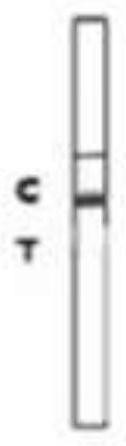Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Pestivirus infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections
InactiveUS20040082574A1Potent and selective activityPotent activityBiocideSugar derivativesPestivirusMedicine
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
Compounds with the bicyclo[4.2.1]nonane system for the treatment of Flaviviridae infections
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
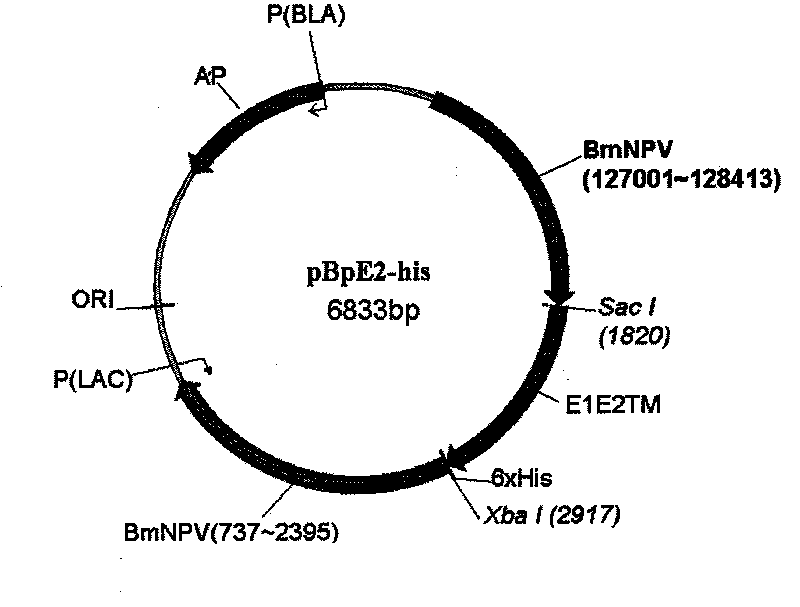
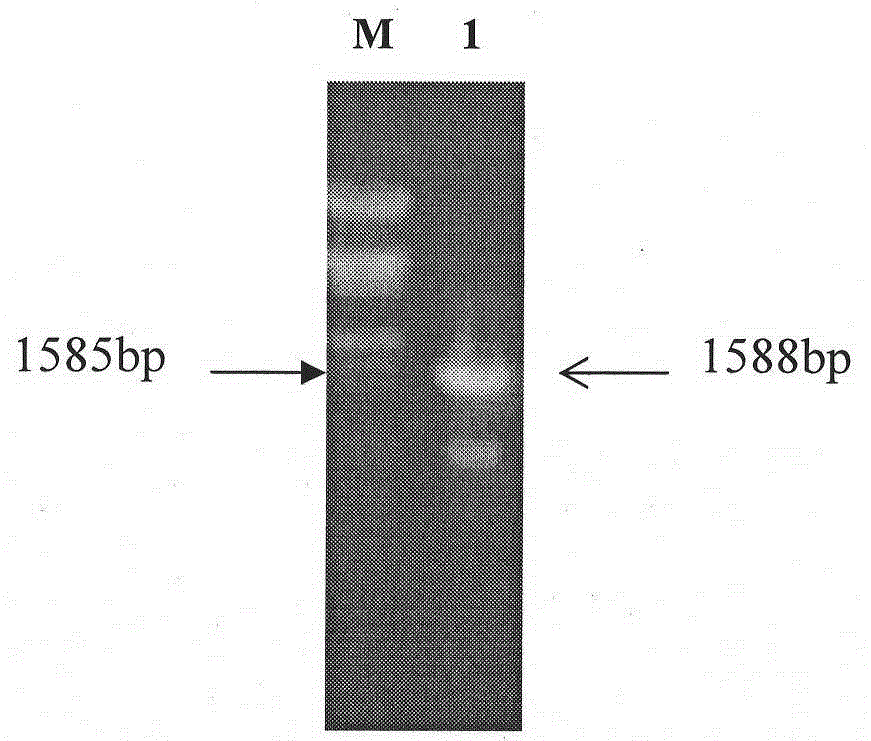
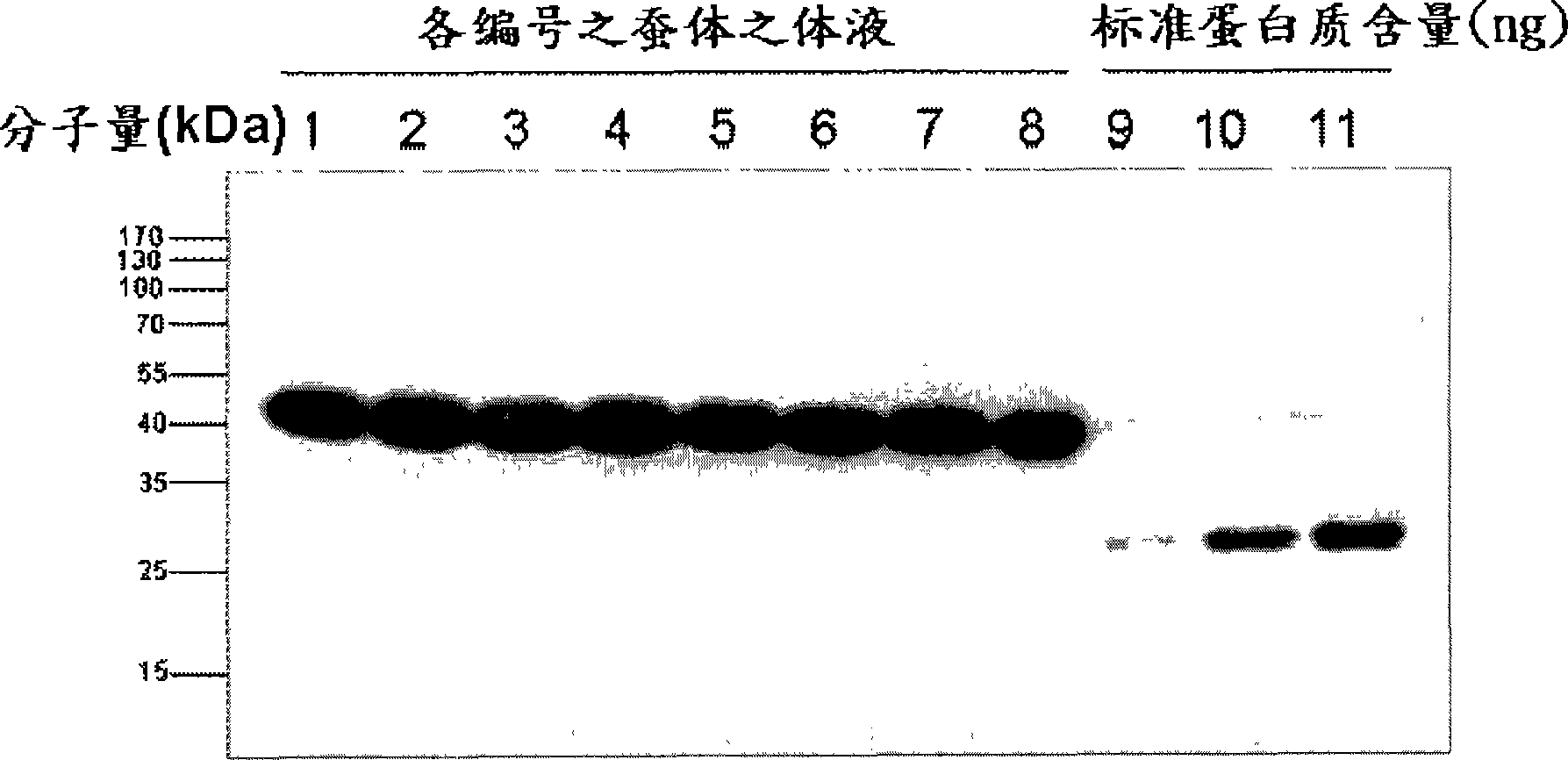
Classical swine fever virus E2 subunit vaccine and preparation thereof
The invention relates to a recombinant E2 protein by using silkworm as a platform to produce a classical swine fever virus gene subgroup 2.1. The invention also provides a subunit vaccine comprising the recombinant protein, which can be used for enhancing the capability of swine to resist classical swine fever virus infection.
