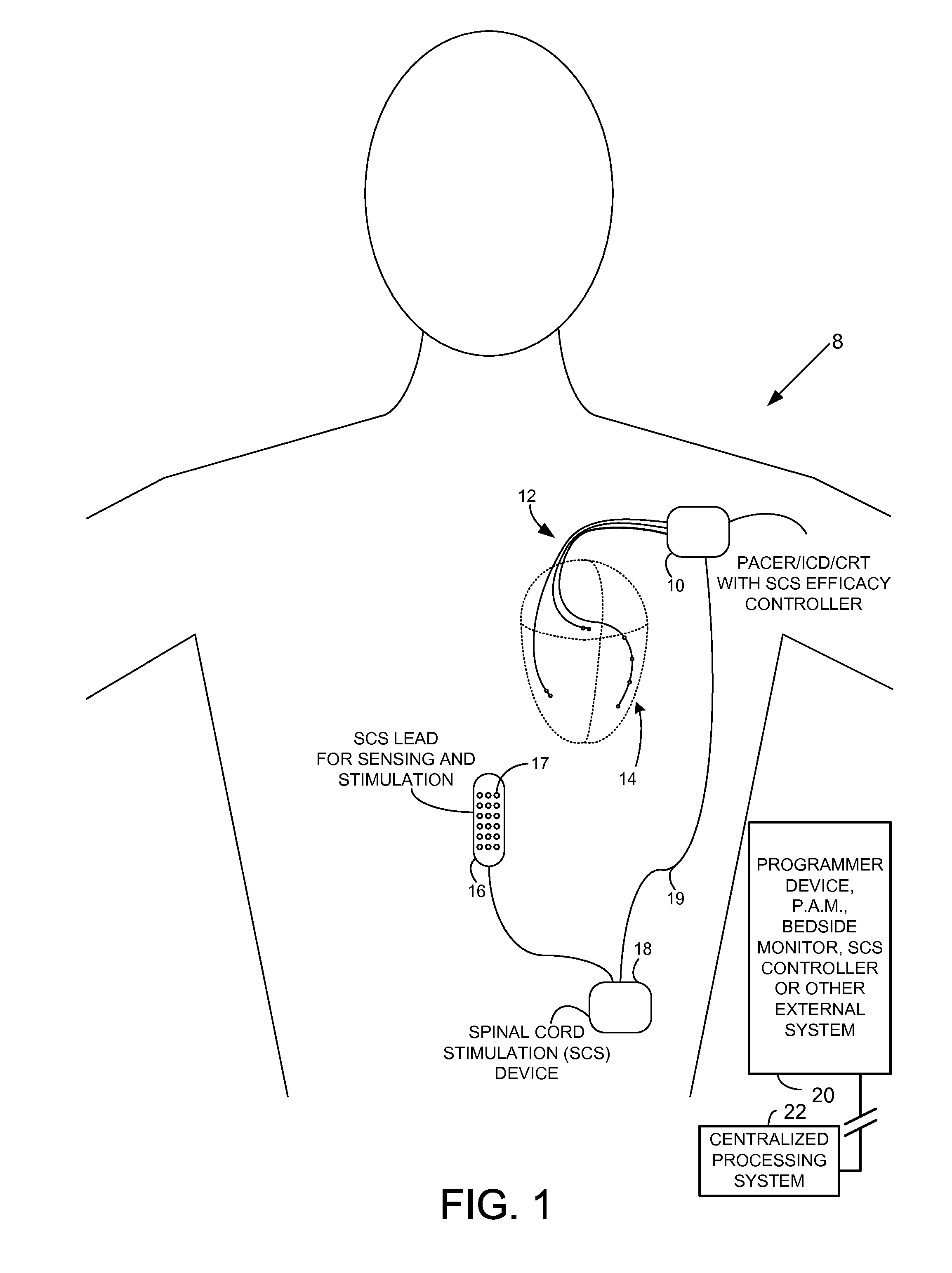
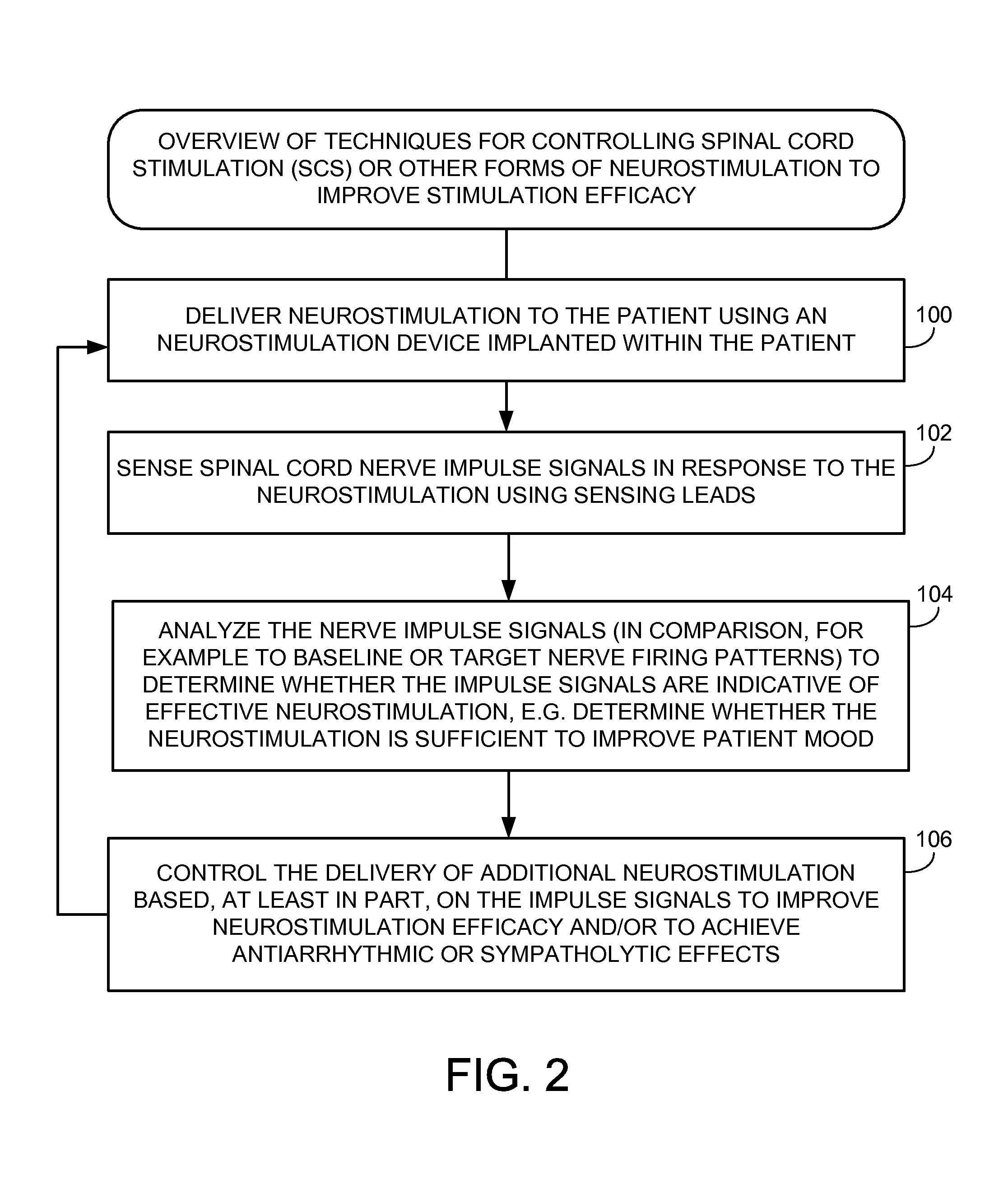
Systems and methods for controlling spinal cord stimulation to improve stimulation efficacy for use by implantable medical devices
ActiveUS20130268016A1Good curative effectRelieve painHeart stimulatorsArtificial respirationNerve impulseRR interval
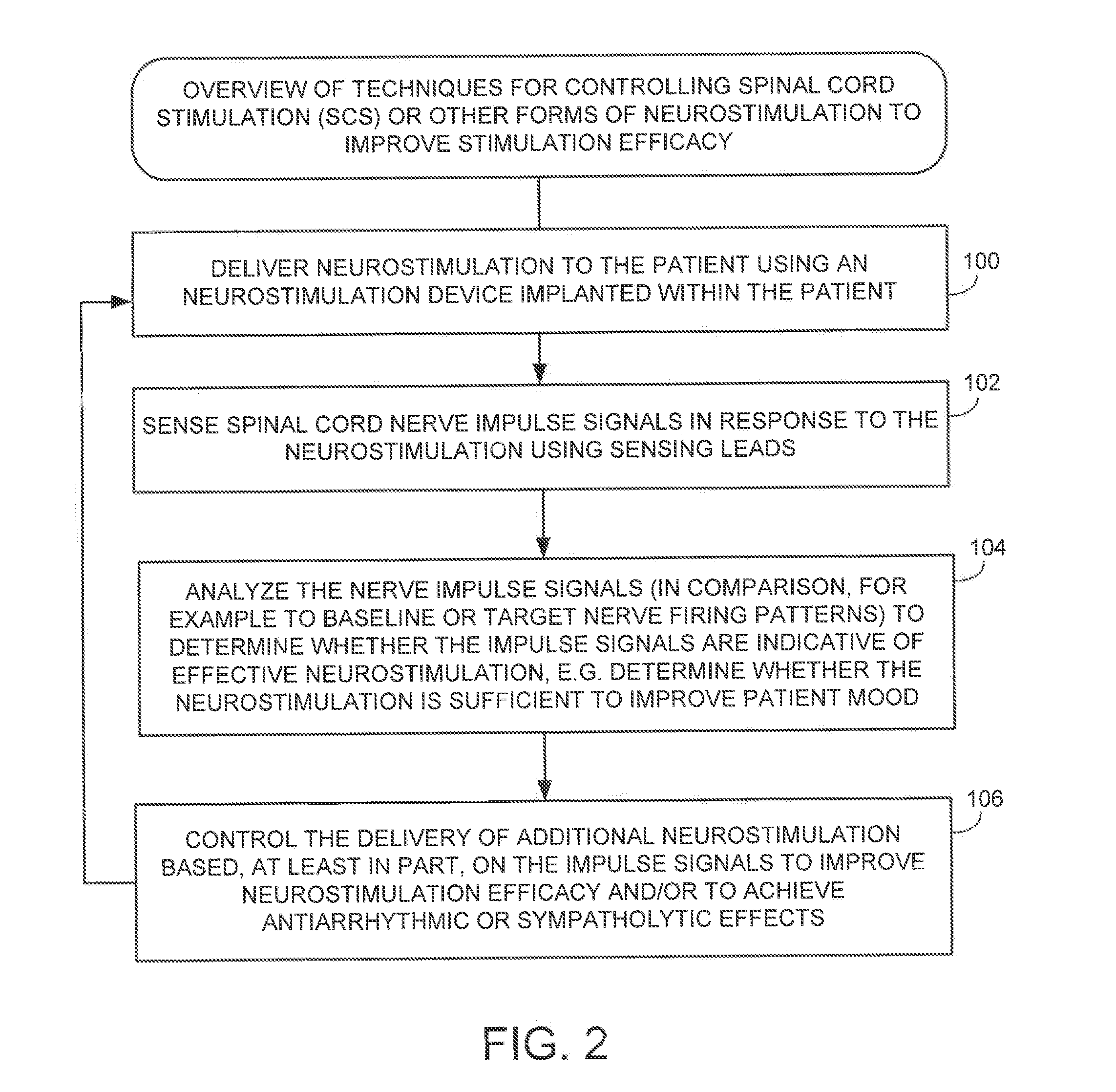
Techniques are provided for controlling spinal cord stimulation (SCS) or other forms of neurostimulation. In one example, SCS treatment is delivered to a patient and nerve impulse firing signals are sensed along the spinal cord following the SCS treatment. The nerve impulse signals are analyzed to determine whether the signals are associated with effective SCS and then the delivery of additional SCS is controlled to improve SCS efficacy. For example, the nerve impulse signals can be analyzed to determine whether the signals are consistent with a positive patient mood associated with pain mitigation and, if not, SCS control parameters are adjusted to improve the efficacy of the SCS in reducing pain. In other examples, heart rate variability (HPV) is also used to control SCS. Still further, adjustments may be made to SCS control parameters to improve antiarrhythmic or sympatholytic effects associated with SCS. Techniques employing baseline / target calibration procedures are also described.
