Swine fever virus subunit vaccine and preparation method and purpose thereof
A swine fever virus and swine fever subunit technology, which is applied in the field of swine fever virus recombinant subunit vaccine and its preparation, can solve problems such as the inability to completely prevent vertical transmission and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
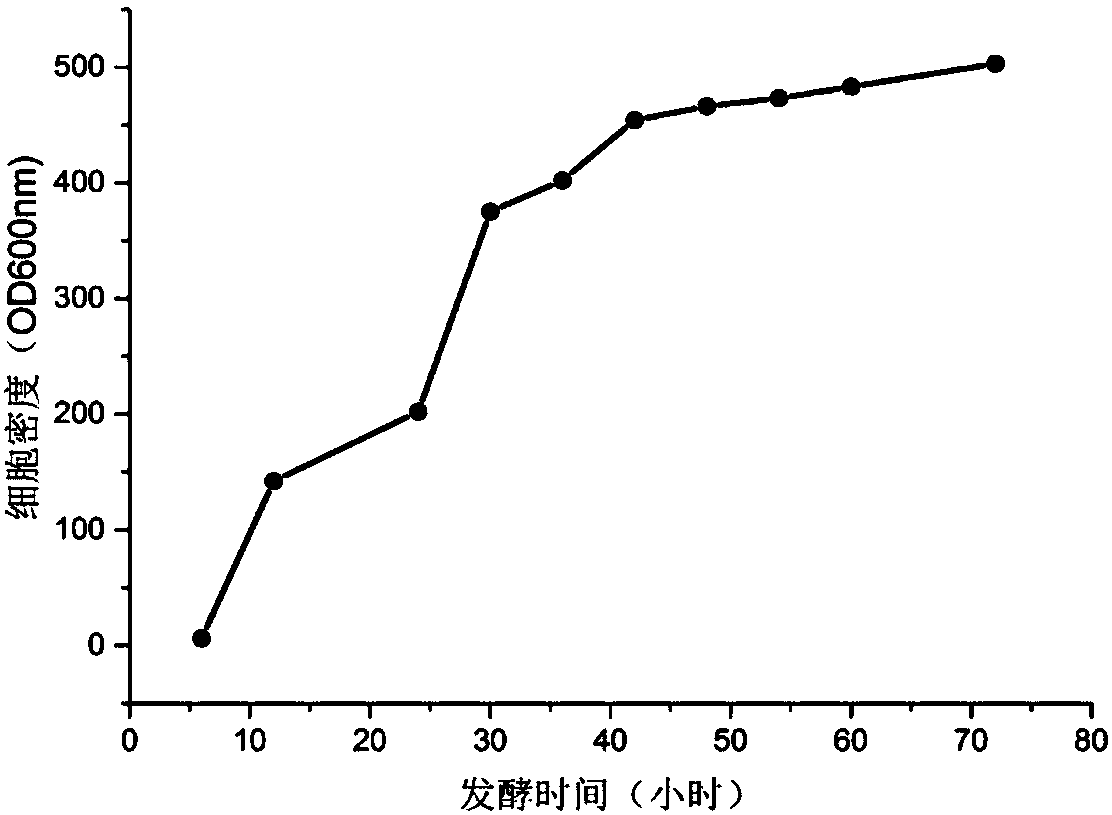
Image
Examples
Embodiment 1
[0052] Embodiment 1, the synthesis and the amplification of the dna sequence of coding swine fever virus mE2 protein
[0053] Comparing the popular strains of classical swine fever virus at home and abroad, the CSFV subtype 2.1b strain (Classical Swine Fever Virus strain Hun23 / 2013, GenBank: KP233071.1) popular in my country was selected. E2 The gene sequence is used as the gene source of the recombinantly expressed classical swine fever virus E2 protein sequence of the present invention.
[0054] According to literature reports, the main antigenic epitope of CSFV is located in the 1-176th position of the E2 protein, so the present invention selects the 1-678th nucleotide sequence of the E2 gene of the CSFV 2.1b strain for recombination express and name mE2 Gene. Referring to the codon preference of Kluyveromyces marx, without changing the amino acid sequence, the mE2 The nucleotide sequence of the gene is codon-optimized, and after optimization mE2 The nucleotide sequence...
Embodiment 2
[0056] Example 2, Construction of Kluyveromyces marx recombinant expression vector for mE2 protein of classical swine fever virus
[0057] restriction enzyme Speech I and Sac II Carry out double enzyme digestion on the Kluyveromyces marx expression vector pUKDN125, and after the digested product undergoes 1% agarose gel electrophoresis, the vector fragment of about 7.2kb is recovered with the SanPrep column DNA gel recovery kit. Using Gibson Assembly traceless connection system (NEB company, item number E2611S / L), the mE2 The gene segment is connected with the carrier segment to obtain the recombinant expression vector pUKDN125-mE2 of the CSFV mE2 protein Kluyveromyces marx. The recombinant vector includes yeast autonomous replication sequence, inulinase promoter, classical swine fever virus mE2 Genes, inulinase terminators, selection marker genes URA 3 promoters and their promoters.
Embodiment 3
[0058] Example 3, Construction of Kluyveromyces marx recombinant strain Fim-1-mE2
[0059] The yeast expression host strain used in the present invention is derived from Kluyveromyces marxense strain Fim-1 (CGMCC No. 10621 of the China General Microorganism Culture Collection Management Center), which is knocked out by homologous recombination. URA3 Gene, and use the YPD containing 5 fluoroorotic acid (1.5g / L) to screen to obtain the expression host bacterium of uracil deficiency, named as Kluyveromyces marxis Fim-1 ura3∆ . Transform pUKDN125-mE2 into Fim-1 using lithium acetate conversion method (World Journal of Microbiology & Biotechnology 16: 653-654, 2000) ura3∆ middle. Spread the transformed product on an SD plate (0.67% amino acid-free yeast nitrogen source, 2% glucose, 2% agar), place the plate in a constant temperature incubator at 30°C and culture it for 2-4 days until colonies are formed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com