Owner:黄金城
Swine fever virus subunit vaccine and preparation method and purpose thereof
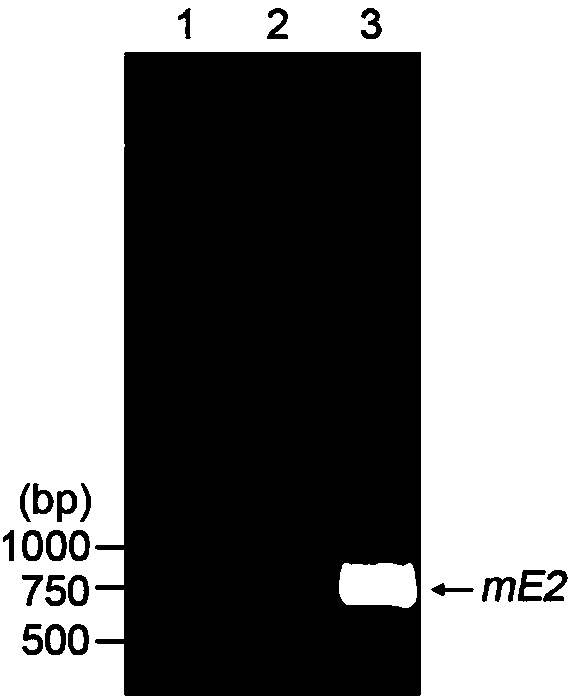
The invention belongs to the technical field of biological medicine, and concretely relates to a swine fever virus subunit vaccine and a preparation method and a purpose thereof. The invention provides a kluyveromyces marxianus yeast recombination strain used for preparing the swine fever virus subunit vaccine. The recombination strain is constructed by the following steps: swine fever virus envelope protein E2 is intercepted, through codon optimization, a coding sequence of the swine fever virus mE2 protein is obtained, and then is cloned to a kluyveromyces marxianus yeast expression vector,and the kluyveromyces marxianus yeast host strain is transformed. The invention also provides the method for preparing the swine fever virus subunit vaccine, which comprises the following steps: the kluyveromyces marxianus yeast host strain is subjected to recombination expression by using mE2, steps of culture, centrifugation, cell disruption, and separating purifying are carried out to obtain the swine fever virus mE2 protein antigen, and the purified antigen and an adjuvant are subjected to complex formulation to prepare the swine fever virus subunit vaccine. The injection immunotherapy ofswine fever virus mE2 protein recombination subunit vaccine can obtain a protective IgG antibody, and the subunit vaccine can reduce and prevent the swine fever virus infection-related disease.
Owner:FUDAN UNIV
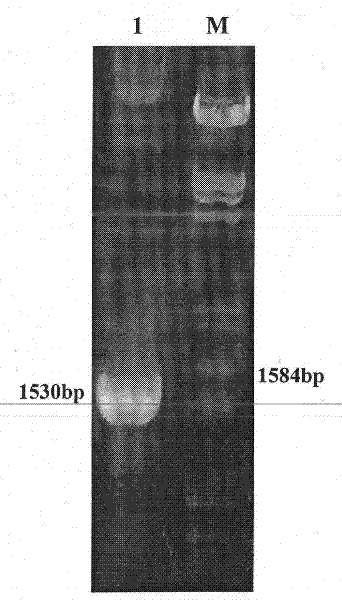
Method for preparing infective hog cholera virus cDNA carrier and use thereof
InactiveCN1603418AViral antigen ingredientsMicrobiological testing/measurementClassical swine fever virus CSFVVaccine research
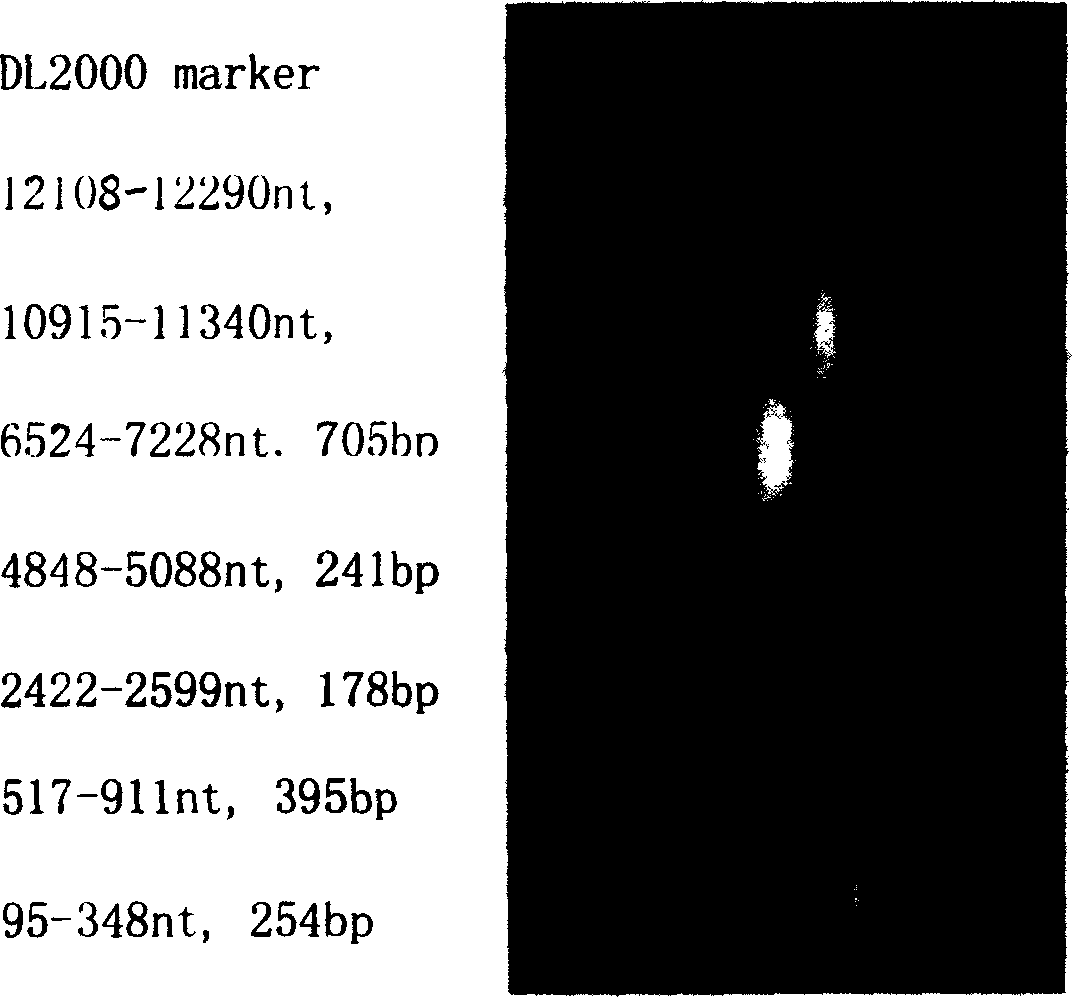
The invention discloses a pig acute communicable disease virus infectious DNA carrier preparation method and the application, this carrier includes T7 to start sub- and pig acute communicable disease virus standard full strength Shimen gene group's DNA, starts sub- and in downriver DNA in T7, in the flaw NS2 gene 1,260 alkali base to, retains the 5' end 3752nd alkali base to to the PshAI position spot 3881st alkali base to the between 130bp NS2 gene fragment, other DNA sequence and a Shimen infectiousness clone DNA to be same. The invention method is simple, the ease of operation, may construct the double price or the multi- prices genetic engineering vaccine, suits the pig acute communicable disease virus the distinction diagnosis and in the vaccine research application.