Owner:PACESETTER INC
Methods of Determining Patient Response By Measurement of HER-2 Expression
Methods are provided for determining or otherwise assessing the response of a patient to treatment, in particular, to cancer treatment. The methods include the analysis of samples for the presence or the absence of HER2 markers alone or in conjunction with other biomarkers, such as HER3 markers. In certain examples, the probable time to progression can be determined by first determining HER2 positive patients and then further stratifying by using the presence or the absence of a second biomarker (e.g, HER3 markers). In addition, the data can be used to track a patient's response to a treatment regimen, assessing the expected success of treating a patient using a particular regiment, determining the effects of a treatment regiment or for categorizing a patient in order to create a homogenous group for a clinical trial.
Owner:LAB OF AMERICA HLDG
Peptides which elicit a high neutralizing antibody titer, cytotoxic T lymphocyte response and T helper cell response in a broad range of MHC type recipients
InactiveUS7094405B1High titerHigh titer of neutralizing antibodyPeptide/protein ingredientsAntibody mimetics/scaffoldsV3 loopT helper cell
Peptide constructs comprised of multideterminant T helper peptides from the envelope glycoprotein of HIV previously identified to induce proliferative responses in four different haplotypes of mice and IL-2 responses in 52-73% of HIV positive, flu positive patients (cluster peptides), were co-linearly synthesized with the peptide 18 of the V3 loop of HIV-1 gp 160, corresponding to the principal neutralizing determinant of HIV-IIIB and also shown to contain a dominant CTL epitope. Cognate help for peptide 18 antibody was elicited following a single immunization in all strains of mice which had previously responded to a T cell epitope encompassed by the peptides. In two strains of mice, the level of neutralizing antibody achieved was comparable to levels adequate for protection from homologous viral challenge in chimpanzees. After a single boost, much higher antibody titers for 90% neutralization in the range of 1:1000 to 1:16,000 were achieved. Spleen cells from mice of three distinct MHC haplotypes sharing the Dd class I MHC molecule but with different class II molecules, immunized with the compound peptides, exhibited enhanced gp160-specific CTL activity.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Systems and methods for controlling spinal cord stimulation to improve stimulation efficacy for use by implantable medical devices
Techniques are provided for controlling spinal cord stimulation (SCS) or other forms of neurostimulation. In one example, SCS treatment is delivered to a patient and nerve impulse firing signals are sensed along the spinal cord following the SCS treatment. The nerve impulse signals are analyzed to determine whether the signals are associated with effective SCS and then the delivery of additional SCS is controlled to improve SCS efficacy. For example, the nerve impulse signals can be analyzed to determine whether the signals are consistent with a positive patient mood associated with pain mitigation and, if not, SCS control parameters are adjusted to improve the efficacy of the SCS in reducing pain. In other examples, heart rate variability (HRV) is also used to control SCS. Still further, adjustments may be made to SCS control parameters to improve antiarrhythmic or sympatholytic effects associated with SCS. Techniques employing baseline / target calibration procedures are also described.
Owner:PACESETTER INC
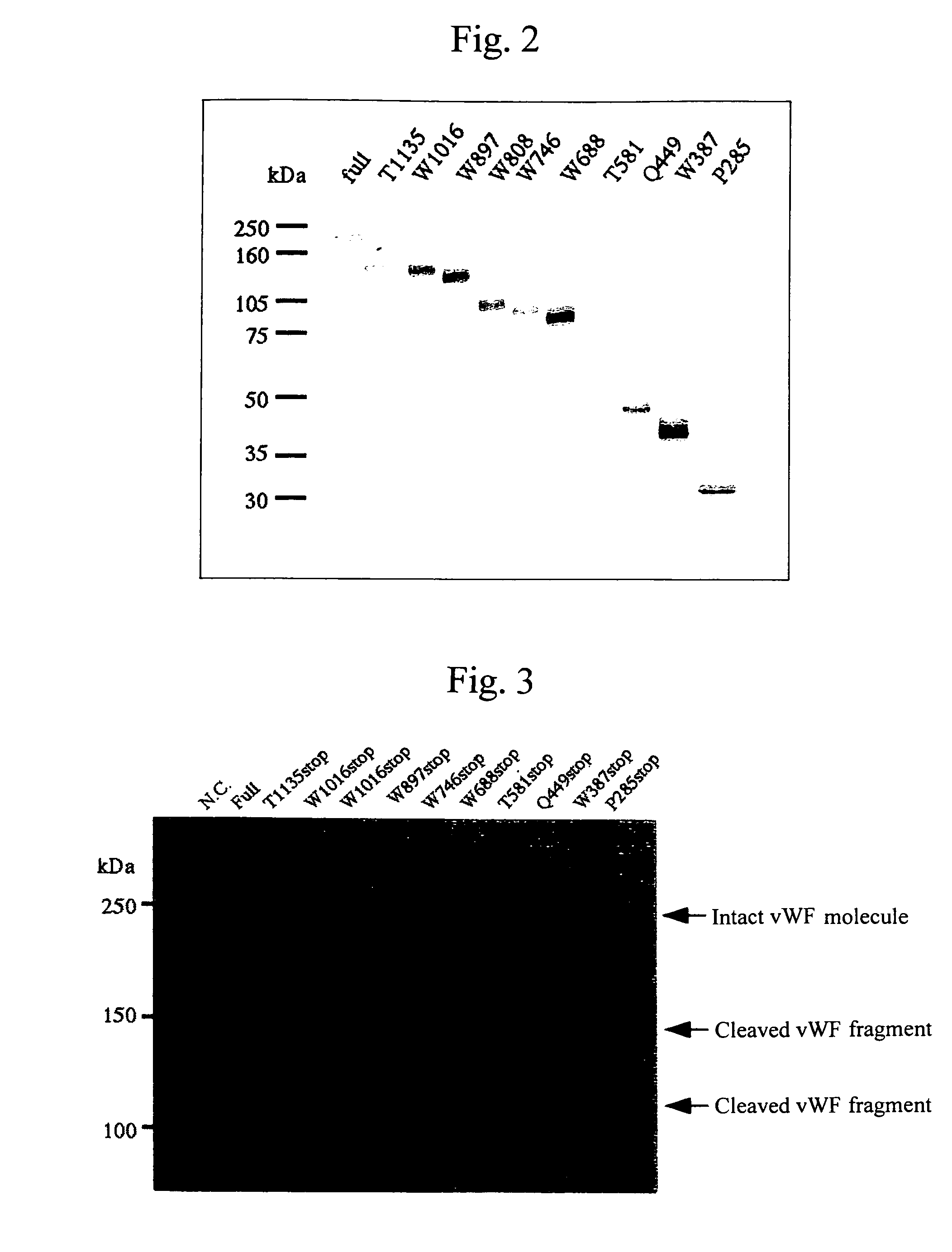
Antibody against von Willebrand factor cleaving enzyme and assay system using the same
ActiveUS7575872B2Easily isolated and sequencedUseful imageAnimal cellsHydrolasesEpitopeFactor VIII vWF
It is intended to provide an antibody showing immunoreactivity selectively to ADAMTS-13 and applications of this antibody in epitope analysis or diagnosis of an ADAMTS-13 autoantibody-positive patient. Alternatively, it is intended to provide a process for producing and use of a modified ADAMTS-13 molecule partially deleted aiming at the application in pharmaceutical products. An antibody specific for ADAMTS-13 which can be obtained from a warm-blooded animal immunized and sensitized with a polypeptide containing a part or the whole of ADAMTS-13 amino acid sequence; a process for producing an antibody comprising a step of immunizing and sensitizing a warm-blooded animal with a polypeptide containing a part or the whole of ADAMTS-13 amino acid sequence; use of the above-described antibody including a method of detecting and purifying ADAMTS-13; and a modified ADAMTS-13 molecule partially deleted are provided.