Owner:WUHAN UNIV
Method for constructing virus live vector recombinant vaccine by utilizing transposon
InactiveCN101850116AGenetic material ingredientsViruses/bacteriophagesSwine Fever VirusRecombinant vaccines
The invention discloses a method for constructing virus live vector recombinant vaccine by utilizing transposon. Green fluorescent protein is taken as a report gene, expression boxes respectively expressing rabies virus glycoprotein and swine fever E2 protein genes are constructed and are cloned to the shuttle vector of the transposon, under the action of mediation of transposase, recombination with purified canine adenovirus type II virus and herpes virus type I entire genome are respectively carried out, then transfection agent (liposome and the like) is utilized to respectively transfect the recombination product with MDCK and Vero cells, thus obtaining four strains of recombinant viruses taking green fluorescent protein as report gene, namely recombinant canine adenovirus type II virus expressing glycoprotein, recombinant canine adenovirus type II virus expressing E2 protein, recombinant herpes virus type I expressing glycoprotein and recombinant herpes virus type I expressing E2 protein. Immunity test shows that the canine adenovirus type II virus expressing E2 gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to swine fever virus infection in swine and canine adenovirus type II virus expressing glycoprotein gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to rabies virus infection in dog.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Swine testicular clone cell line and production method of classical swine fever live vaccine
ActiveCN102965332AMicroorganism based processesAntiviralsBovine Viral Diarrhea VirusesInfection rate
The invention provides a highly classical swine fever virus infective swine testicular clone cell strain ST-B2, which has a preservation number of CCTCC NO.C2011101, and a preparation method of the swine testicular clone cell. The method comprises the steps of: 1) subjecting a swine testicular cell to limited dilution, conducting cell cloning and subcell cloning, thus obtaining a subcellular clone strain; and 2) selecting a subclone strain of the swine testicular cell with a highest classical swine fever virus infection rate, i.e. the highly infective swine testicular clone cell of the classical swine fever virus. The invention also provides a method for preparation of a high titer classical swine fever virus solution and a classical swine fever live vaccine from the swine testicular clone cell. The swine testicular clone cell provided in the invention has a high classical swine fever virus infection rate, and the classical swine fever vaccine produced from the swine testicular cell line ST clone cell strain ST-B2 by a virus-carrying and virus transmission technique has high virus titer, hard exposure to BVDV (bovine viral diarrhea virus) pollution and good pureness. By the virus-carrying and virus transmission technique, a resurgent cell can undergo continuous passage to at least 15 generations. The cell resurrection and virus inoculation times can be reduced, the production process is simplified, and the production efficiency is improved.
Owner:PU LIKE BIO ENG
Recombinant duck plague virus of expressing duck tembusu virus E protein as well as construction method and application of recombinant duck plague virus
InactiveCN105039268AHigh expressionEliminate steps such as connectionViral antigen ingredientsAntiviralsTembusu virusFibroblast
The invention discloses a recombinant duck plague virus of expressing duck tembusu virus E protein as well as a construction method and application of the recombinant duck plague virus, wherein the gene of the duck tembusu virus E protein is interpolated inside the genome of the recombinant duck plague virus; and the nucleotide sequence of the duck tembusu virus E protein gene is as shown in SEQ ID NO. 9. The construction method comprises the following steps: (1) substituting the CMV promoter of gfp gene in pDEV-vac with an EF1 promoter so as to obtain pDEV-EF1; (2) interpolating a Pcmv-E-BGH-pA expression cassette into the pDEV-EF1 so as to obtain pDEV-E; and (3) transfecting the pDEV-E with chicken embryo fibroblasts and rescuing so as to obtain the recombinant duck plague virus. The recombinant duck plague virus, compared with a parent strain, has no significant difference in the size of virus plaque, showing that the diffusion of the duck plague virus on the chicken embryo fibroblasts is not affected by the interpolation of the duck tembusu virus E protein gene. After transfecting with the chicken embryo fibroblasts, the recombinant duck plague virus can successfully express E protein, so as to lay a foundation for developing duck plague virus-duck tembusu virus bivalent vaccine.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Vaccine peptides
There is provided a polypeptide having an amino acid sequence selected from SEQ ID NOs:1, 2 and 3 or a functional fragment thereof, or comprising an amino acid sequence at least 65% identical to any one of SEQ ID NOs:1, 2 or 3. There is also provided a method of rapidly vaccinating an animal against infection by a pestivirus comprising administering to the animal an effective amount of a polypeptide comprising an E2 epitope polypeptide and / or an NS3 epitope polypeptide; the E2 epitope polypeptide may be SEQ ID NO:1 and the NS3 epitope polypeptide may be SEQ ID NO:2 or 3, or functional fragments thereof.
Owner:THE UK SEC FOR ENVIRONMENT FOOD & RURAL AFFAIRS
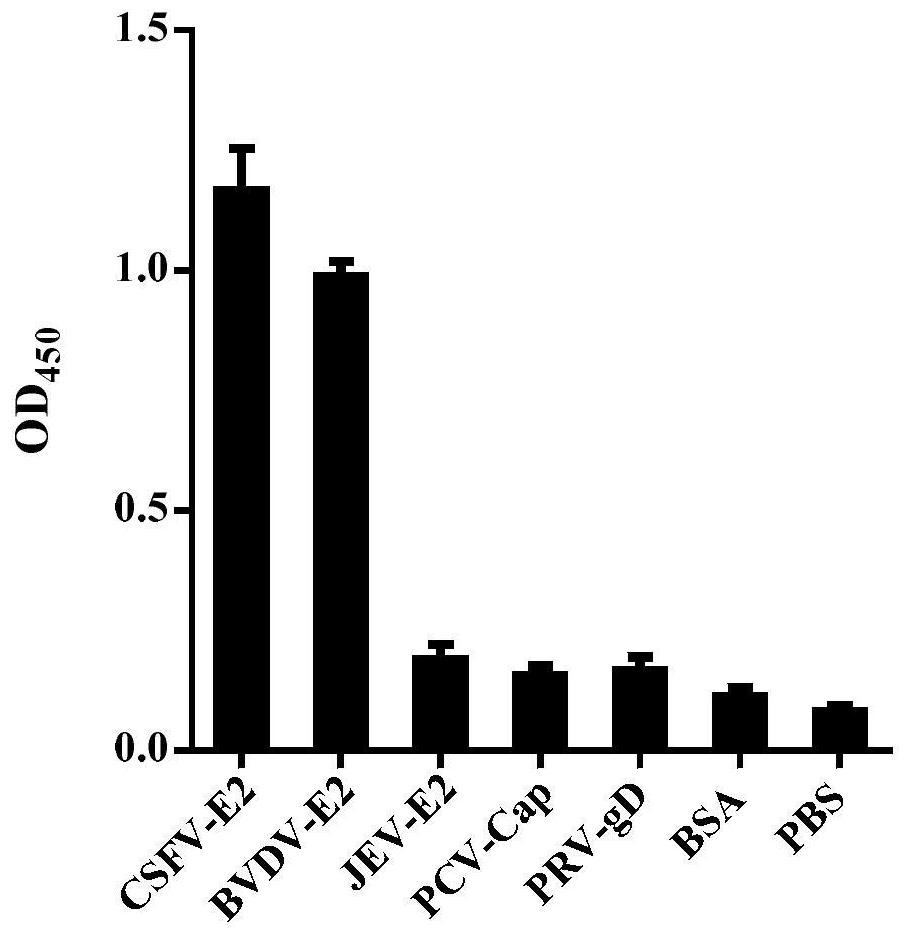
Enzyme linked immunosorbent assay (ELISA) kit for detecting atypical porcine pestivirus (APPV) antibody based on E2 protein
The invention discloses an enzyme linked immunosorbent assay (ELISA) kit for detecting an atypical porcine pestivirus (APPV) antibody based on E2 protein. The ELISA kit comprises a reaction plate enveloped by the E2 protein of the APPV shown in SEQ ID No. 1, an enzyme-labeled antibody, a color development solution, a stop solution, positive serum and negative serum. The invention creates the ELISAkit for detecting the APPV antibody for the first time and can rapidly, specifically and sensitively detect the APPV antibody in serum; the serum antibody detection sensitivity of the ELISA kit is 1to 1000. The ELISA kit provided by the invention is simple in use method, low in cost, easy in observation of reaction results and good in specificity and suitable for monitoring of APPV infection, epidemiological survey and detection of veterinary clinical samples, thus being suitable for wide-range popularization and application.
Owner:SOUTH CHINA AGRI UNIV
Non-spreading pestivirus
InactiveUS20050220813A1Prevent negative consequenceSafely vaccinatedSsRNA viruses positive-senseViral antigen ingredientsPestivirusWild type
The invention relates to vaccines used in the eradication or control of pestivirus infections, particularly those used in pigs or ruminants. The invention provides nucleic acid, pestivirus-like particles and a pestivirus vaccine, comprising the nucleic acid or particles, which is capable of eliciting a proper immune response without having the ability to spread throughout the vaccinated animal, thereby avoiding the negative consequences of viral spread. Preferably, the immune response allows for serological discrimination between vaccinated animals and wild-type pestivirus infected animals.
Owner:INTERVET INT BV
Non-spreading pestivirus
InactiveUS6923969B2Prevent negative consequenceSafely vaccinatedSsRNA viruses positive-senseSugar derivativesWild typeViral Vaccine
The invention relates to vaccines used in the eradication or control of pestivirus infections, particularly used in pigs or ruminants. The invention provides nucleic acid, pestivirus-like particles and a pestivirus vaccine, comprising the nucleic acid or particles, which is capable of eliciting a proper immune response without having the ability to spread throughout the vaccinated animal, thereby avoiding the negative consequences of viral spread. Preferably, the immunological response allows for serological discrimination between vaccinated animals and wild-type pestivirus infected animals.
Owner:INTERVET INT BV
Specific inference hog cholera virus genome sequence and method for efficiently curing hog cholera virus infection
InactiveCN101407809AAvoid infectionHigh suppression efficiencyGenetic material ingredientsAntiviralsDiseasePolymerase L
The invention provides a special genome sequence for disturbing a hog cholera virus and simultaneously discloses a method for effectively curing the infection of the hog cholera virus. The invention relates to a medicament for curing the infection of the hog cholera virus; the effective and special interruption RNA molecules for restraining the hog cholera virus can be obtained through designing the DNA template sequence of the special interruption RNA molecules which aim at the different genes of the hog cholera virus and utilizing T7, T3 or SP6RNA polymerase in vitro transcription systems and an in vitro transcription plasmid expression system which comprises an H1 or U6 promoter. The invention is suitable for industrial production. The invention also provides a biological agent produced by utilizing the method; the inhibition efficiency of the biological agent to CSFV achieves as high as 95.1 to 99.0 percent; and the infection of CSFV can be remarkably stopped in the body of sensitive animals for preventing the animals from disease and death.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Clone of IRG6 gene having potential anti-classical swine fever virus effect, and construction of stable expression cell line of IRG6 gene
ActiveCN102899338AConvenient researchHealth effectsTransferasesMicroorganism based processesPestivirus infectionsVirus infection mechanism
The present invention relates to clone of IRG6 gene having a potential anti-classical swine fever virus effect, and construction of a stable expression cell line of the IRG6 gene. The construction is characterized by comprising the following steps: (1) obtaining a gene fragment; (2) constructing a vector; (3) screening a stable IRG6 protein expression cell line; and (4) detecting reporter gene expression. The cell line can be used for classical swine fever virus infection mechanism research, wherein drugs for treating classical swine fever virus can be developed by revealing the anti-classical swine fever virus mechanism of the protein gene.
Owner:GUANGXI UNIV
Cloning of irg6 gene with potential anti-swine fever virus effect and construction of its stable expression cell line
ActiveCN102899338BConvenient researchHealth effectsTransferasesMicroorganism based processesSwine Fever VirusPestivirus infections
The present invention relates to clone of IRG6 gene having a potential anti-classical swine fever virus effect, and construction of a stable expression cell line of the IRG6 gene. The construction is characterized by comprising the following steps: (1) obtaining a gene fragment; (2) constructing a vector; (3) screening a stable IRG6 protein expression cell line; and (4) detecting reporter gene expression. The cell line can be used for classical swine fever virus infection mechanism research, wherein drugs for treating classical swine fever virus can be developed by revealing the anti-classical swine fever virus mechanism of the protein gene.