Owner:KM BIOLOGICS CO LTD
Ctl inducer composition
ActiveUS20100278851A1Wide rangeEffective treatmentBiocideSsRNA viruses positive-senseCancer preventionDisease
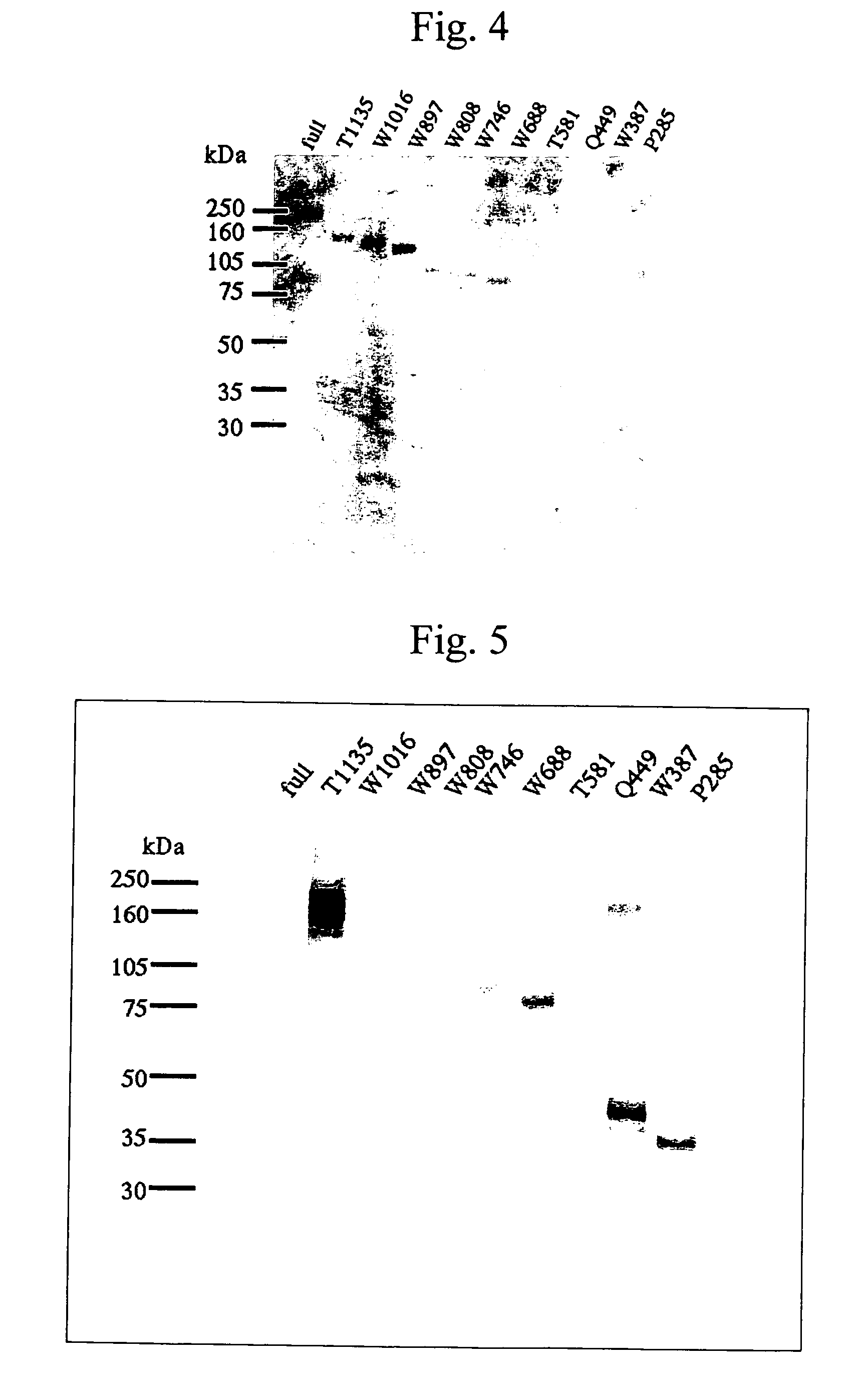
The present invention provides a CTL inducer composition which comprises one or more peptides selected from the group consisting of the peptides of SEQ ID NOS: 1 to 27 in the Sequence Listing, and can be used for the treatment or prevention of cancer or a hepatitis C virus-related disease in two or more patient groups selected from the group consisting of an HLA-A2 positive patient group, an HLA-A24 positive patient group, an HLA-A26 positive patient group, and an HLA-A3 supertype positive patient group.
Owner:BRIGHTPATH BIOTHERAPEUTICS CO LTD
Serum/plasma miRNA composition and use thereof
InactiveCN102021169AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSerum igeProtein markers