Owner:GUANGXI UNIV
TPR structural motifs and cellular localization of swine ISG60 gene associated with antiviral immunity
InactiveCN102559688ANo significant homologyAltered specific responseBiological testingFermentationSwine Fever VirusTetratricopeptide
The invention provides TPR (tetratricopeptide repeat) structural motifs of swine ISG60 gene associated with antiviral immunity, which contains viral infection-related regulatory element sequences that has no significant homology with existing reported TPR structure of a swine gene. The invention also specifically relates to the strategy of swine ISG60 gene, and identifies cellular location of ISG60 gene. The TPR structural motifs of swine ISG60 gene can be used for studying on the infection mechanism of classical swine fever virus. A medicine for treating swine fever can be developed through revealing the anti-swine fever virus mechanism of the gene.
Owner:GUANGXI UNIV
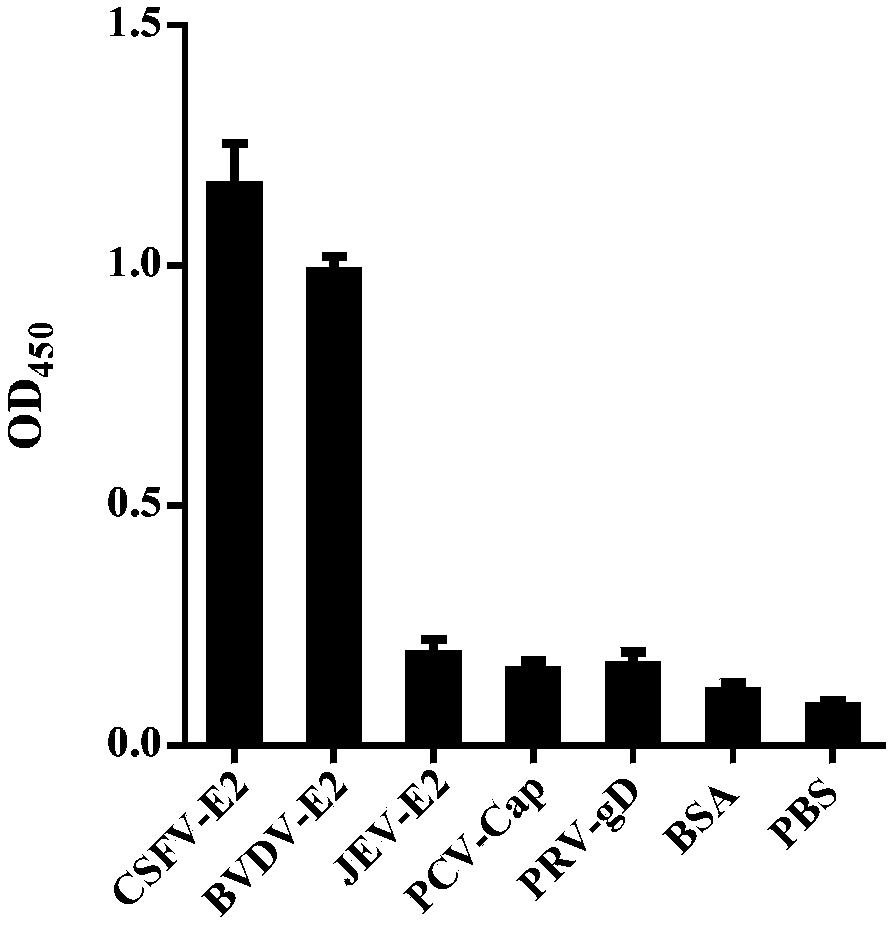
Protection of an animal against pestivirus infection
InactiveUS20100221275A1Antibacterial agentsSsRNA viruses positive-sensePestivirusPestivirus infections
This invention involves compositions and methods for protecting animals from pestivirus infection and for treating animals infected with pestivirus. Pharmaceutical compositions containing E2 and NS3 from a pestivirus are used to protect animals or treat animals.
Owner:THE ROYAL VETERINARY COLLEGE +1
Polypeptide for inhibiting hog cholera virus infection activity and application thereof
ActiveCN108586578AHigh affinityImprove bindingPeptide/protein ingredientsBiological material analysisAnti virusBovine Viral Diarrhea Viruses
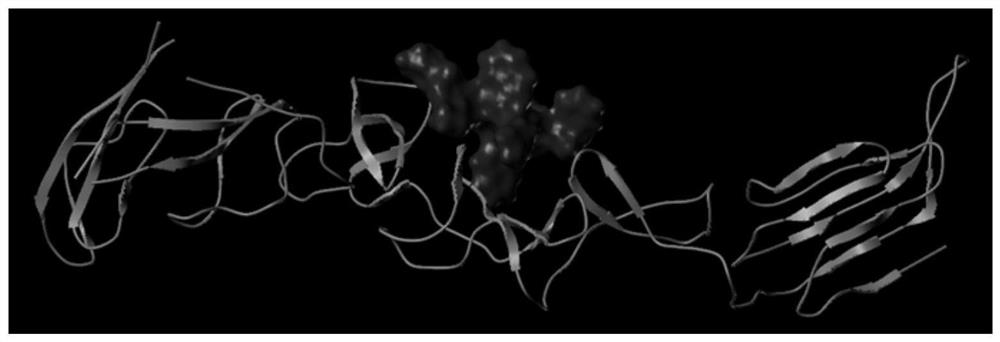
The invention provides a polypeptide for inhibiting hog cholera virus infection activity and application thereof. The sequence of the polypeptide is KRWWSHK (P8). The bovine viral diarrhea virus E2 protein crystal structure is used as a template; homology modeling is performed to obtain a three-dimensional structure of the hog cholera virus E2 protein; a virtual molecular butt joint technology isused for finally obtaining a polypeptide sequence with the high score value, i.e., P8. The artificially synthesized P8 sequences uses ELISA and SPR test for identifying the affinity and specificity ofthe mutual effect with the E2 protein. The result shows that the P8 and the E2 have higher affinity and specificity for combination; then, the virus infection inhibition activity is verified throughqRT-PCR and IPMA tests; the results show that the P8 sequence has relatively good activity for inhibiting the hog cholera virus infection PK-15 cells. The reliable theoretical basis and new idea are provided for further studying virus receptor and anti-virus medicine design.
Owner:HENAN ACAD OF AGRI SCI
Porcine pestvirus, vaccines, and assays
ActiveUS10555995B2SsRNA viruses positive-senseViral antigen ingredientsPorcine pestivirusClassical swine fever virus CSFV
Porcine pestivirus designated herein as atypical porcine pestivirus (“APPV”) (Genbank accession no. KR011347.1). Immunogenic compositions to induce an immune response against porcine pestivirus infection in a pig are described, which APPV antigenic agents (e.g., isolated whole virus, derivatives thereof, functional fragments thereof, and combinations of the foregoing). Methods of vaccinating against porcine pestivirus infection using the immunogenic compositions are also described. The methods can be also applied for clinical research and / or study, including diagnostic methods for detecting pestivirus infection using monoclonal antibodies specifically binding to APPV epitopes.
Owner:KANSAS STATE UNIV RES FOUND
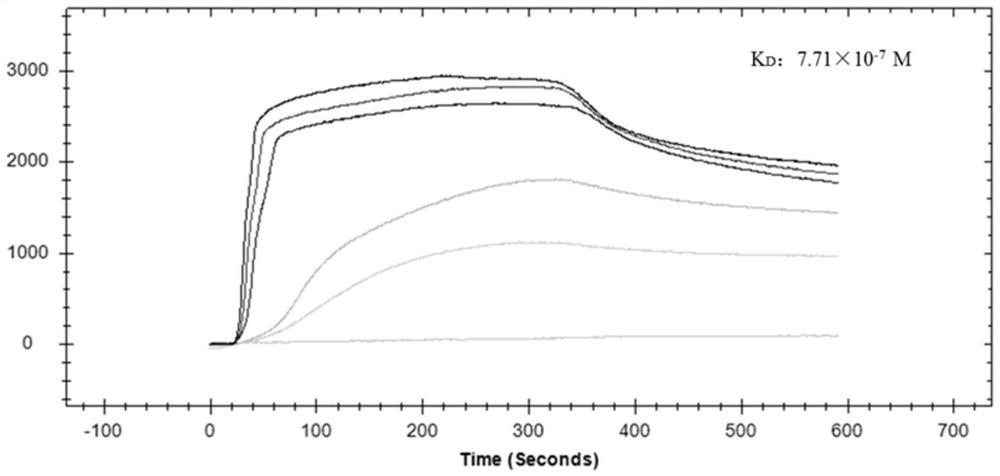
An affinity peptide capable of binding to classical swine fever virus e2 protein and its application
ActiveCN108383895BInhibitory activityHigh affinityPeptide/protein ingredientsBiological material analysisClassical swine fever virus CSFVBovine Viral Diarrhea Viruses
The present invention mainly relates to an affinity peptide that can be combined with classical swine fever virus E2 protein and its application. The sequence of the affinity peptide is HRKWKSKWK(P 7 ). The present invention uses the crystal structure of the E2 protein of bovine viral diarrhea virus as a template, and obtains the three-dimensional structure of the E2 protein of classical swine fever virus through homology modeling, and finally obtains a polypeptide sequence with a high score value, which is P 7 ; Synthetic P 7 ELISA and SPR tests were used to identify the affinity and specificity of its interaction with E2 protein, and the results showed that P 7 It has a high affinity and specific binding to E2; then its activity of inhibiting virus infection is verified by qRT‑PCR and IPMA tests, and the results show that P 7 The sequence has better activity of inhibiting the infection of PK‑15 cells by classical swine fever virus. The invention can provide a reliable theoretical basis and new ideas for further research on viral receptors and antiviral drug design.
Owner:HENAN ACAD OF AGRI SCI
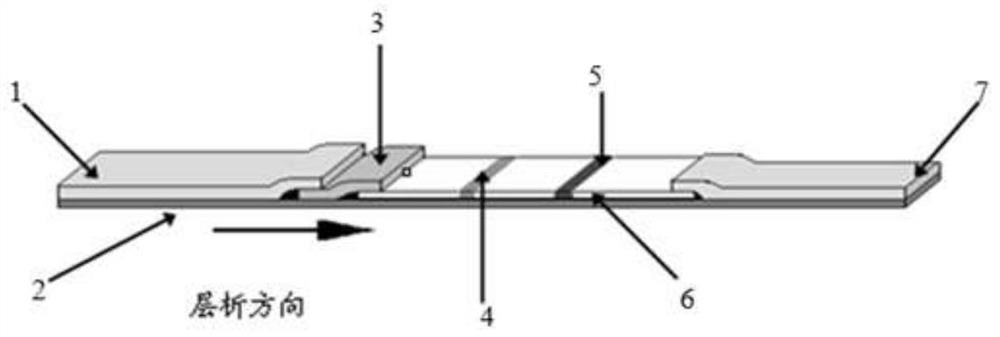
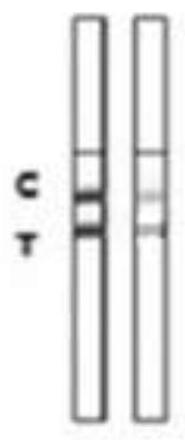
A colloidal gold detection card for rapid detection of rabbit plague virus and its preparation method
ActiveCN109856397BEffective controlAccurate detection of infectionMaterial analysisRabiesColloidal au
The invention relates to a colloidal gold detection card for rapidly detecting rabbit plague virus and a preparation method thereof. The specific steps are: (1) preparation of paired anti-rabbit plague virus monoclonal antibodies, including immune procedures, cell fusion, hybridoma screening and Cloning, antibody preparation and purification; (2) colloidal gold detection card for rapid detection of rabbit plague virus and its preparation method, including preparation of colloidal gold monoclonal antibody, sample pad treatment, spray film, C and T line determination, tested Sample processing, performance measurement. The invention can quickly, sensitively and accurately detect rabbit plague virus infection in the liver and kidney of rabbits, overcomes the domestic method of detecting rabbit plague virus mainly by red blood cell agglutination test, greatly shortens the detection time, and provides convenience for on-site detection.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Classical swine fever virus E2 subunit vaccine and preparation thereof
The invention relates to a recombinant E2 protein by using silkworm as a platform to produce a classical swine fever virus gene subgroup 2.1. The invention also provides a subunit vaccine comprising the recombinant protein, which can be used for enhancing the capability of swine to resist classical swine fever virus infection.
Owner:黄金城
Duck Tembusu virus e protein truncated gene, recombinant duck plague virus and its construction method and application
ActiveCN107312782BEfficient expressionSsRNA viruses positive-senseViral antigen ingredientsNucleotideTembusu virus
The invention discloses a duck Tembusu virus E protein truncated gene, a recombinant duck plague virus, a construction method and an application. The nucleotide sequence of the duck Tembusu virus E protein truncated gene is shown in SEQ ID No.4. A recombinant duck plague virus, wherein the duck Tembusu virus E protein truncated gene is inserted into the genome of the recombinant duck plague virus. The present invention screens and obtains a truncated gene of duck Tembusu virus E protein, inserts the E protein truncated gene into the genome of recombinant duck plague virus to obtain a new recombinant duck plague virus, and infects CEFs After cells, the E protein truncated gene is highly expressed. The recombinant duck plague virus inserted with the E protein truncated gene can be used for the preparation of duck plague virus and duck Tembusu virus dual vaccine.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
A polypeptide for inhibiting the infection activity of classical swine fever virus and its application
ActiveCN108586578BHigh affinityImprove bindingPeptide/protein ingredientsBiological material analysisClassical swine fever virus CSFVBovine Viral Diarrhea Viruses
A polypeptide of the present invention that inhibits the infection activity of classical swine fever virus and its application, the polypeptide sequence is KRWWSHK(P 8 ). The present invention uses the crystal structure of the E2 protein of bovine viral diarrhea virus as a template, and obtains the three-dimensional structure of the E2 protein of classical swine fever virus through homology modeling, and finally obtains a polypeptide sequence with a high score value, which is P 8 ; Synthetic P 8 ELISA and SPR tests were used to identify the affinity and specificity of its interaction with E2 protein, and the results showed that P 8 It has a high affinity and specific binding to E2; then its activity of inhibiting virus infection is verified by qRT‑PCR and IPMA tests, and the results show that P 8 The sequence has better activity of inhibiting the infection of PK‑15 cells by classical swine fever virus. The invention can provide a reliable theoretical basis and new ideas for further research on viral receptors and antiviral drug design.