Owner:NANJING UNIV
Antibodies binding to an intracellular prl-1 or prl-3 polypeptide
ActiveCN101820910ABiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeLymphatic Spread
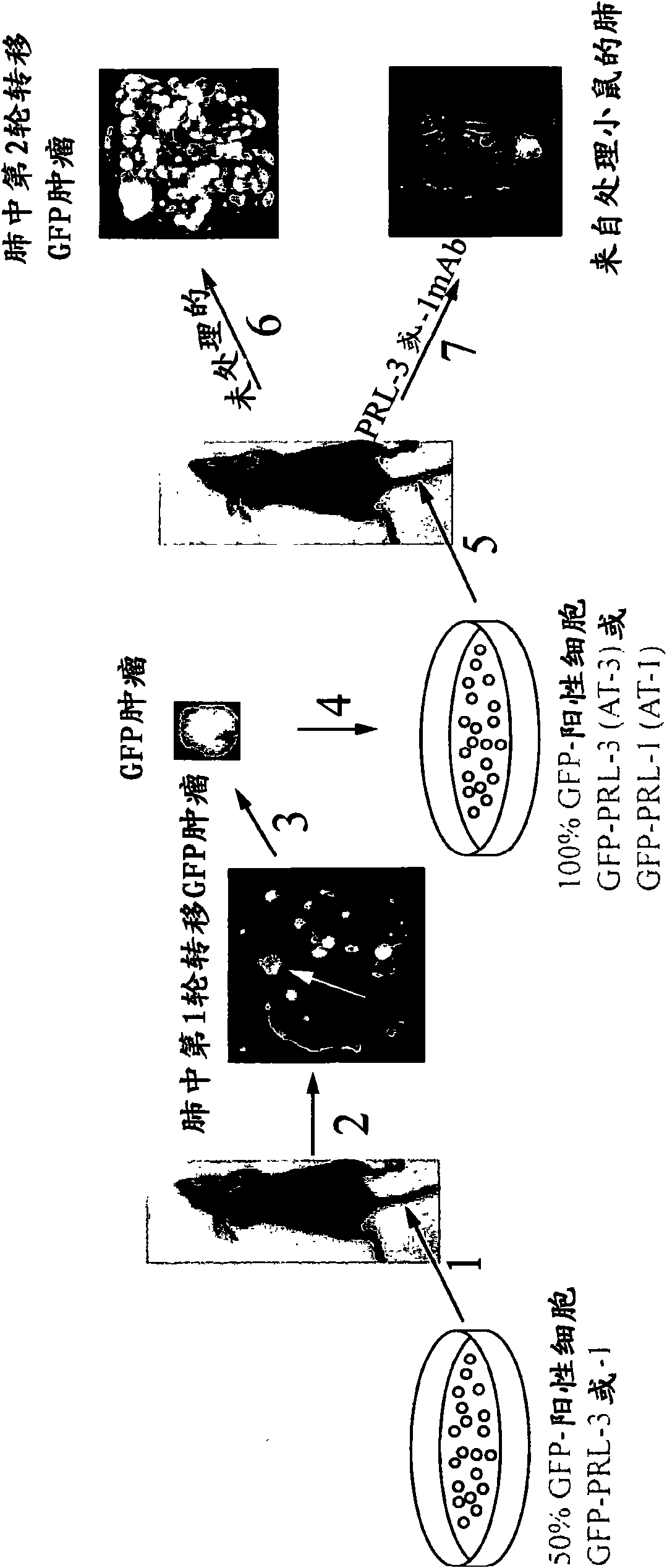
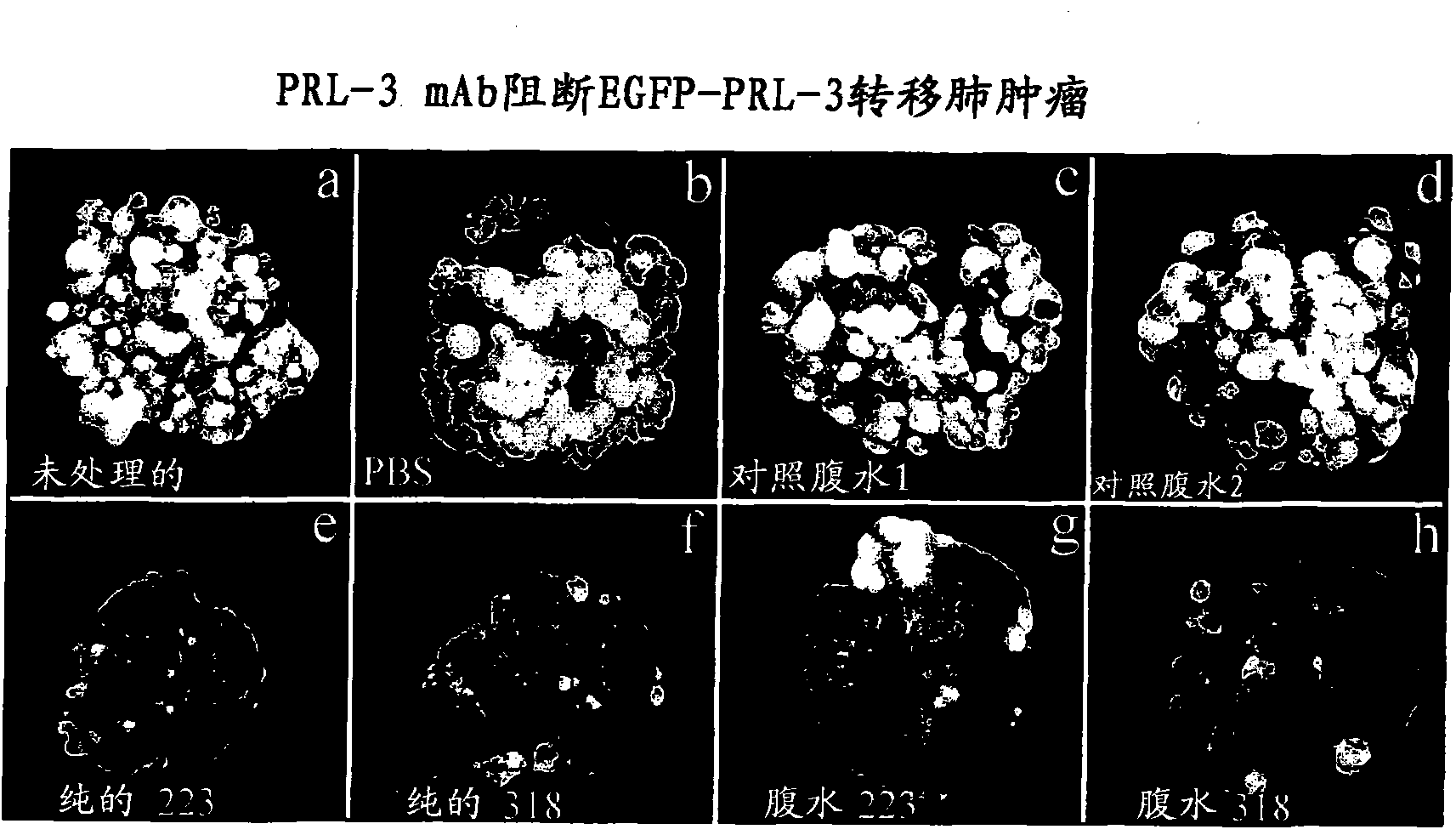
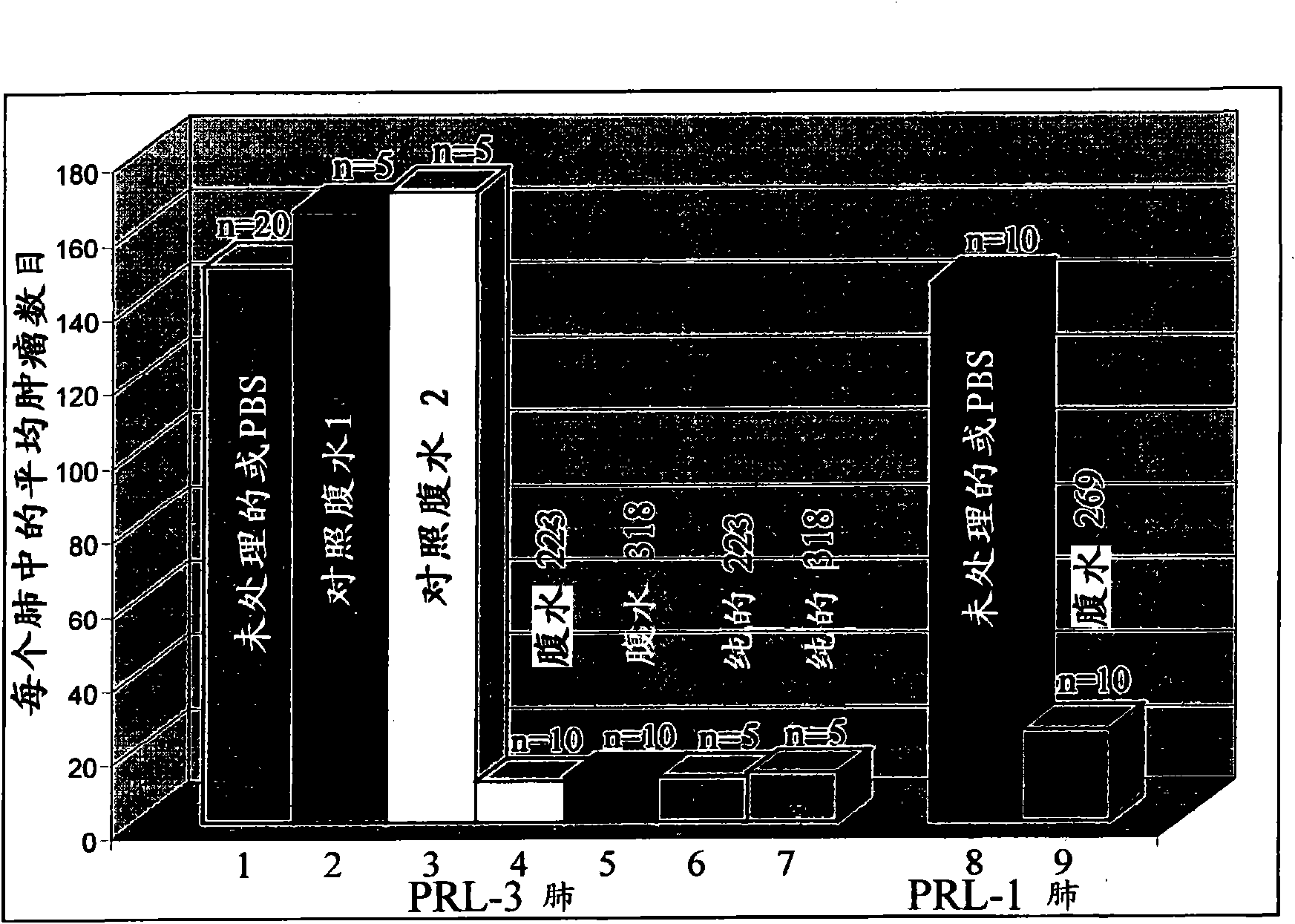
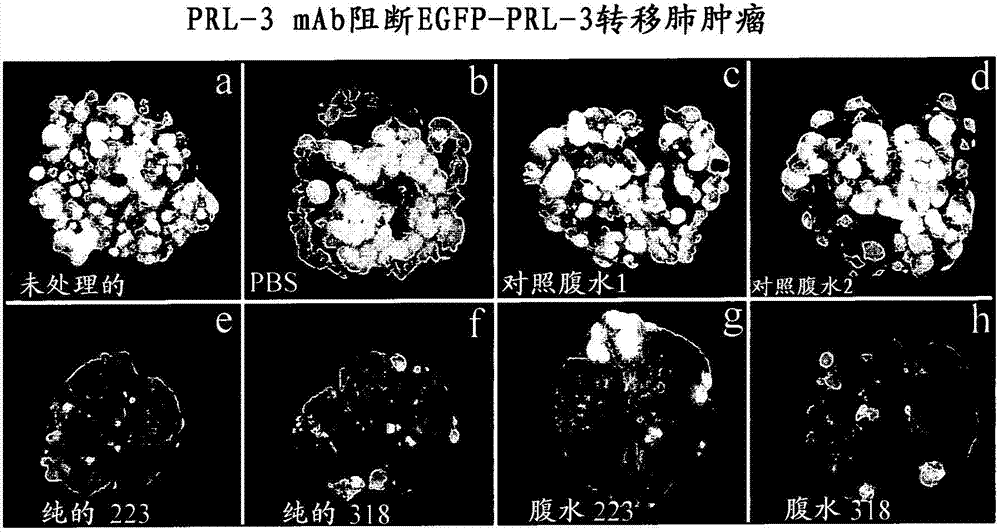
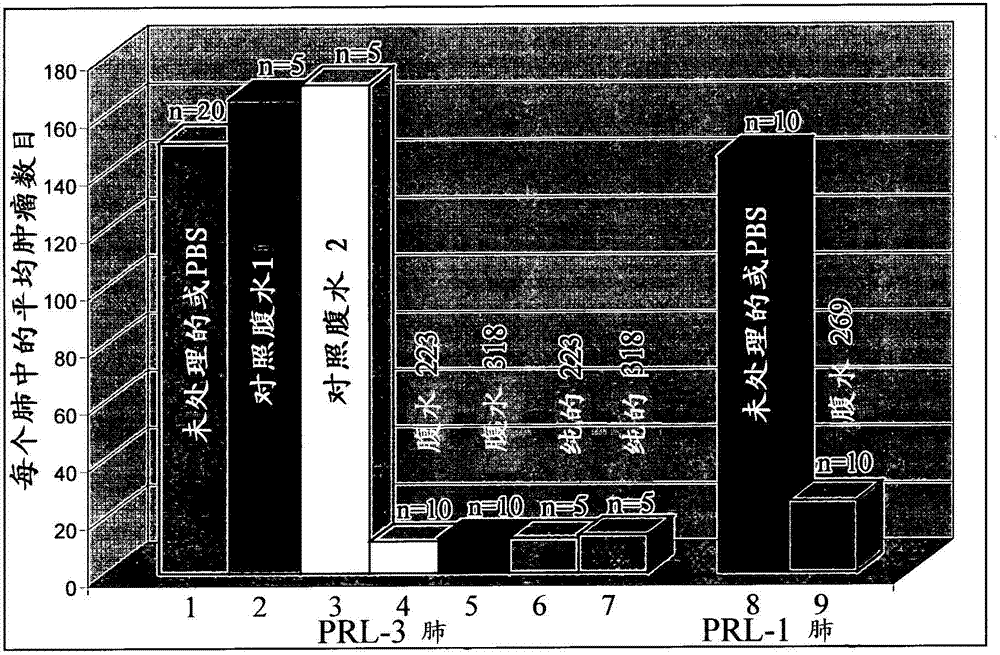
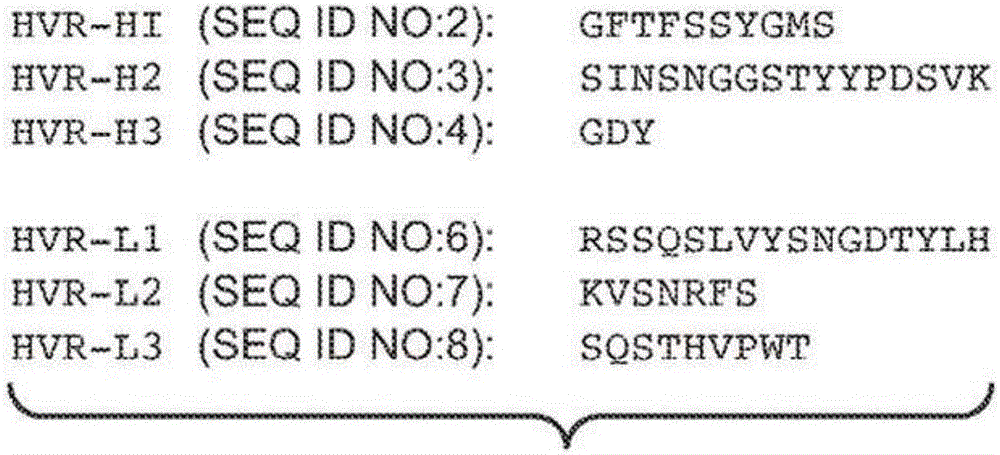
We provide an antibody capable of binding to an intracellular PRL-I or PRL-3 polypeptide, in which the antibody is capable of binding to an epitope bound by antibody 269, antibody 223 or antibody 318. Such anti-PRL antibodies may be capable of binding to intracellular PRL-I or PRL-3. They may be suitable for use as therapies against cancer or metastasis thereof, or in clinical diagnosis to identify PRL-3 or PRL-I positive patients.
Owner:AGENCY FOR SCI TECH & RES
CTL inducer composition
The present invention provides a CTL inducer composition which comprises one or more peptides selected from the group consisting of the peptides of SEQ ID NOS: 1 to 27 in the Sequence Listing, and can be used for the treatment or prevention of cancer or a hepatitis C virus-related disease in two or more patient groups selected from the group consisting of an HLA-A2 positive patient group, an HLA-A24 positive patient group, an HLA-A26 positive patient group, and an HLA-A3 supertype positive patient group.
Owner:BRIGHTPATH BIOTHERAPEUTICS CO LTD
Methods for determining anti-drug antibody isotypes
The present invention provides assay methods for the determination of one or more anti-drug antibody (ADA) isotypes in a sample. As a non-limiting example, the assays of the present invention are particularly useful for determining different ADA isotypes in samples from ADA-positive patients receiving an anti-TNFa drug such as REMICADETM (infliximab) or HUMIRATM (adalimumab). The present invention also provides methods for optimizing therapy and / or reducing toxicity in subjects receiving TNFa inhibitors for the treatment of TNFa-mediated disease or disorders.
Owner:SOC DES PROD NESTLE SA
Health-care tea and preparation method thereof
The invention discloses a health-care tea and a preparation method thereof. The health-care tea is prepared from the following raw materials by weight: 18-22 kg of negundo chastetree fruit, 15-25 kg of semen cassiae, 10-15 kg of chastetree fruit, 15-25 kg of fruits of slovenia dulcis, 20-30 kg of glossy privet fruit, 15-25 kg of red date, 9-11 kg of codonopsis pilosula, 15-25 kg of medlar, 4-6 kg of great burdock achene, 18-22 kg of radix astragali, 20-30 kg of cherokee rose fruit and 1-5 kg of chrysanthemum, wherein the raw materials are cleaned respectively, air-dried, then baked, sterilized, ground and finally packaged into tea bags. The health-care tea has excellent color, smell, taste and form, is mellow and fragrant, induces saliva, slakes thirst, is refreshing, invigorates the vitality, has the effects of clearing intestinal toxin, managing intestine and stomach, enriching blood, invigorating vital energy, nourishing kidney, dispelling wind, eliminating dampness and nourishing and protecting liver, and is particularly suitable for people who drink and smoke more and stay up late frequently, HBsAg, HBeAg and Anti-HBc positive and HBsAg, Anti-HBe and Anti-HBc positive patients and sub-health people due to environmental pollution.
Owner:广东五子神科技有限公司
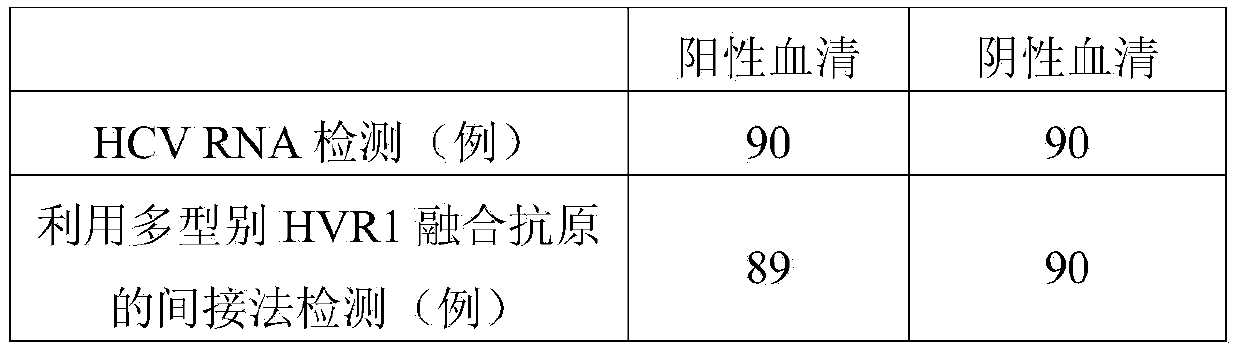
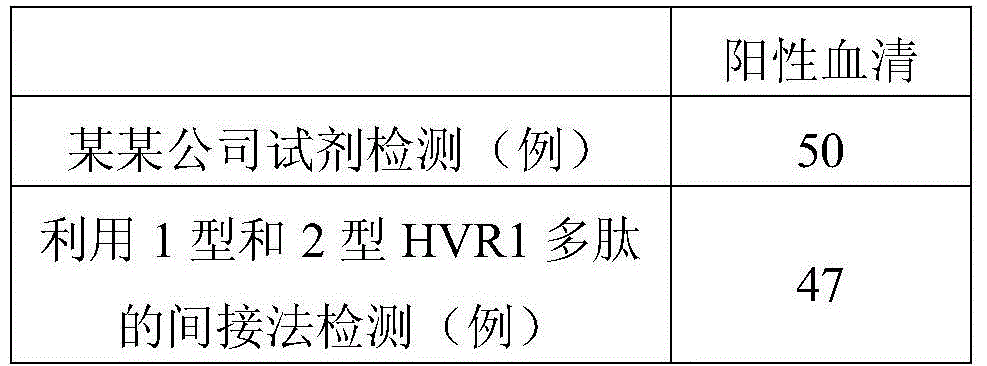
Hepatitis C virus HVR1 (Hypervariable Region 1) fusion antigen and application thereof
ActiveCN104193827AAvoid disadvantagesEliminate missed detectionBiological testingHybrid peptidesHcv hepatitisAntibody hypervariable region
The invention discloses a hepatitis C virus HVR1 (Hypervariable Region 1) fusion antigen. An amino acid sequence of the fusion antigen is shown as SEQ ID No.1. The invention also discloses application of the fusion antigen in preparation of a hepatitis C virus HVR1 antibody detection kit. The experiment proves that the cross reactivity of the fusion antigen is obviously improved compared with that of a single segment HVR1 antigen, and the detection coincidence rate of hepatitis C virus RNA positive patients is 99 percent. Because the HVR1 is a main neutralizing antigen epitope in the hepatitis C virus, the antigen can be used for achieving an aim of comprehensively detecting HCV infection for hepatitis C virus HVR1 antibody detection and effectively solving the problems such as outcome and prognosis of HCV diseases and prediction of interferon curative effect and has extremely great clinical application prospects.
Owner:山东莱博生物科技有限公司
Broad spectrum anti-HPV-L1 and E6/E7-IgY, small molecule antibody and application thereof
PendingCN110054686ASolve the problem of "turning yang into yin"Egg immunoglobulinsImmunoglobulins against virusesHuman papillomavirusDisinfectant
The invention discloses broad spectrum or multivalent anti-HPV-L1 and E6 / E7-IgY, a small molecule antibody, a preparation method, a composition and application thereof. The genetic recombination technology is adopted to prepare prophylactic broad spectrum anti-HPV-L1-IgY and therapeutic broad spectrum or multivalent anti-HPV-E6 / E7-IgY and the small molecule antibody, and the broad spectrum or multivalent anti-HPV small molecule antibody is subjected to long-term modification with liposomes and cell penetrating peptides coupled. The invention also provides a liquid crystal microcapsule, a gel,a suppository, a cream, a lotion, a tablet, an effervescent tablet, a capsule, a soft capsule and an injection prepared by the broad spectrum or multivalent anti-HPV-IgY, the small molecule antibody and the composition, as well as the application in drugs, disinfectants, health products and medical devices for prevention and treatment of human papillomavirus (HPV) infections. The majority of HPV infections can be prevented, and HPV-infected positive patients can be treated to achieve the positive-to-negative conversion effect.