Owner:HENAN ACAD OF AGRI SCI
Method for constructing virus live vector recombinant vaccine by utilizing transposon
InactiveCN101850116BGenetic material ingredientsViruses/bacteriophagesRecombinant vaccinesSwine Fever Virus
The invention discloses a method for constructing virus live vector recombinant vaccine by utilizing transposon. Green fluorescent protein is taken as a report gene, expression boxes respectively expressing rabies virus glycoprotein and swine fever E2 protein genes are constructed and are cloned to the shuttle vector of the transposon, under the action of mediation of transposase, recombination with purified canine adenovirus type II virus and herpes virus type I entire genome are respectively carried out, then transfection agent (liposome and the like) is utilized to respectively transfect the recombination product with MDCK and Vero cells, thus obtaining four strains of recombinant viruses taking green fluorescent protein as report gene, namely recombinant canine adenovirus type II virus expressing glycoprotein, recombinant canine adenovirus type II virus expressing E2 protein, recombinant herpes virus type I expressing glycoprotein and recombinant herpes virus type I expressing E2 protein. Immunity test shows that the canine adenovirus type II virus expressing E2 gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to swine fever virus infection in swine and canine adenovirus type II virus expressing glycoprotein gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to rabies virus infection in dog.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
An ELISA kit for detecting porcine atypical fever virus antibody based on e2 protein
The invention discloses an enzyme linked immunosorbent assay (ELISA) kit for detecting an atypical porcine pestivirus (APPV) antibody based on E2 protein. The ELISA kit comprises a reaction plate enveloped by the E2 protein of the APPV shown in SEQ ID No. 1, an enzyme-labeled antibody, a color development solution, a stop solution, positive serum and negative serum. The invention creates the ELISAkit for detecting the APPV antibody for the first time and can rapidly, specifically and sensitively detect the APPV antibody in serum; the serum antibody detection sensitivity of the ELISA kit is 1to 1000. The ELISA kit provided by the invention is simple in use method, low in cost, easy in observation of reaction results and good in specificity and suitable for monitoring of APPV infection, epidemiological survey and detection of veterinary clinical samples, thus being suitable for wide-range popularization and application.
Owner:SOUTH CHINA AGRI UNIV
Genetically modified pestiviruses and use thereof as a marker vaccine
PendingCN112020509ASsRNA viruses positive-senseViral antigen ingredientsDiseaseBovine virus diarrhoea
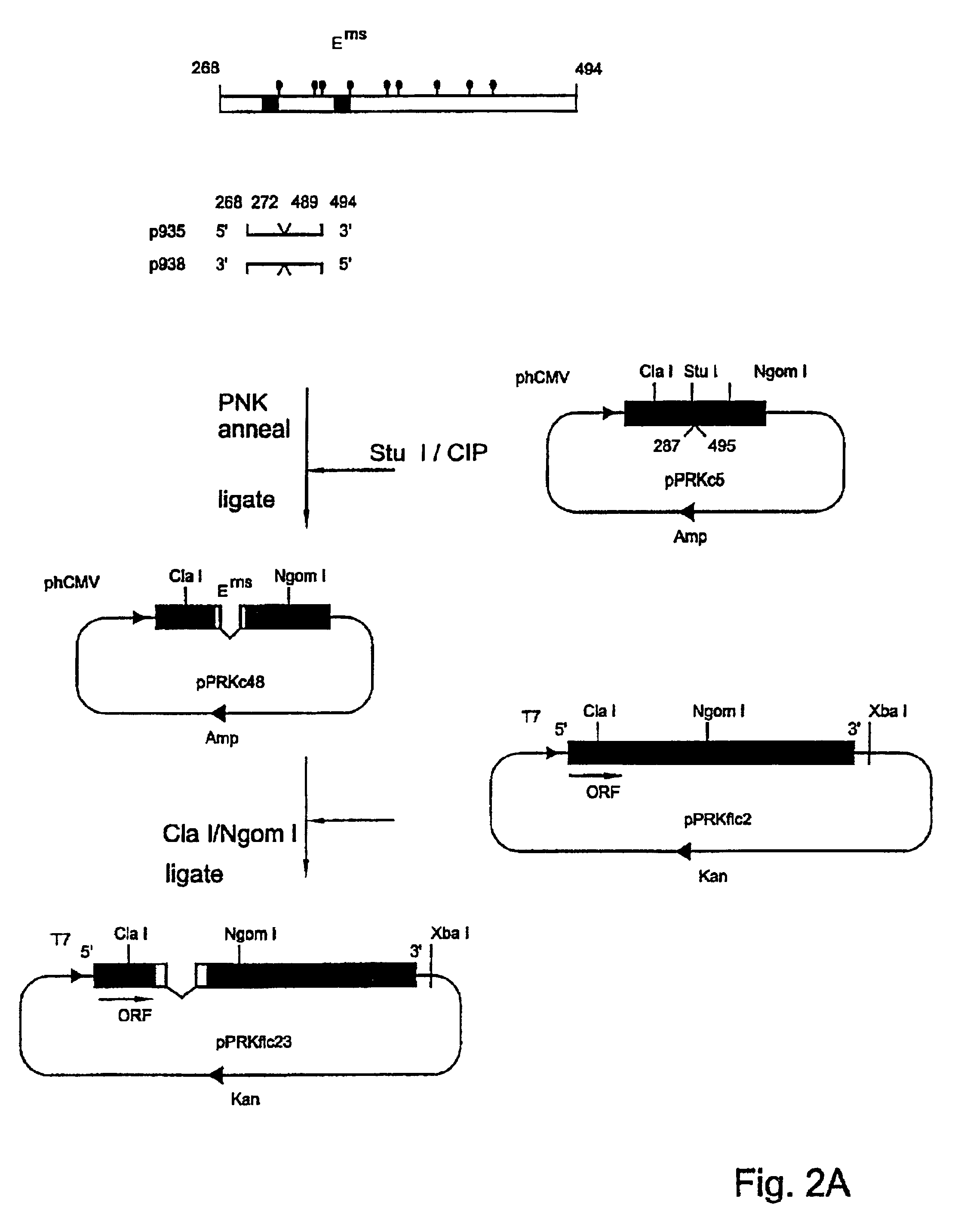
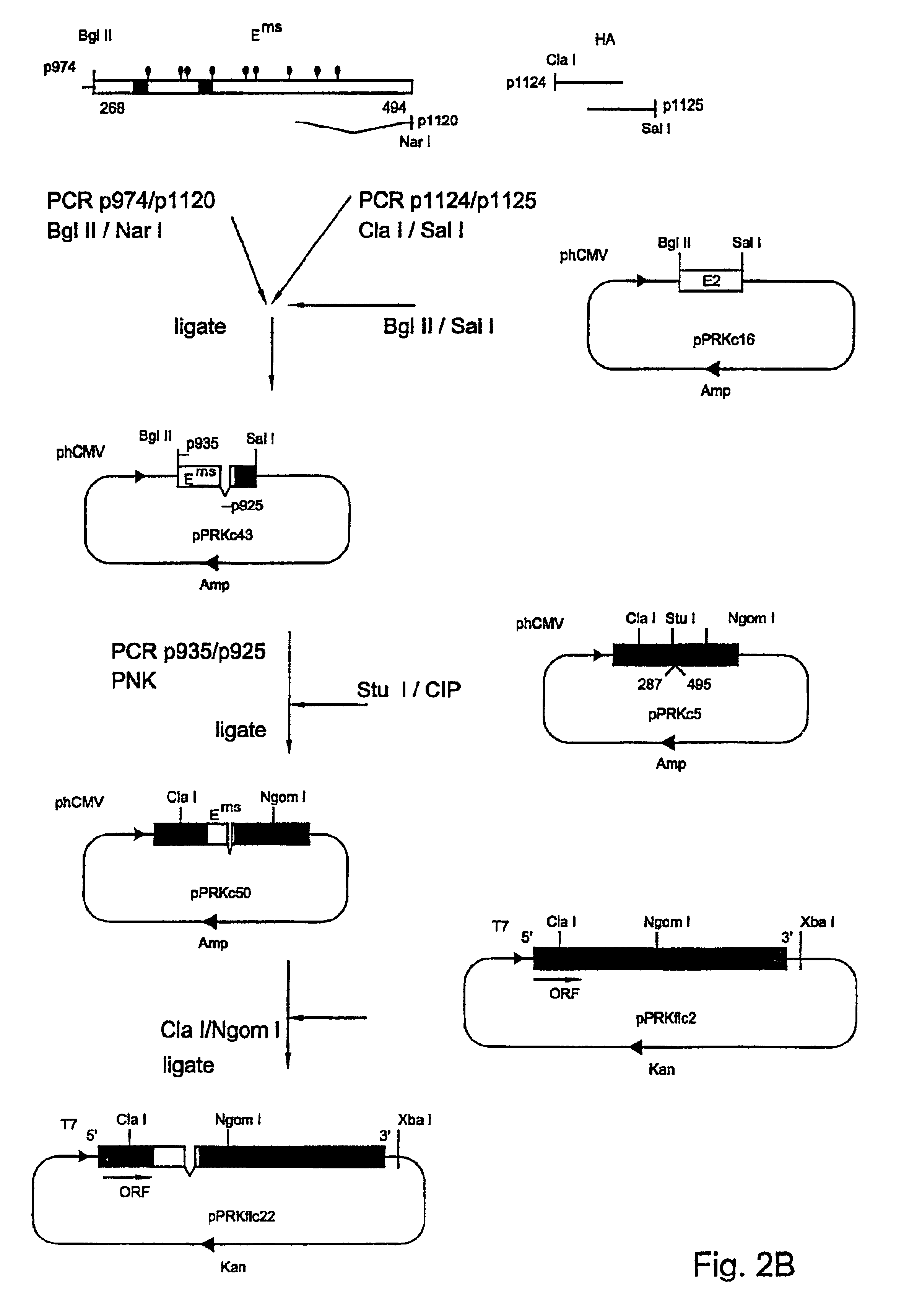
In a first aspect, the present invention relates to a genetically modified pestivirus, which is characterized by the fact that it is modified in such a way that at least the gene segment coded for theEms peptide originates from a distantly related pestivirus or from a plurality of pestiviruses that are distantly related to the genetically modified pestivirus. In a further aspect, the invention relates to an infected host cell or its cell culture supernatant containing a pestivirus genetically modified according to the invention. The invention is also relates to a vaccine and to its use and tothe use of the genetically modified pestivirus as a vaccine, in particular as a vaccine for animals and as a vaccine against classical swine fever (CSF), bovine virus diarrhoea (BVD), border disease(BD) and other illnesses caused by pestiviruses. The invention further relates to a recombinant chimaeric Ems peptide, and to detection systems for detecting pestiviruses or antibodies induced thereby, in particular for differentiating between vaccinated animals and animals after a natural infection with pestiviruses. Finally, the invention relates to a method for determining the extent to which an animal has been vaccinated with a vaccine according to the invention, and also to diagnostic kits for this purpose and to methods for controlling an infection with a pestivirus in a population of animals from the order of Artiodactyla.