Owner:SHENZHEN JASON INTELLIGENT BIOTECH CO LIMLTED PRC
A kind of oral powder for treating hepatitis B
InactiveCN102274438AEnhanced inhibitory effectImprove your own anti-virus abilityPowder deliveryAnthropod material medical ingredientsOral PowderCurative effect
The "an oral powder for treating hepatitis B" of the present invention has strict compatibility. The curative effect is definite in the treatment of hepatitis B virus carriers or patients with hepatitis B, improving the self-immunity of hepatitis B patients, rapidly reducing enzymes, and repairing damaged liver cells. After being taken by more than a thousand patients, no virus mutation was found in one case; the negative conversion rate for patients with large and small three yang Compared with similar drugs, it is relatively improved, up to about 60%.
Owner:莫桂森
Medicament for treating HPV infection and application of medicament in preparation of medicament for treating HPV infection
InactiveCN102416175ANot easy to relapseSsRNA viruses negative-senseViral antigen ingredientsHuman papilloma virus infectionHuman papillomavirus
A medicament for treating HPV infection and an application of the medicament in the preparation of medicaments for treating HPV infection. The invention is characterized in that the active component of the medicament is rabies vaccine for human, and a pharmaceutically-acceptable carrier is added to prepare the medicament; the invention also provides an application of the medicament in the preparation of medicaments for treating HPV infection, that is, a new application of rabies vaccine for human in the preparation of medicaments for treating human papillomavirus infection. The medicament (rabies vaccine for human) for treating human papillomavirus infection provided by the invention has obvious treatment effect on human papillomavirus infection; through animal experiments and preclinical observation, the medicament has satisfied and beneficial effect, has the characteristics of safety, effectiveness, and difficult recurrence after cure, and has a treatment negative conversion rate of up to 90% for human papillomavirus infection positive patients.
Owner:LIAONING CHENGDA BIOTECH
Methods and compositions for cancer prevention and treatment
ActiveUS20130331365A1Preventing and delaying onsetMaintains genome stabilityOrganic active ingredientsBiocideProgestin AntagonistCancer prevention
The present invention provides methods of preventing or delaying the development of cancer (e.g., breast cancer) in BRCA1 mutation positive patients by beginning progesterone receptor antagonist treatment at an early age (e.g., by age 35, 30, or 25). In certain embodiment, such early treatment is long-term treatment, which may substitute or delay a preventative ovariectomy, single or double mastectomy (e.g., in patients wishing to delay or avoid a mastectomy, or patients that cannot afford a mastectomy).
Owner:RGT UNIV OF CALIFORNIA
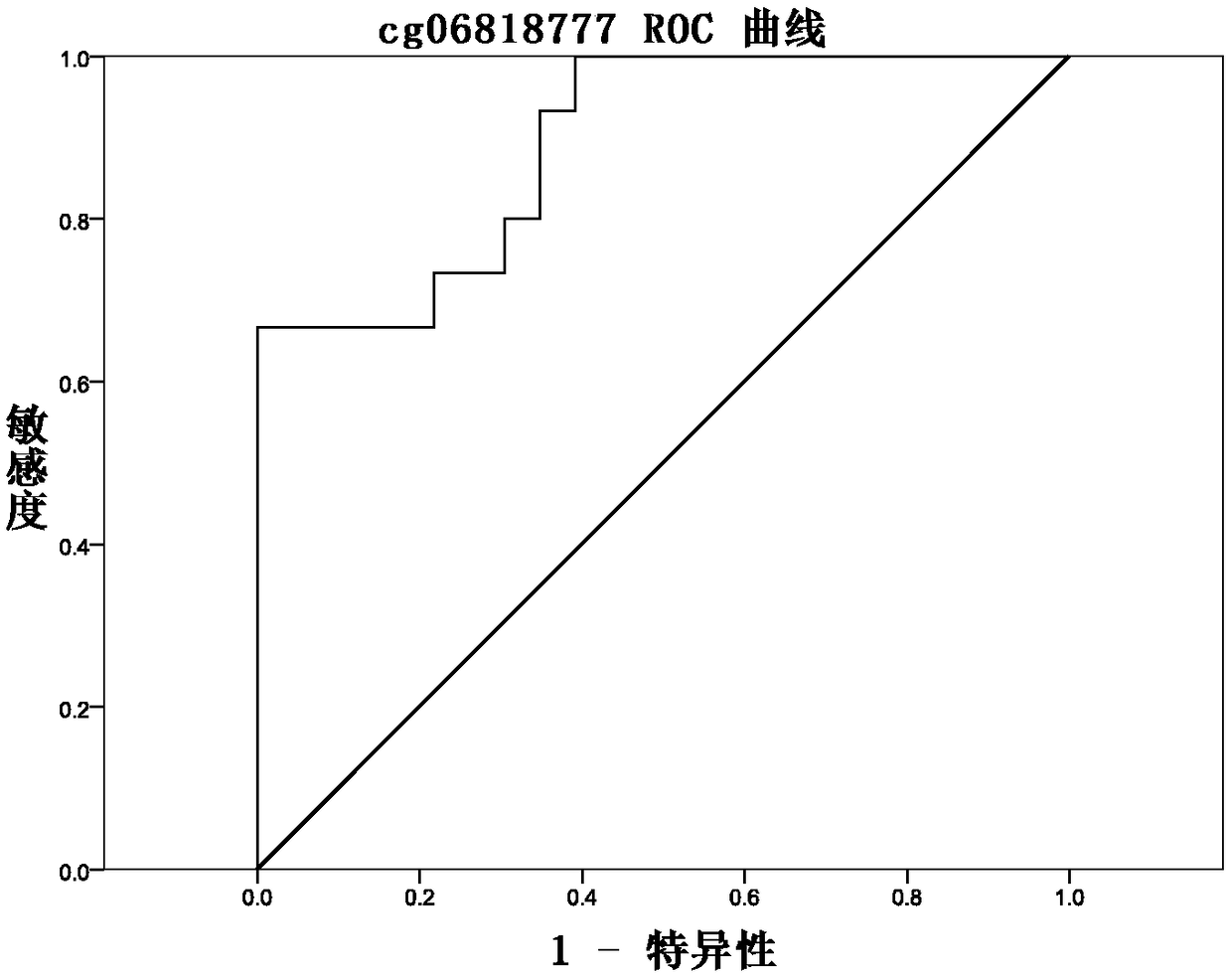
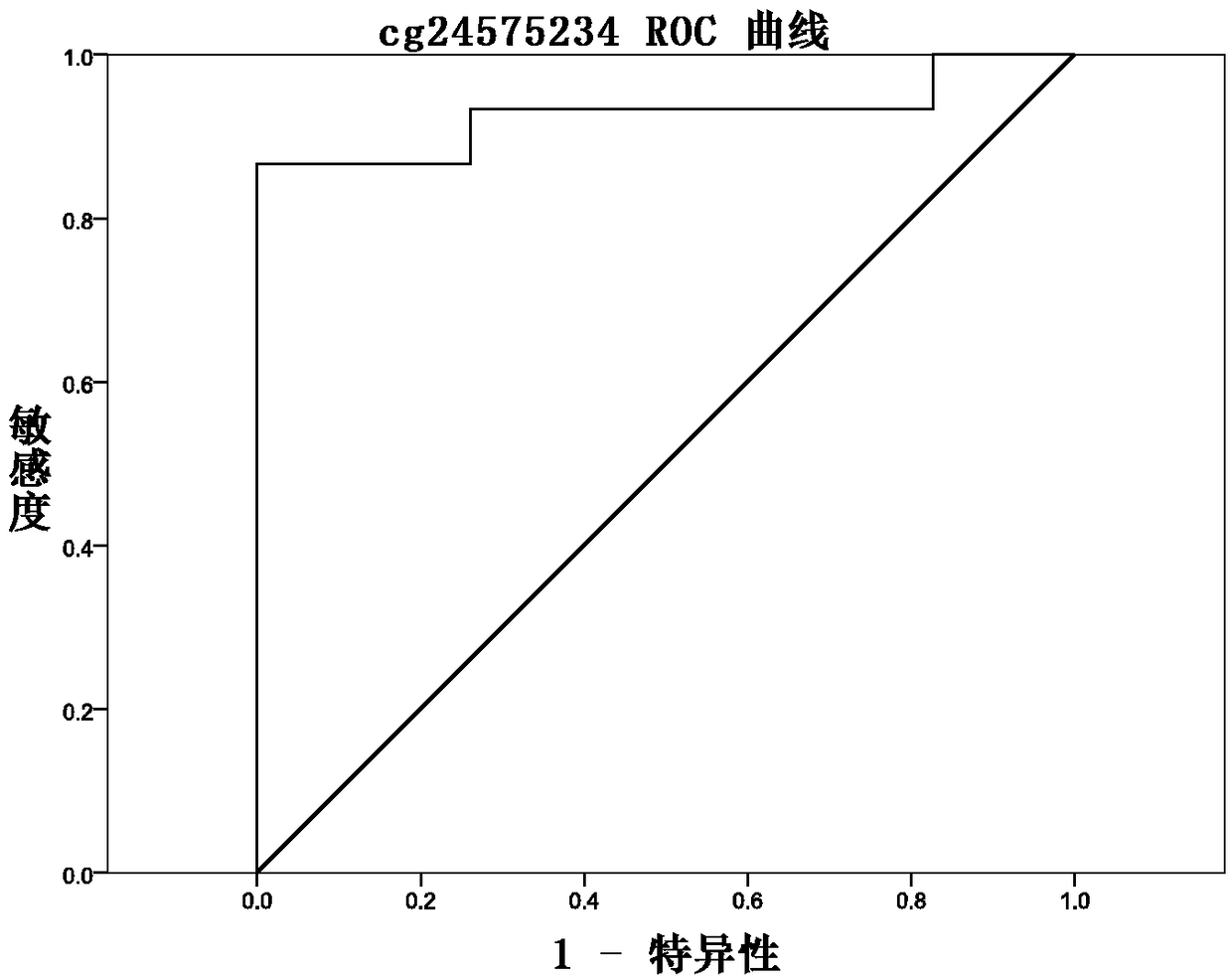
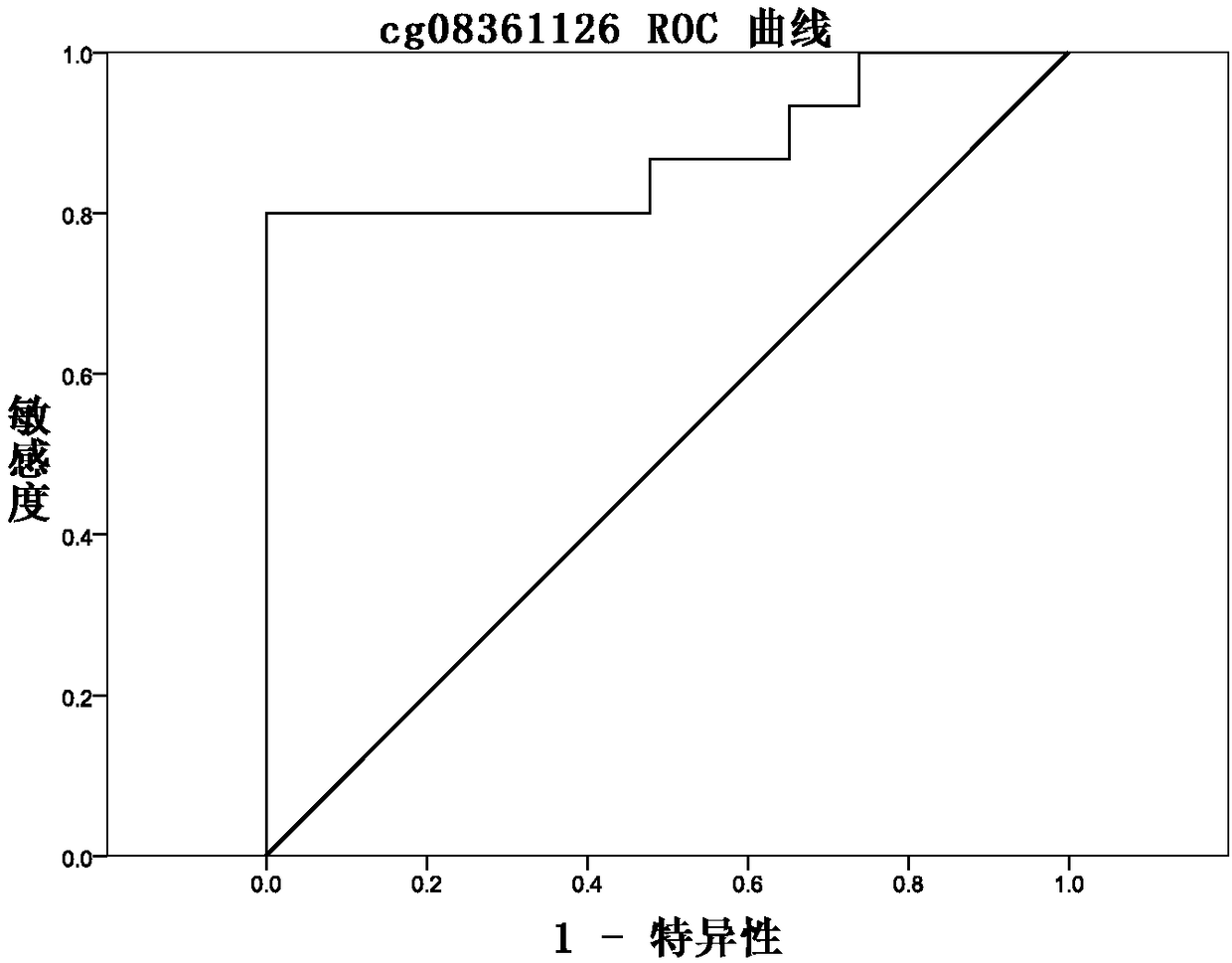
Methylation site for cervical cancer screening and detection primer thereof
ActiveCN109468381AImprove featuresThe result is objectiveMicrobiological testing/measurementDNA/RNA fragmentationCervical cancer screeningMethylation Site
The invention discloses a methylation site for cervical cancer screening. The methylation site is a cg24575234 site of a CHRM2 gene, a cg06818777 site of a CHAD gene, a cg08361126 site of an FGF10 gene, and a cg08976810 site of an ITGA8 gene. The invention also discloses a primer pair included in a kit for detecting a methylation level of a sample gene of cervical cancer. According to the invention, a whole process can be automated, the result is objective and is easy to interpret, and the influence of subjective factors is avoided; and the specificity of the methylation site, provided by theinvention, for detecting and screening cervical cancer is 100%, which is greater than the specificity (80%) of an HPV gene detection method, and the positive prediction value is increased from 0.0474%to 100%, so that the screening result is objective and is easy to interpret. The methylation site provided by the invention can also be used in parallel with a current cervical cancer screening experiment, thereby greatly saving the examination cost and avoiding economic losses and psychological burdens of too many false positive patients due to positive results.
Owner:兰州市第一人民医院
Antibodies binding to an intracellular PRL-1 OR PRL-3 polypeptide
InactiveCN102847148ABiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeClinical diagnosis
We provide an antibody capable of binding to an intracellular PRL-I or PRL-3 polypeptide, in which the antibody is capable of binding to an epitope bound by antibody 269, antibody 223 or antibody 318. Such anti-PRL antibodies may be capable of binding to intracellular PRL-I or PRL-3. They may be suitable for use as therapies against cancer or metastasis thereof, or in clinical diagnosis to identify PRL-3 or PRL-I positive patients.
Owner:AGENCY FOR SCI TECH & RES
Methods of treating alzheimer's disease
InactiveCN106456729ANervous disorderMicrobiological testing/measurementPredictive biomarkerPediatrics
Methods of treating Alzheimer's Disease (AD) in patients suffering from mild to moderate AD, including ApoE4 positive patients and patients suffering from mild AD are provided. Also provided are methods of selecting or identifying patients for treatment with an anti-Abeta antibody. Methods include the use of prognostic and / or predictive biomarkers.
Owner:F HOFFMANN LA ROCHE & CO AG
Application of L858R+V834L gene mutation primary tumor transplantation tumor model for human non-small cell lung cancer
The invention discloses application of an L858R+V834L gene mutation primary tumor transplantation tumor model for a human non-small cell lung cancer. A transplantation tumor generated by the L858R+V834L gene mutation primary tumor transplantation tumor model for the human non-small cell lung cancer can be restrained by Iressa; the L858R+V834L gene mutation primary tumor transplantation tumor model provided by the invention can be used for screening a targeted drug for treating an EGRF (estimated glomerular filtration rate) mutation positive patient suffered from the non-small cell lung cancer; and an experimental technical platform is provided for the deep exploration of the action of epidermal growth factor receptor (EGFR) mutation generated in the non-small cell lung cancer.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Method for establishing HIV (human immunodeficiency virus) virus antibody yeast display library, method for screening virus broad-spectrum neutral antibody and application thereof
InactiveCN104725501AQuality improvementImprove efficiencyImmunoglobulins against virusesAntiviralsYeast AntibodyYeast display
The invention provides a method for screening a virus broad-spectrum neutral antibody and application thereof. The method comprises the following steps: preparing or providing a virus antigen protein; marking the virus antigen protein by using a fluorescence marker; preparing a cDNA (complementary deoxyribonucleic acid) template of a virus positive patient, designing primers, and amplifying the antibody gene; establishing a yeast antibody display library by using the obtained antibody gene; carrying out antigen protein mixed incubation on yeast cells in the obtained antibody display library and the obtained virus with the fluorescence marker, setting a cell separation door, and separating fluorescence positive yeast cells by a flow cytometry; and carrying out sequencing identification on the fluorescence positive yeast cells to obtain the virus broad-spectrum neutral antibody. The invention also provides a method for establishing an HIV (human immunodeficiency virus) virus antibody yeast display library and application thereof.
Owner:SHENZHEN INST OF ADVANCED TECH
Method, biomarker and diagnostic agent for detection of high-risk prostate cancer
ActiveUS20190049451A1High riskLow detection levelBiological material analysisPsa testSpecific lectin
[Problem] To provide a method for detecting high-risk prostate cancer, for the purpose of providing useful information, such as necessity of biopsy, to a test-positive patient in a PSA test.[Solution] The method for detecting high-risk prostate cancer according to the present invention comprises reacting a PSA contained in a sample composed of urine collected from a human body which is suspected to be suffering from prostate cancer with (1) a fucose α1→6 affinitive lectin which has a characteristic property that the lectin has affinity expressed by a binding constant of 1.0×104 M−1 or more (at 25° C.) for an α1→6 fucose sugar chain No. 405. The fucose α1→6 affinitive lectin is preferably (2) a fucose α1→6 specific lectin which has a characteristic property that the lectin has a binding constant of 1.0×104 M−1 or less (at 25° C.) for a sugar chain No. 003 that does not contain α1→6 fucose and a glycolipid-type sugar chain No. 909 that does not contain α1→6 fucose.