Owner:汉诺威兽医学院基金会
ELISA kit for detecting porcine atypical pestivirus antibody based on Npro protein
The invention discloses an ELISA kit for detecting a porcine atypical pestivirus antibody based on an Npro protein. The kit comprises a reaction plate coated with porcine atypical pestiviruses havingsequences shown in the formula of SEQ ID NO. 1, an enzyme-labeled antibody, a developing solution, a stop buffer, positive serum and negative serum. The invention creates the ELISA kit for detecting aporcine atypical pestivirus antibody. The kit can fast, specifically and sensitively detect the porcine atypical pestivirus antibody and has antibody detection sensitivity of 1: 1000. The kit is easyto use, realizes a low cost, reaction result observation easiness and good specificity, is suitable for the monitoring of the atypical pestivirus infection, epidemiological investigation and clinicalveterinary sample detection and can be widely used.
Owner:SOUTH CHINA AGRI UNIV
one based on n pro ELISA kit for detection of porcine atypical fever virus antibody by protein
ActiveCN107677816BQuick checkSpecific detectionBiological material analysisSerum igeEpidemiologic survey
The invention discloses an ELISA kit for detecting a porcine atypical pestivirus antibody based on an Npro protein. The kit comprises a reaction plate coated with porcine atypical pestiviruses havingsequences shown in the formula of SEQ ID NO. 1, an enzyme-labeled antibody, a developing solution, a stop buffer, positive serum and negative serum. The invention creates the ELISA kit for detecting aporcine atypical pestivirus antibody. The kit can fast, specifically and sensitively detect the porcine atypical pestivirus antibody and has antibody detection sensitivity of 1: 1000. The kit is easyto use, realizes a low cost, reaction result observation easiness and good specificity, is suitable for the monitoring of the atypical pestivirus infection, epidemiological investigation and clinicalveterinary sample detection and can be widely used.
Owner:SOUTH CHINA AGRI UNIV
Nucleotide sequences of pestivirus strains, polypeptides encoded by these sequences and use thereof for diagnosis and prevention of pestivirus infections
The invention provides a nucleotide sequence corresponding to a classical swine fever virus (CSFV) genome or a part or a mutant thereof, which comprises at least a part of the nucleotide sequence of the CSFV C-strain depicted in SEQ ID No. 1, or a complement or RNA equivalent of such nucleotide sequence, or which comprises a nucleotide sequence encoding at least the amino acid sequence 268-494 of the amino acid sequence depicted in SEQ ID No. 1, or a complement or RNA equivalent of such nucleotide sequence. Also provided is a pestivirus polypeptide corresponding to the amino acid sequence 690-1063 of SEQ ID No. 1 or part thereof, which contains a mutation in one of the epitopes within amino acid sequences 691-750 or 785-870, said mutation altering said epitope. Further provided is a method of determining the presence of a test substance capable of specifically binding with a binding site of a binding partner, in a sample, by means of competition of said test substance with a measurable amount of a reference substance capable of specifically binding with the same binding site of said binding partner, comprising: (1) contacting said sample with (a) said reference substance bound to a solid carrier, (b) the binding partner of said reference substance, said binding partner molecule containing at least two identical binding sites for said reference substance, and (c) said reference substance provided with a label; (2) measuring the degree of binding of said label to said carrier.
Owner:动物保健研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00002.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00003.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-D00000.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-D00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-C00001.png)