Owner:MGC WOODCHEM CORP +1
Oral powder for treating hepatitis B
InactiveCN102266517BEnhanced inhibitory effectImprove your own anti-virus abilityAnthropod material medical ingredientsDigestive systemOral PowderMedicine
Owner:莫桂森
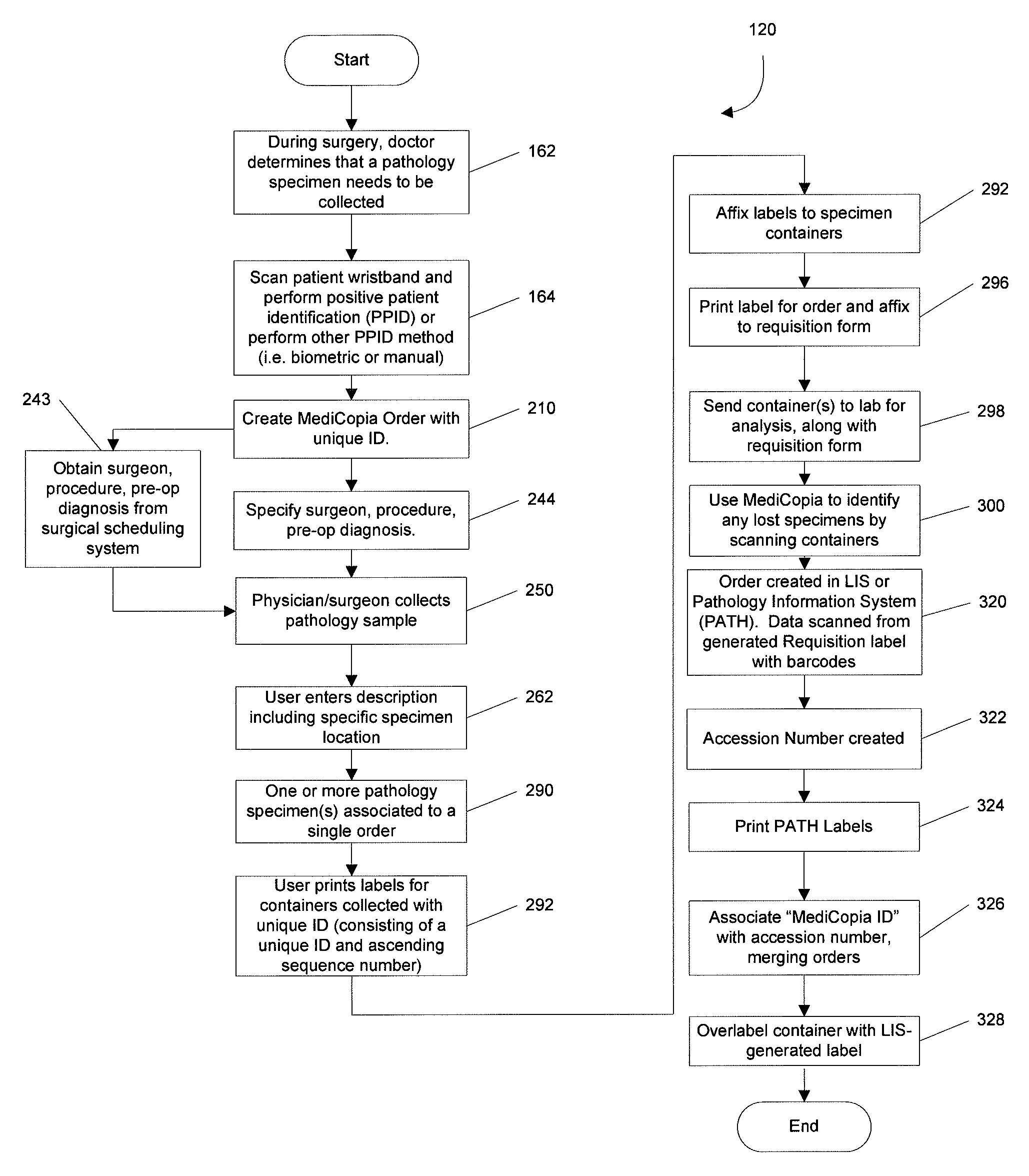
System and method for pathology specimen collection
InactiveUS9058636B2Computer-assisted medical data acquisitionLogisticsPathology specimensComputer science
A computer-implemented method for labeling and tracking a pathology specimen after collection and positive patient identification comprises the step of creating a pathology specimen record with a unique specimen identification code for at least one specimen. The method further includes the step of creating a label for application to a container holding the specimen, wherein the label includes the unique specimen identification code and creating an order with a unique accession number after the container is forwarded for processing, by scanning the label. The unique identification code is utilized for tracking purposes to ensure that pathology specimens for the positively identified patient are not lost in transit to the pathology laboratory for further processing. This improves quality to the patient by the elimination of transcription errors, identification of missing or lost specimens, and confirmation that pathology specimens are correctly matched to a patient.
Owner:LATTICE
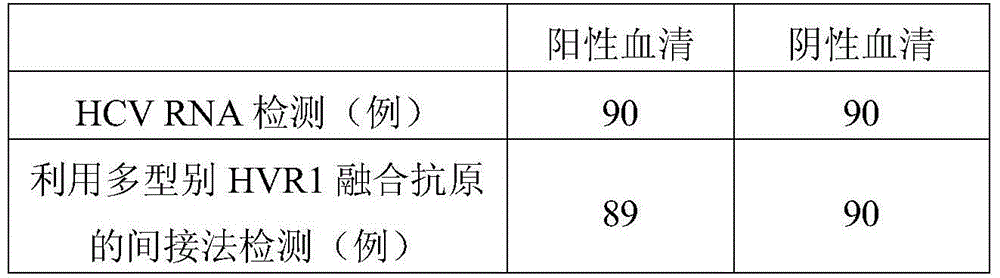
A fusion antigen of the first hypervariable region of hepatitis C virus and its application
ActiveCN104193827BHigh positive serum coverageIncreased cross-reactivityBiological testingHybrid peptidesAntibody hypervariable regionPositive patient
The invention discloses a fusion antigen of the first hypervariable region of hepatitis C virus. The amino acid sequence of the fusion antigen is shown in SEQ ID No.1. The invention also discloses the detection of the fusion antigen in the preparation of hepatitis C virus HVR1 antibody. application in the kit. It is verified by experiments that the cross-reactivity of the fusion antigen of the present invention is significantly improved compared with the single fragment HVR1 antigen, and the detection coincidence rate with hepatitis C virus RNA-positive patients is as high as 99%. Since HVR1 is the main neutralizing epitope in hepatitis C virus, through the application of the antigen of the present invention, for the detection of hepatitis C virus HVR1 antibody, the purpose of comprehensive detection of HCV infection can be realized, and it can be used for the transformation of HCV disease. Problems such as reconciliation prognosis and interferon curative effect prediction can be effectively solved, and have great clinical application prospects.
Owner:山东莱博生物科技有限公司
Methods of Determining Patient Response by Measurement of HER-2 Expression
Methods are provided for determining or otherwise assessing the response of a patient to treatment, in particular, to cancer treatment. The methods include the analysis of samples for the presence or the absence of HER2 markers alone or in conjunction with other biomarkers, such as HER3 markers. In certain examples, the probable time to progression can be determined by first determining HER2 positive patients and then further stratifying by using the presence or the absence of a second biomarker (e.g, HER3 markers). In addition, the data can be used to track a patient's response to a treatment regimen, assessing the expected success of treating a patient using a particular regiment, determining the effects of a treatment regiment or for categorizing a patient in order to create a homogenous group for a clinical trial.
Owner:LAB OF AMERICA HLDG
Pair of primers for amplifying LINC02253 gene and application thereof
ActiveCN109266746ARapid responseAccurate responseMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceLesion
The invention discloses a pair of primers for amplifying LINC02253 gene and application thereof, which is mainly used for detecting LINC02253 / GAPDH value by real-time fluorescence quantitative PCR oncolonoscopic sample or operation sample, if the ratio>=0. 00018 is positive, (0.00018 is negative. This method is an auxiliary diagnostic tool. Positive patients should be reexamined periodically if the pathological test is negative. At first, that PCR detection method is convenient, simple and suitable for hospital at all levels to popularize and use. The biopsy samples are helpful to determine the lesion and localize the tumor. Therefore, LINC02253 gene has high accuracy, specificity and practicability in the diagnosis of colorectal cancer.
Owner:NANJING MEDICAL UNIV
High dose treatments for Alzheimer's disease
InactiveCN108602883AIncrease incidenceReduce incidenceNervous disorderMicrobiological testing/measurementHigh dosesHigh dose treatment
Owner:GENENTECH INC
HA-1 epitopes and uses thereof
InactiveUS20080206268A1Improve anti-tumor effectPeptide/protein ingredientsMammal material medical ingredientsImmune therapyHematopoietic Tumor
Peptide sequences constituting T-cell epitopes of minor Histocompatibility antigen, HA-1. HA-1 is associated with Graft versus Host Disease. The peptides and their derivatives find many uses, for instance, in bone marrow transplantation, organ transplantation and in treatment of leukemia and non-hematopoietic tumors. The peptide and / or its derivatives can be incorporated in vaccines, in pharmaceutical formulations and they can be used in diagnostic test kits. HA-1 is expressed by non-hematopoietic tumor cells. While absent in normal epithelial cells, tumor cells and tumor cell lines, particularly from epithelial origin, express HA-1 and are recognized by HA-1 cytotoxic T-cells. The invention provides means and methods for HA-1 specific immunotherapy for HA-1-positive patients with non-hematopoietic tumor cells.
Owner:GOULMY ELSA A J M +2
Method for detecting pulmonary embolism
InactiveCN107860702AMature technologySimple and fast operationIndividual particle analysisDiagnostic SpecificityMicroparticle
The invention discloses a method for detecting pulmonary embolism. Absolute counting of platelet microparticles in blood plasma is detected to serve as auxiliary diagnosis of the pulmonary embolism bythe method based on standard flow cytometry. Through ROC curve analysis, the critical value of the platelet microparticles for diagnosing the pulmonary embolism is 716.90 per mu l; the sensitivity and the specificity at the critical value are respectively 58.82 percent and 95.92 percent. The positive likelihood ratio is 14.41, and the negative likelihood ratio is 0.43. The positive predictive value is 93.75 percent, and the negative predictive value is 69.12 percent; the Youden's index is 54.74 percent. The method is a non-invasive operation and is low in cost, easy to perform and high in specificity, and can further screen patients with a positive D-dimer result clinically, improves diagnostic specificity, and can serve as an evaluation means before CTPA examination is performed on a patient with the pulmonary embolism, so that adverse reactions caused by the CTPA examination are reduced, and the burden of the patient is lightened.
Owner:傅应云
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com